James C. Watson
INTRODUCTION
Neurologic injury following regional anesthesia is an uncommon, but dreaded, complication that creates high levels of anxiety in the patient and anesthesiologist. Most deficits will be sensory predominant and limited in duration and severity and can be handled with reassurance and appropriate follow-up.
Discerning these cases from the rare complications that require emergent imaging, neurologic or neurosurgical consultation, or treatment is vital. This section focuses on recognition of postoperative neurologic complications, recognition of barriers to their recognition and evaluations, and an efficient, structured clinical approach to postanesthesia neurologic complications.
BARRIERS TO RECOGNITION OF POSTOPERATIVE NEUROLOGIC INJURY
Neurologic deficits in the postoperative setting may result from perioperative anesthetic procedures, surgical factors or iatrogenic injury, nerve compression occurring in the operative theater or during postoperative recuperation, or recognition of preexisting, but previously unappreciated, neurologic disease. While recognizing a neurologic complication immediately postoperatively would strongly implicate a perioperative complication (surgical, anesthetic, or positioning), there are many barriers to early recognition of perioperative neurologic complications. Postoperative sedation or analgesia may mask the complication.
Given expected neurologic symptoms postoperatively after regional anesthesia, patients and caregivers may presume that the patient’s symptoms are block related. Patients therefore fail to complain of symptoms that may be unrelated to the block, and caregivers may fail to pursue reported symptoms because they presume they are block related, when in fact they may be distinct in distribution from the expected neurologic deficit.
Patients also are unaware of what to expect postoperatively and may presume that postoperative symptoms are normal. Surgical dressings, drains, castings, and postoperative activity restrictions limit a patient’s activity level postoperatively such that a postoperative neurologic deficit may be unrecognized until more normal activity levels can be resumed.
Finally, patients often see the postoperative period as a single time epoch (the perioperative blur) instead of individual days where precise recognition of symptom onset would have been useful in finetuning the differential diagnosis regarding the cause of a postoperative neurologic complication. In a prospective study of postoperative ulnar neuropathy, some patients in follow-up reported that their symptoms were noted “immediately” following surgery, while the prospective evaluations had clearly documented an onset of signs and symptoms more than 48 hours after surgery, hence exonerating the surgical and anesthesia operative teams and implicating a postoperative convalescence complication.
Given these barriers, only 77%–90% of sensorimotor and 20% of sensory nerve injury complications following total hip and knee arthroplasties were recorded during the procedural hospitalization. Studies that only include early neurologic injury (less than 48 hours postoperatively) likely underestimate risk.
Conversely, injuries recognized late often have (or perhaps more likely have) nonanesthetic/operative-related causes, including infection, postoperative inflammation, and consequences of immobilization or compression in the recovery period. The frequency of ulnar neuropathy in surgical cohorts more than 2 days postoperatively, for example, is similar to the frequency in medical patients hospitalized for the same duration. These nonanesthetic/operative complications are often obvious as they occur in a distribution distinct from the surgical or anesthetic site, but when they do not, they further confuse the clinical picture.
BARRIERS TO NEUROLOGIC EVALUATION OF A POSTOPERATIVE NEUROLOGIC COMPLICATION
Neurologic evaluation of an identified postoperative neurologic complication is limited by many of the same factors that interfere with recognition. Dressings, casting, drains, and activity restrictions limit the ability to perform a comprehensive neurologic examination and hence the ability to localize a nerve injury. Electrophysiologic testing has the same limitations in being able to adequately access muscles and nerves that may be discriminatory for localization. The operating room and specifically regional anesthetic and surgical approaches to various problems are generally foreign to most neurologists, and as such they may be unaware of which structures were most at risk or which mechanism of injury may be most probable from a given surgical or anesthetic technique. In many institutions, the anesthetic record may be unavailable to the consulting neurologist or when available formatted in a way that makes extracting usable information challenging for the nonanesthesiologist. Useful neurologic consultation is facilitated by direct and frank discussion between the anesthesia, surgical, and neurologic services.
Mechanisms of Injury
The potential mechanisms of anesthesia-related neurologic injury have been previously articulated; however, the mechanism of injury is pertinent to workup and prognosis. Documentation and recognition of preexisting neurologic disease is important, as it may explain falsely localizing neurologic signs evident during the assessment of an apparent postoperative nerve injury. For example, hyperreflexia and a Babinski sign from preexisting cervical spinal stenosis may falsely suggest a central nervous system etiology in a patient with a peripheral nerve injury (PNI). Preexisting neurologic disease, while sometimes insufficient alone to cause clinical symptoms, limits the neurologic reserve of a nerve, meaning that it is more susceptible to developing clinical deficits from a second injury. This has been shown to be particularly relevant in postanesthesia nerve injuries, which are more common in at-risk nerves (the double-crush phenomenon).
Similarly metabolic derangements that are frequently associated with peripheral neuropathy, such as diabetes mellitus, may have caused unrecognized PNI previously insufficient to have caused clinical symptoms, but sufficient to decrease the neurologic reserve and put a peripheral nerve at risk from a second hit. This likely explains the frequent association of diabetes as a risk factor for post–regional anesthesia neurologic complications. Preexisting systemic diseases associated with neurogenic impairment (diabetes with neuropathy, for example) likely also impair the potential for recovery following a PNI.
Vascular injuries are the most catastrophic of possible postanesthesia complications. Ischemic vascular injuries may be related to an embolic phenomenon, direct trauma, or vasoconstriction of the artery of Adamkiewicz causing an anterior spinal artery syndrome (ASAS) or from watershed ischemia related to hypotension or vasoconstriction. Notably, spinal cord blood flow is autoregulated, and hypotension would need to be extreme (mean arterial pressure < 50 mmHg) or occur in the setting of impaired autoregulation to cause a watershed spinal cord ischemic event. Hematoma formation is critical to recognize, as it is treatable; it is devastating if unrecognized. Anticoagulation or a bleeding diathesis predispose to hematoma risk, and consensus recommendations exist for antiplatelet and anticoagulation use in the setting of regional anesthesia.
Infectious processes can cause neurologic impairment from diffuse involvement (meningitis or a polyradiculopathy) or from abscess formation and compression (epidural abscess). Infectious processes are obviously treatable, but potentially devastating when unrecognized.
Direct neurogenic injury (spinal cord or peripheral nerve) from needle or catheter trauma, local anesthetic toxicity, or surgical trauma is variable in its severity and prognosis. Unfortunately, once it has occurred, there is little that can be done to intervene on or improve its natural history and likelihood of recovery.
Some peripheral nerve injuries are unrelated to the anesthetic or surgical intervention, although the anesthesiologist or surgeon are often erroneously blamed. For example, there would be appropriate concern for a neuraxial complication in a patient with an epidural catheter who awoke with a foot drop, but careful evaluation may show a simple peroneal compressive neuropathy at the fibular head unrelated to the epidural catheter. In addition, while compressive neuropathies can occur in the operative theater, they commonly occur during postoperative hospitalization and the period of convalescence. Similarly, there has been increasing recognition that some postsurgical neuropathies are related to an inappropriate inflammatory response directed at the peripheral nervous system. These are important to recognize as they are unrelated to a specific anesthetic or surgical intervention and are potentially treatable.
Neuraxial Complications
Neuraxial complications from anesthetic techniques include epidural hematoma (EH), spinal epidural abscess (SEA), ASAS, and direct cord trauma. Fortunately, these are very rare (EH, 2 per 100,000 to 1 per 140,000–220,000 neuraxial techniques; SEA, 1 in 40,000 to 1 in 100,000 neuraxial anesthetics), but they can be neurologically devastating if unrecognized. With all neuraxial complications, the longer it takes to diagnose and treat, the worse the prognosis is. As such, unless the neurologic deficit is distinctly limited to the distribution of a peripheral nerve susceptible to compression (ulnar or peroneal nerve), in the setting of a postoperative neurologic complication occurring following neuraxial anesthesia, the patient needs emergent advanced spine neuroimaging.
Magnetic resonance imaging (MRI) is the imaging modality of choice given its ability to localize the catheter (if an epidural catheter intervention), differentiate soft tissue structures, define neurogenic impingement of adjacent structures (cord or root[s]), and identify preexisting, but pertinent, comorbidities (such as spinal stenosis). Computed tomography (CT) of the spine is sufficient to identify space-occupying lesions such as EHs or abscesses that require emergent neurosurgical intervention but lacks the soft tissue discriminatory sensitivity of MRI. As such, CT would not be able to identify an intrinsic spinal cord injury such as ASAS or direct needle trauma. If MRI is not immediately available on recognition of a postanesthetic complication following neuraxial intervention, CT of the spine should be pursued. It will exclude a neurosurgical emergency (SEA or EH), but if the CT is negative without resolving neurologic deficits, MRI should be arranged as soon as possible to assess for an intrinsic cord process even if it requires transfer to a facility with more immediate resources.
NYSORA Tips
- In the setting of neuraxial anesthesia, any concern for spinal cord dysfunction requires emergent neuroimaging.
- MRI is the preferred imaging modality. However, imaging should not be delayed to arrange MRI or to obtain neurologic consultation. CT or CT myelography is acceptable as initial imaging to exclude a compressive lesion.
Epidural hematoma in the setting of neuraxial anesthesia generally presents more fulminantly (75% present with deficits maximizing over 24 hours) than when EH is related to anticoagulation or unknown causes. However, 25% present with a slower evolution of symptoms, and, as such, the absence of a fulminant presentation should not reassure the anesthesia team. Emergent imaging should be pursued for any unexplained neurologic deficit following neuraxial intervention.
Typically, EH is heralded by localized spinal pain at the time of bleeding onset; however, anesthesia or analgesics frequently mask this. Patients progress from vague sensory symptoms below the site of the hematoma to a dense sensory loss below this level (a sensory level) that can be identified with cold (alcohol swab) or pinprick. A flaccid lower extremity paralysis develops concomitantly as the sensory deficits become more severe, and two-thirds of patients develop neurogenic bowel and bladder as a late complication.
Risk factors include anticoagulation (most common), antiplatelet usage, bleeding diathesis, an emergency operation, technically challenging epidural or spinal anesthesia, and older age. Spinal epidural abscess risk factors include diabetes, immunosuppression, systemic cancer, preexisting infection, intravenous drug abuse, alcoholism, trauma, and prolonged duration of epidural catheter maintenance. Like EH, localized spinal pain is frequently the first sign for SEA, but this is often masked by postoperative analgesia.
Fevers and elevated serologic inflammatory markers follow, but in patients who are immunosuppressed, these signs may not develop. The first neurologic sign is usually root irritation in a distinct radicular pain pattern. Neurologic deficits, including sensory level, paraparesis, and neurogenic bowel and bladder, develop below the level of the SEA with time, although a smaller percentage than with EH progress to frank paralysis before diagnosis.
SEA may also seed the leptomeninges, causing frank meningitis, with such patients showing signs of sedation, confusion, headache, nuchal rigidity, light sensitivity, and possibly seizure.
Anterior spinal artery syndrome results from a complication involving the anterior spinal artery causing ischemia to the anterior two-thirds of the spinal cord. This has been reported most commonly with neuraxial pain interventions, particularly transforaminal epidural steroid injections, but could conceivably occur with paravertebral procedures or neuraxial anesthetic procedures during which the needle is placed laterally in the interlaminar space. Mechanisms include embolization (particulate steroid, vertebroplasty cement, or atherosclerotic debris), dissection, vasospasm, or direct trauma to the artery of Adamkiewicz in the thoracolumbar spine or to the vertebral, ascending, or deep cervical arteries in the cervical spine. Patients present fulminantly and progress rapidly to para- or tetraplegia, with a sensory level limited to pain and temperature modalities (spinothalamic tract dysfunction) with relative sparing of the posterior columns (proprioception). Hyperreflexia and spasticity will eventually develop, but in the hyperacute phase, spinal shock causes areflexia and a flaccid tone.
Direct needle trauma to the spinal cord with neuraxial anesthesia may be the most difficult of neuraxial complications to recognize. Needle insertion into the cord without injection may not cause pain. While pain would be expected with increased intramedullary pressure with injection into the cord, this may not be evident in a sedated patient. In addition, paresthesias are not uncommon with properly performed epidural anesthetics, so their presence alone does not indicate cord trauma. ASA closed-claim data indicate these are most common in cervical spine procedures (including pain interventions) and are commonly associated with major morbidity or mortality. The clinical manifestations and prognosis of direct needle trauma to the cord are variable depending on the site of injury and whether an injection was performed.
A diagnostic algorithm for the evaluation of postneuraxial anesthesia neurologic complications is presented in Figure 1.
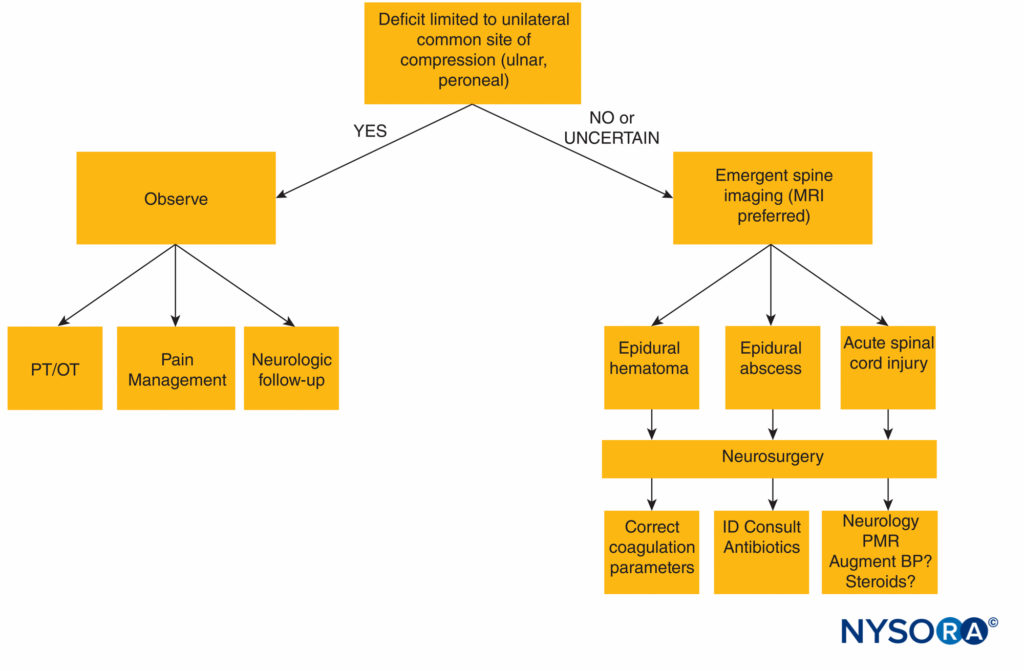
FIGURE 1. Diagnostic algorithm for neurologic deficit following neuraxial anesthesia. BP = blood pressure; MRI = magnetic resonance imaging; OT = occupational therapy; PT = physical therapy; ID = infectious disease, PMR = polymyalgia rheumatica.
TREATMENT AND PROGNOSIS OF NEUROLOGIC COMPLICATIONS OF NEURAXIAL PROCEDURES
The prognosis following a neurologic complication of neuraxial anesthesia can be dire: 5.5% of patients with EH and 15% with SEA die. For EH and SEA, early recognition and intervention improve neurologic functional outcome. For EH, of those evacuated within 8–12 hours, 40%–66% make a complete recovery, whereas when evacuation occurs more than 12 hours after presentation more than half of patients are left with no improvement or severe residual neurologic deficits. For SEA, functional recovery is significantly improved in those treated definitively before paralysis or in those with paralysis for less than 36 hours. Patients with paralysis for more than 48 hours at the time of evacuation are unlikely to recover. Of all patients with SEA, almost one-third remain paralyzed.The severity of the neurologic deficit at the time of evacuation predicts outcome, with more severe deficits at evacuation having a lower likelihood of a good recovery. As such, for EH or SEA, the goal should be surgical evacuation as soon as possible after diagnosis, and neurosurgical consultation should be obtained immediately even if it requires transfer to a facility with more immediate access to neurosurgical services.
NYSORA Tips
- Diagnosis of a compressive lesion (EH or SEA) within or near the neuraxis demands emergent neurosurgical consultation for consideration of decompression.
- Outcomes for compressive lesions (EH or SEA) are dependent on the severity of neurologic impairment and the duration of symptoms at the time of neurosurgical decompression. Neurologic recovery is improved with early decompression (<8–12 hours from symptom onset in EH and < 36 hours from symptom onset for SEA) and with milder preoperative neurologic deficits.
Unlike EH and SEA, for which early intervention can improve neurologic outcome, there is no proven treatment for ASAS or for direct cord trauma. Almost two-thirds with ASAS do not improve, minimally improve, or die. Those that survive often require gait aides or a wheelchair for motor and sensory deficits and are left with neurogenic bowel and bladder functional deficits.
Corticosteroids are frequently used in the setting of acute traumatic spinal cord injury. Treatment is with methylprednisolone at a dosage of 30 mg/kg over 15 minutes within 8 hours of the injury, followed by a maintenance infusion of 5.4 mg/kg/h for an additional 23 hours, having been shown in meta-analysis and Cochrane review to improve motor outcomes at 1 year postinjury. Others have questioned the validity of this practice or the assertion that it has a benign side effect profile and thus concluded that the evidence is insufficient to recommend corticosteroids as a standard guideline to treat acute spinal cord injury. Steroids are commonly empirically given intraoperatively with variable dosing in the setting of presumed neurologic injury. The role of corticosteroids in the setting of neuraxial complications of regional anesthesia is unknown. Considerations to their unproven use in this setting include that the postoperative neurologic complication may have been delayed in recognition, whereas the supportive data for traumatic spinal cord injury are in the hyperacute phase immediately following the injury. In addition, corticosteroids may increase the risk of postoperative infection or impaired wound healing.
Peripheral Nerve Injury
There are multiple risk factors for PNI in the setting of regional anesthesia (Table 1). These include patient characteristics that make regional anesthetic procedures more challenging (body mass index) or lead to limited neurogenic reserve, putting nerves at risk from a second perioperative insult (double-crush syndrome), as well as perioperative factors.
TABLE 1. Risk factors associated with perioperative peripheral nerve injury.
| Patient Characteristics | Perioperative Characteristics |
|---|---|
| - reexisting neurologic diseasea | - Paresthesia with needle placement |
| - Diabetesa | - Pain with injection |
| - Smoker | - Prolonged tourniquet time |
| - Body mass index (BMI) extremes | - Positioning: compression or stretch |
| - Elderly | - Sedated patient during regional block |
| - Prolonged hospitalization |
There are three steps to evaluating a postanesthetic or operative peripheral nerve injury (PNI). (1) Is there an active process (hematoma or compartment syndrome) causing neurologic impairment that can be treated? (2) Is the PNI surgically related? (3) Localize the neurologic deficit.
Similar to neuraxial complications, a bleeding complication (perineural or retroperitoneal hematoma) should be considered in patients whose perioperative interventions were performed or maintained while on anticoagulation or antiplatelet agents, in the setting of thrombocytopenia or bleeding diathesis, or if the procedure was performed in close proximity to vascular structures (particularly if the regional anesthetic was performed without ultrasound guidance). When considered, urgent imaging (CT or ultrasound) should be pursued. If identified, coagulation parameters should be corrected and, if severe, surgical evacuation to be considered.
The second issue to be considered is whether the deficit is iatrogenic, but surgically related. In a study of 1614 axillary blocks, surgical variables were thought responsible for 89% of the identified PNIs, most commonly related to direct trauma or stretch. Of PNIs serious and lasting enough to require peripheral nerve exploration, 17% were for iatrogenically induced neuropathies, of which 94% of these were originally injured intraoperatively. The surgical team would be aware of suspicious sutures, clips, or instrumentation placed intraoperatively, whether any nerve relevant to the patient’s symptoms was directly at risk intraoperatively from direct trauma or transection, whether excessive traction was necessary, or whether there were intraoperative concerns raised regarding vascular structures, hemodynamics, or intraoperative monitoring. Cases with severe neurologic deficits and a concern for a potential surgical complication may require urgent surgical exploration. Patient care is facilitated by direct, nonincriminatory discussion between the anesthesia and surgical teams.
The final step in evaluation is localization and characterization of the PNI. This will help stratify which patients can be reassured and simply followed and which patients deserve early neurologic consultation and further workup. Localization is important as PNI is often in a distribution distinct from where the peripheral regional anesthetic was performed.
While this may exonerate the anesthesiologist, it still requires further assessment and arrangements for appropriate follow-up. If the clinical symptoms are sensory in nature (two-thirds of PNIs) and limited to the distribution in which the block or infusion was performed, the prognosis is excellent, and patients can be reassured that these symptoms should resolve over days to weeks. Appropriate follow-up should be arranged to ensure symptom resolution. In the rare case where symptoms persist at follow-up several weeks after symptom onset, further neurologic and electrophysiologic assessment should be considered.
If symptoms are in a territory distinct from the distribution of the block, it should be determined if the clinical findings are limited to a nerve that is vulnerable to compression in the operative theater (most commonly, ulnar nerve at the elbow and peroneal nerve at the fibular head). If the symptoms are limited to this distribution, then the presumed mechanism of injury is neurapraxia, whereby there is localized myelin sheath dysfunction, usually from compression. Even if associated with weakness, these usually improve over a period of days to weeks and can be managed with reassurance and scheduled follow-up to direct further assessment if symptoms or deficits persist. Nerve conduction studies (NCSs) can identify conduction block indicating neurapraxia at a common site of compression in the acute postoperative period and can be useful to confirm compressive mononeuropathy if there is clinical uncertainty in the immediate postoperative period.
Beyond this, electrophysiology is of limited utility in the immediate postoperative period (see the section on the role of electrophysiology).
If clinical signs and symptoms are limited to a single nerve distribution (mononeuropathy) but severe (defined as a neurologic deficit causing functional limitations), motor predominant, or progressing, then early (in-hospital) neurologic assessment is appropriate, as is consideration of active treatable causes (hematoma, compartment syndrome, inflammatory neuropathy) or of a surgical complication. If the PNI cannot be localized to an individual nerve territory and is diffuse or multifocal (or simply challenging to localize), then early neurologic assessment is appropriate, especially if associated with functional impairment or progressive signs and symptoms (Table 2).
TABLE 2. Indications for early neurologic consultation for PNI.
| • Deficit(s) are • Severe • Functionally limiting • Progressivea • Multifocal of difficult to localizea |
| • Unexplained neurologic impairment outside the block region or region of common compression |
| • Associated severe pain (disproportionate to typical postoperative course)a |
| • Intervening return to neurologic baseline after surgery before development of PNIa |
Figure 2 outlines an algorithmic approach to peripheral nerve injuries following regional anesthesia.
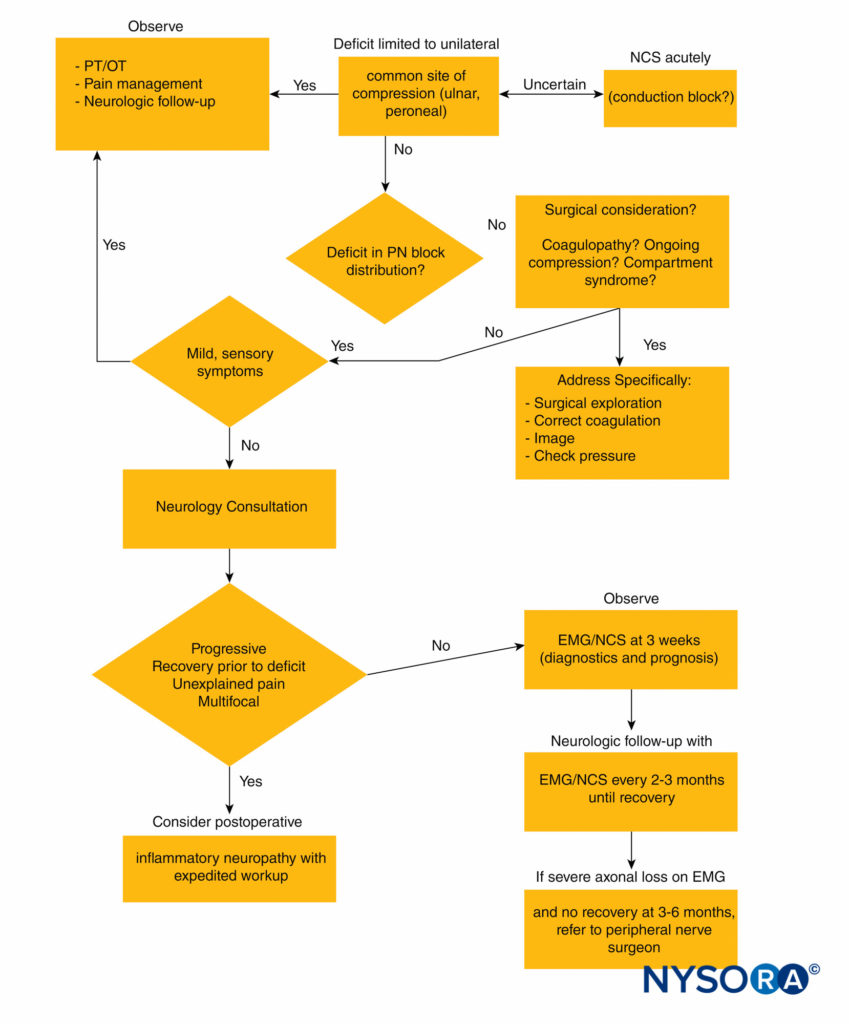
FIGURE 2. Diagnostic algorithm for peripheral nerve injury following regional anesthesia. EMG = electromyography; NCS = nerve conduction studies; OT = occupational therapy; PN = peripheral nerve; PT = physical therapy.
Clinical Pearl
- Once an active process that requires urgent intervention to remove something causing ongoing damage has been excluded, unfortunately there is nothing further that can be done that will materially affect the patient’s neurologic functional outcome.
Postsurgical Inflammatory Neuropathies
There is an evolving literature on postoperative peripheral nerve deficits arising from an inflammatory-immune response triggered by the stressor of surgery or anesthesia that inappropriately targets the nerves of the peripheral nervous system. Postsurgical inflammatory neuropathies are most often multifocal or diffuse within a limb, but mononeuropathies have been reported as well. Most commonly, they are recognized in a distribution distinct from the surgery or regional anesthetic, but they can occur within the same distribution, which makes discerning them from a direct surgical or regional anesthetic complication challenging. The temporal onset of the neurologic symptoms can be useful in distinguishing a postsurgical inflammatory neuropathy from other causes of PNI.
Classically, inflammatory neuropathies such as Parsonage-Turner syndrome (idiopathic brachial plexopathy), diabetic or nondiabetic lumbosacral radiculoplexus neuropathy, or postsurgical inflammatory neuropathie have severe pain at their onset, developing hours to weeks after a stressor (surgery, anesthesia, vaccination, illness). The pain is usually poorly localized but often affects the proximal more than the distal limb. While it can be confused for a radiculopathy, back or neck pain is absent or limited and, in the context of a postoperative complication, mechanistically unlikely. Weakness will develop subsequently, although sometimes is not appreciated until pain has started to improve. The weakness is variable in distribution but usually multifocal within the limb. Sensory deficits accompany the motor weakness, but clinically the pain and weakness predominate the clinical picture, and there are notable functional limitations related to both.
In cases occurring during the postoperative hospitalization, perioperative analgesia and regional anesthesia (particularly infusion catheters) make recognition of the painful onset of an inflammatory neuropathy challenging. A contextual cue of an inflammatory cause of PNI in the immediate perioperative period would be unexplained, refractory severe postoperative pain distinct from that typically expected or a period of good pain control followed by the emergence of severe, unexplained limb pain. Similarly, severe pain in a region distinct from the surgery or regional anesthetic that progresses to neurologic deficits would suggest the possibility of an inflammatory postsurgical neuropathy, as would neurologic symptoms emerging, in a painful limb, after a period of documented return to baseline neurologic function postoperatively. When clinical features raise the possibility of an inflammatory postsurgical neuropathy, early neurologic consultation is appropriate (Table 2).
Clinical Pearl
- Clinical cues of a possible postoperative inflammatory neuropathy include atypical, unexplained, refractory postoperative pain; severe limb pain and emerging neurologic dysfunction after a period of documented return to baseline neurologic function postoperatively; and multifocal or difficult-to-localize, progressive postoperative neurologic deficits.
The cause of postsurgical inflammatory neuropathies is unknown, but biopsy of affected nerves in postoperative cases demonstrated perivascular lymphocytic inflammation consistent with a microvasculitis. There is significant axonal loss with this form of PNI, and as such the recovery is protracted. The process is usually monophasic, and functional prognosis has been reported to be good (90% have good functional recovery within 3 years in Parsonage-Turner syndrome).
However, patients whose peak deficits are severe or located distally may have incomplete recovery. Given the pathologically proven inflammatory etiology, corticosteroids are mechanistically a rationale as a potential treatment (and often used in practice) but have not been evaluated in randomized controlled trials.
The Role of Electrophysiology in Evaluating Postoperative Nerve Injuries
Electrophysiologic studies consist of NCSs and electromyography (EMG). NCSs are electrophysiologic tests whereby a peripheral motor, sensory, or mixed sensorimotor nerve is stimulated and a recording made of the motor or sensory response. Normal values have been established using standard techniques to define abnormalities of the response amplitude (corresponding with axonal loss) and speed of transmission along a nerve (conduction velocity and distal latency, corresponding to demyelination). EMG is an electrophysiologic test whereby a small (commonly 26-gauge) concentric recording needle is placed into muscles innervated by different spinal roots, pathways through the plexus, and peripheral nerves to identify characteristic electrical changes of neurogenic, myopathic, or neuromuscular transmission disorders. Pertinent to PNI, by evaluating different muscles innervated by different peripheral nerves, EMG can help localize the site of a nerve injury as well as assess the severity of the injury and whether recovery is occurring. EMG and NCS are complementary to one another and are almost always performed together and in common practice are referred to collectively simply as EMG.
Nerve injury (from any source) can be categorized, and these categories are important prognostically (Table 3). Most perioperative PNIs are related to compression or transient dysfunction of the myelin in a focal area of the nerve (neurapraxia) without any underlying damage to the nerve axon. Neurapraxia is evident on NCS, almost immediately after injury and symptom onset, as conduction block: There is normal nerve conduction distal to the site of injury and abnormal conduction proximal and through the site of injury. As such, NCS can be used in the acute perioperative period to identify patients with a common compression neuropathy (usually ulnar or peroneal) by identifying conduction block at a common site of compression. Because sensory fascicles of nerves are more susceptible to injury than motor fascicles, most perioperative compression neuropathies are sensory predominant. Patients with predominantly sensory symptoms or evidence of neurapraxia (conduction block) on NCS have an excellent prognosis, with expected complete recovery over days to a couple of months.
TABLE 3. Classification of nerve injury.62
| Pathology | Electrophysiology | Prognosis | |
|---|---|---|---|
| Neurapraxia | Localized myelin derangement - Axons intact Sensory > motor | Conduction block or focal slowing | Excellent |
| Axonotmesis | Axonal integrity and transport impaired → Wallerian degeneration - Endoneurial tube intact | NCS: low amplitude/absent motor and sensory responses EMG: denervation | Slow recovery |
| Neurotmesis | Axonal and connective tissue strata (neural tube) destroyed | Similar to axonotmesis, but no reinnervation on serial studies | No recovery |
When the PNI results from more severe or longer duration compression, from direct trauma to the nerve (needle, scalpel, suture, staple, or cautery), or from ischemia, the injury affects not only the myelin sheath but also the axons within the nerve. When the axon is injured, axonoplasmic flow is disrupted such that the axon cannot be maintained and will degenerate (Wallerian degeneration). Because Wallerian degeneration occurs over a few weeks following axonal nerve injury, its effects on electrophysiologic testing are not evident in the acute perioperative period, and the extent (severity) of axonal injury cannot be determined acutely with electrodiagnostic testing. As such, in cases of functionally significant PNIs that are not purely sensory or localized to a single nerve territory, the role of electrophysiologic testing in the acute perioperative period is limited. It could be used to identify preexisting neurogenic injury, but the electrophysiologic effects of the acute axonal injury from the PNI will not be definitively identifiable until approximately 2 to 3 weeks following the injury. Therefore, definitive electrophysiologic localization of a PNI and determination of injury severity cannot be determined electrophysiologically in the immediate perioperative period and can be done only 2 to 3 weeks postinjury. Importantly, while electrophysiologic testing localizes a lesion, defines its severity, and provides prognostic information, it does not elucidate the cause of the injury.
NYSORA Tips
- Electrodiagnostic studies (EMG and NCSs) may help confirm neurapraxia with conduction block or define preexisting disease when performed acutely.
- Axonal loss (prognostic) and the extent of a perioperative neurogenic injury will be better clarified by electrodiagnostic studies performed 3 weeks after injury.
The prognosis is much less favorable when there is significant axonal injury and degeneration compared to when there is only evidence of neurapraxia. Axonal injury can be classified depending on whether the injury is only to the axon (axonotmesis) or whether the connective tissue strata around the axon (the neural tube) has been damaged as well (neurotmesis), such as in transection. Peripheral nerve axons will regenerate back through the neural tube to their original targets of innervation if the neural tube is intact (axonotmesis), but not in neurotmesis. A single EMG (here used collectively to refer to NCS and EMG) study cannot differentiate these categorizations of axonal injury (any other clinical or imaging test also cannot). However, in axonotmesis, serial studies performed every 2–3 months will show axonal regeneration and reinnervation into muscles adjacent to the area of injury initially and proceeding distally with time. Electrodiagnostic evidence of reinnervation and recovery will be evident before clinical recovery. In cases of more severe axonal injury (neurotmesis), no recovery will be seen on serial studies. When this occurs, patients should be referred to a peripheral nerve neurosurgeon for surgical options. Functional improvement is improved if surgical intervention occurs before 6 to 9 months following the time of injury.
Clinical Pearl
- Nerve lesions that fail to resolve 3–5 months after initial neurologic evaluation should prompt consideration of consultation with a peripheral nerve neurosurgeon.
CONCLUSION
Perioperative central or peripheral nervous system injuries from anesthetic or surgical factors are fortunately rare, and most are sensory predominant and transient. Neurologic complications in the setting of neuraxial anesthesia require urgent evaluation as delay in the diagnosis of EH, epidural abscess, or spinal cord injury contributes to long-term morbidity. In the setting of peripheral nerve injuries, early neurologic evaluation should be considered when deficits are severe, progressive, or difficult to localize.
Unfortunately, once an active process has been excluded as the cause of a perioperative neurologic injury, there is nothing that can be done to improve the neurologic outcome. However, patient perceptions of a perioperative neurologic injury can be managed by providing adequate patient education, pain management, functional assistance through physical and occupational therapy, and scheduled follow-up with neurologic and electrophysiologic consultation if appropriate. All of these should be in place before dismissal for a patient with a significant perioperative nerve injury. For patients with only sensory symptoms, clinical follow-up to ensure symptom resolution should be arranged.
REFERENCES
- Horlocker TT, Wedel DJ, Rowlingson JC, et al: Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (third edition). Reg Anesth Pain Med 2010;35(1):64–101.
- Brull R, McCartney CJ, Chan VW, El-Beheiry H: Neurological complications after regional anesthesia: contemporary estimates of risk. Anesth Analg 2007;104(4):965–974.
- Moen V, Dahlgren N, Irestedt L: Severe neurological complications after central neuraxial blocks in Sweden 1990–1999. Anesthesiology 2004;101(4):950–959.
- Warner MA, Warner DO, Matsumoto JY, Harper CM, Schroeder DR, Maxson PM: Ulnar neuropathy in surgical patients. Anesthesiology 1999;90(1):54–59.
- Jacob AK, Mantilla CB, Sviggum HP, Schroeder DR, Pagnano MW, Hebl JR: Perioperative nerve injury after total hip arthroplasty: regional anesthesia risk during a 20-year cohort study. Anesthesiology 2011;115(6):1172–1178.
- Jacob AK, Mantilla CB, Sviggum HP, Schroeder DR, Pagnano MW, Hebl JR: Perioperative nerve injury after total knee arthroplasty: regional anesthesia risk during a 20-year cohort study. Anesthesiology
2011;114(2):311–317. - Welch MB, Brummett CM, Welch TD, et al: Perioperative peripheral nerve injuries: a retrospective study of 380,680 cases during a 10-year period at a single institution. Anesthesiology 2009;111(3):490–497.
- Warner MA, Warner DO, Harper CM, Schroeder DR, Maxson PM: Ulnar neuropathy in medical patients. Anesthesiology 2000;92(2): 613–615.
- Fredrickson MJ, Kilfoyle DH: Neurological complication analysis of 1000 ultrasound guided peripheral nerve blocks for elective orthopaedic surgery: a prospective study. Anaesthesia 2009;64(8):836–844.
- Neal JM: Anatomy and pathophysiology of spinal cord injury associated with regional anesthesia and pain medicine. Reg Anesth Pain Med 2008;33(5):423–434.
- Hebl JR, Horlocker TT, Sorenson EJ, Schroeder DR: Regional anesthesia does not increase the risk of postoperative neuropathy in patients undergoing ulnar nerve transposition. Anesth Analg 2001;93(6):
1606–1611, table of contents. - Neal JM, Bernards CM, Hadzic A, et al: ASRA practice advisory on neurologic complications in regional anesthesia and pain medicine. Reg Anesth Pain Med 2008;33(5):404–415.
- Staff NP, Engelstad J, Klein CJ, et al: Post-surgical inflammatory neuropathy. Brain 2010;133(10):2866–2880.
- Li SL, Wang DX, Ma D: Epidural hematoma after neuraxial block: a retrospective report from China. Anesth Analg 2010;111(5):1322–1324.
- Ruppen W, Derry S, McQuay H, Moore RA: Incidence of epidural hematoma, infection, and neurologic injury in obstetric patients with epidural analgesia/anesthesia. Anesthesiology 2006;105(2):394–399.
- Renck H: Neurological complications of central nerve blocks. Acta Anaesthesiol Scand 1995;39(7):859–868.
- Aromaa U, Lahdensuu M, Cozanitis DA: Severe complications associated with epidural and spinal anaesthesias in Finland 1987–1993. A study based on patient insurance claims [see comment]. Acta Anaesthesiol
Scand 1997;41(4):445–452. - Cook TM, Counsell D, Wildsmith JA: Major complications of central neuraxial block: report on the Third National Audit Project of the Royal College of Anaesthetists. Br J Anaesth 2009;102(2):179–190.
- Hebl JR, Niesen AD: Infectious complications of regional anesthesia. Curr Opin Anaesthesiol 2011;24(5):573–580.
- Boukobza M, Haddar D, Boissonet M, Merland JJ: Spinal subdural haematoma: a study of three cases. Clin Radiol 2001;56(6):475–480.
- Boukobza M, Guichard JP, Boissonet M, et al: Spinal epidural haematoma: report of 11 cases and review of the literature. Neuroradiology 1994;36(6):456–459.
- Braun P, Kazmi K, Nogues-Melendez P, Mas-Estelles F, Aparici-Robles F: MRI findings in spinal subdural and epidural hematomas. Eur J Radiol 2007;64(1):119–125.
- Ackland HM, Cameron PA, Varma DK, et al: Cervical spine magnetic resonance imaging in alert, neurologically intact trauma patients with persistent midline tenderness and negative computed tomography results.
Ann Emerg Med 2011;58(6):521–530. - Boye S, Schumacher J: Diagnosis of vertebral canal haematoma by myelography and spiral computer tomography in a patient with an implantable cardioverter-defibrillator contraindicating magnetic resonance imaging. Br J Anaesth 2009;103(1):137–138.
- Kreppel D, Antoniadis G, Seeling W: Spinal hematoma: a literature survey with meta-analysis of 613 patients. Neurosurg Rev 2003;26(1):1–49.
- Vandermeulen EP, Van Aken H, Vermylen J: Anticoagulants and spinalepidural anesthesia. Anesth Analg 1994;79(6):1165–1177.
- Practice advisory for the prevention, diagnosis, and management of infectious complications associated with neuraxial techniques: a report by the American Society of Anesthesiologists Task Force on Infectious
Complications Associated With Neuraxial Techniques. Anesthesiology 2010;112(3):530–545. - Rigamonti D, Liem L, Wolf AL, et al: Epidural abscess in the cervical spine. Mt Sinai J Med 1994;61(4):357–362.
- Reihsaus E, Waldbaur H, Seeling W: Spinal epidural abscess: a meta-analysis of 915 patients. Neurosurg Rev 2000;23(4):175–204; discussion 205.
- Silver JR, Buxton PH: Spinal stroke. Brain 1974;97(3):539–550.
- Mutch JA, Johansson JE: Occlusion of the artery of Adamkiewicz after hip and knee arthroplasty. J Arthroplasty 2011;26(3):e505–e508.
- Charles YP, Barbe B, Beaujeux R, Boujan F, Steib JP: Relevance of the anatomical location of the Adamkiewicz artery in spine surgery. Surg Radiol Anat 2011;33(1):3–9.
- Singh U, Silver JR, Welply NC: Hypotensive infarction of the spinal cord. Paraplegia 1994;32(5):314–322.
- Tsai YD, Liliang PC, Chen HJ, Lu K, Liang CL, Wang KW: Anterior spinal artery syndrome following vertebroplasty: a case report. Spine (Phila Pa 1976) 2010;35(4):E134–E136.
- Huntoon MA: Anatomy of the cervical intervertebral foramina: vulnerable arteries and ischemic neurologic injuries after transforaminal epidural injections. Pain 2005;117(1–2):104–111.
- Horlocker TT, McGregor DG, Matsushige DK, Schroeder DR, Besse JA: A retrospective review of 4767 consecutive spinal anesthetics: central nervous system complications. Perioperative Outcomes Group. Anesth
Analg 1997;84(3):578–584. - Rathmell JP, Michna E, Fitzgibbon DR, Stephens LS, Posner KL, Domino KB: Injury and liability associated with cervical procedures for chronic pain. Anesthesiology 2011;114(4):918–926.
- Lawton MT, Porter RW, Heiserman JE, Jacobowitz R, Sonntag VK, Dickman CA: Surgical management of spinal epidural hematoma: relationship between surgical timing and neurological outcome. J Neurosurg 1995;83(1):1–7.
- Cheshire WP, Santos CC, Massey EW, Howard JF Jr: Spinal cord infarction: etiology and outcome. Neurology 1996;47(2):321–330.
- Salvador de la Barrera S, Barca-Buyo A, Montoto-Marques A, Ferreiro- Velasco ME, Cidoncha-Dans M, Rodriguez-Sotillo A: Spinal cord infarction: prognosis and recovery in a series of 36 patients. Spinal Cord
2001;39(10):520–525. - Kumral E, Polat F, Gulluoglu H, Uzunkopru C, Tuncel R, Alpaydin S: Spinal ischaemic stroke: clinical and radiological findings and short-term outcome. Eur J Neurol 2011;18(2):232–239.
- Bracken MB: Steroids for acute spinal cord injury. Cochrane Database Syst Rev 2012;1:CD001046.
- Bracken MB, Shepard MJ, Collins WF, et al: A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinalcord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med 1990;322(20):1405–1411.
- Bracken MB, Shepard MJ, Holford TR, et al: Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National
Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA 1997;277(20):1597–1604. - Markandaya M, Stein DM, Menaker J: Acute treatment options for spinal cord injury. Curr Treat Options Neurol 2012 Feb 3. [Epub ahead of print]
- Pharmacological therapy after acute cervical spinal cord injury. Neurosurgery 2002;50(3 Suppl):S63–S72.
- Sayer FT, Kronvall E, Nilsson OG: Methylprednisolone treatment in acute spinal cord injury: the myth challenged through a structured analysis of published literature. Spine J 2006;6(3):335–343.
- Short DJ, El Masry WS, Jones PW: High dose methylprednisolone in the management of acute spinal cord injury—a systematic review from a clinical perspective. Spinal Cord 2000;38(5):273–286.
- Selander D, Brattsand R, Lundborg G, Nordborg C, Olsson Y: Local anesthetics: importance of mode of application, concentration and adrenaline for the appearance of nerve lesions. An experimental study of
axonal degeneration and barrier damage after intrafascicular injection or topical application of bupivacaine (Marcain). Acta Anaesthesiol Scand 1979;23(2):127–136. - Lundborg G, Dahlin LB: Anatomy, function, and pathophysiology of peripheral nerves and nerve compression. Hand Clin 1996;12(2): 185–193.
- Kalichman MW, Calcutt NA: Local anesthetic-induced conduction block and nerve fiber injury in streptozotocin-diabetic rats. Anesthesiology 1992;77(5):941–947.
- Winfree CJ, Kline DG: Intraoperative positioning nerve injuries. Surg Neurol 2005;63(1):5–18; discussion 18.
- Horlocker TT, Kufner RP, Bishop AT, Maxson PM, Schroeder DR: The risk of persistent paresthesia is not increased with repeated axillary block. Anesth Analg 1999;88(2):382–387.
- Kretschmer T, Heinen CW, Antoniadis G, Richter HP, Konig RW: Iatrogenic nerve injuries. Neurosurg Clin N Am 2009;20(1):73–90, vii.
- Borgeat A, Ekatodramis G, Kalberer F, Benz C: Acute and nonacute complications associated with interscalene block and shoulder surgery: a prospective study. Anesthesiology 2001;95(4):875–880.
- Urban MK, Urquhart B: Evaluation of brachial plexus anesthesia for upper extremity surgery. Reg Anesth 1994;19(3):175–182.
- Ben-David B: Complications of peripheral block. Anesthesiol Clin North America 2002;20(3):695–707.
- Ahn KS, Kopp SL, Watson JC, Scott KP, Trousdale RT, Hebl JR: Postsurgical inflammatory neuropathy. Reg Anesth Pain Med 2011;36(4): 403–405.
- Laughlin RS, Dyck PJ, Watson JC, et al: Ipsilateral inflammatory neuropathy after hip surgery. Mayo Clin Proc 2014;89:454–461.
- van Alfen N, van Engelen BG: The clinical spectrum of neuralgic amyotrophy in 246 cases. Brain 2006;129(Pt 2):438–450.
- Dyck PJ, Windebank AJ: Diabetic and nondiabetic lumbosacral radiculoplexus neuropathies: new insights into pathophysiology and treatment. Muscle Nerve 2002;25(4):477–491.
- Sunderland S: The anatomy and physiology of nerve injury. Muscle Nerve 1990;13(9):771–784.
- Robinson LR: Traumatic injury to peripheral nerves. Muscle Nerve 2000;23(6):863–873.
- Aminoff MJ: Electrophysiologic testing for the diagnosis of peripheral nerve injuries. Anesthesiology 2004;100(5):1298–1303.
- Gilchrist JM, Sachs GM: Electrodiagnostic studies in the management and prognosis of neuromuscular disorders. Muscle Nerve 2004;29(2): 165–190.
- Kandenwein JA, Kretschmer T, Engelhardt M, Richter HP, Antoniadis G: Surgical interventions for traumatic lesions of the brachial plexus: a retrospective study of 134 cases. J Neurosurg 2005;103(4): 614–621.


