Adrian Chin and André van Zundert
THE HISTORY OF SPINAL ANESTHESIA
Carl Koller, an ophthalmologist from Vienna, in 1884 first described the use of topical cocaine for analgesia of the eye. William Halsted and Richard Hall, surgeons at Roosevelt Hospital in New York City, took the idea of local anesthesia a step further by injecting cocaine into human tissues and nerves to produce anesthesia for surgery. James Leonard Corning, a neurologist in New York City, in 1885 described the use of cocaine for spinal anesthesia. Because Corning was a frequent observer at Roosevelt Hospital, the idea of using cocaine in the subarachnoid space may have come from observing Halsted and Hall performing cocaine injections. Corning first injected cocaine intrathecally into a dog and within a few minutes the dog had marked weakness in the hindquarters. Next, Corning injected cocaine into a man at the T11–T12 interspace into what he thought was the subarachnoid space. Because Corning did not notice any effect after 8 minutes, he repeated the injection.
Ten minutes after the second injection, the patient complained of sleepiness in his legs but was able to stand and walk. Because Corning made no mention of cerebrospinal fluid (CSF) efflux, most likely he inadvertently gave an epidural rather than a spinal injection to the patient.
The presence of a neuraxial fluid was first noted by Galen in AD 200, and CSF was later studied in the 1500s by Antonio Valsalva. Dural puncture was described in 1891 by Essex Wynter followed shortly by Heinrich Quincke 6 months later.
Augustus Karl Gustav Bier, a German surgeon, used cocaine intrathecally in 1898 on six patients for lower extremity surgery. In true scientific fashion, Bier decided to experiment on himself and developed a postdural puncture headache (PDPH) for his efforts. His assistant, Dr. Otto Hildebrandt, volunteered to have the procedure performed after Bier was unable to continue due to the PDPH. After injection of spinal cocaine into Hildebrandt, Bier conducted experiments on the lower half of Hildebrandt’s body. Bier described needle pricks and cigar burns to the legs, incisions on the thighs, avulsion of pubic hairs, strong blows with an iron hammer to the shins, and torsion of the testicles. Hildebrandt reported minimal to no pain during the experiments; however, afterward, he suffered nausea, vomiting, PDPH, and bruising and pain in his legs. Bier attributed the PDPH to loss of CSF and felt the use of small-gauge needles would help prevent the headache.
Dudley Tait and Guido Caglieri performed the first spinal anesthetic in the United States in San Francisco in 1899. Their studies included cadavers, animals, and live patients to determine the benefits of lumbar puncture, especially in the treatment of syphilis. Tait and Caglieri injected mercuric salts and iodides into the CSF, but worsened the condition of one patient with tertiary syphilis. Rudolph Matas, a vascular surgeon in New Orleans, described the use of spinal cocaine on patients and possibly was the first to use morphine in the subarachnoid space. Matas also described the complication of death after lumbar puncture. Theodore Tuffier, a French surgeon in Paris, studied spinal anesthesia and reported on it in 1900. Tuffier felt that cocaine should not be injected until CSF was recognized.
Tuffier taught at the University of Paris at the same time that Tait was a medical student there and most likely was one of Tait’s mentors. Tuffier’s demonstrations in Paris helped popularize spinal anesthesia in Europe.
Arthur Barker, a professor of surgery at the University of London, reported on the advancement of spinal techniques in 1907, including the use of a hyperbaric spinal local anesthetic, emphasis on sterility, and ease of midline over paramedian dural puncture. Advancement of sterility and the investigation of decreases in blood pressure after injection helped make spinal anesthesia safer and more popular. Gaston Labat was a strong proponent of spinal anesthesia in the United States and performed early studies on the effects of Trendelenburg position on blood pressure after spinal anesthesia. George Pitkin attempted to use a hypobaric local anesthetic to control the level of spinal nerve block by mixing procaine with alcohol. Lincoln Sise, an anesthesiologist at the Lahey Clinic in Boston, used Barker’s technique of hyperbaric spinal anesthesia with both procaine and tetracaine.
Spinal anesthesia became more popular as new developments occurred, including the introduction in 1946 of saddle nerve block anesthesia by Adriani and Roman-Vega. However, in 1947 the well-publicized case of Woolley and Roe (United Kingdom) resulted in two patients becoming paraplegic in one day. Across the Atlantic, reports of paraplegia in the United States similarly caused anesthesiologists to discontinue the use of spinal anesthesia. The development of novel intravenous anesthetic agents and neuromuscular blockers coincided with the decreased use of spinal anesthesia. In 1954, Dripps and Vandam described the safety of spinal anesthetics in more than 10,000 patients, and spinal anesthesia was revived.
In the field of obstetrics, over 500,000 spinals had been performed on American women by the mid-1950s. Despite spinal anesthesia being the most frequently used technique for vaginal delivery and cesarean section in the 1950s, subsequent improvements in epidural technology resulted in a decline in obstetric spinal anesthesia in the late 1960s. The Third National Audit Project (NAP3) estimated 133,525 obstetric spinals were performed in 2006 in the United Kingdom.
The early development of spinal needles paralleled the early development of spinal anesthesia. Corning chose a gold needle that had a short bevel point, flexible cannula, and set screw that fixed the needle to the depth of dural penetration. Corning also used an introducer for the needle, which was right angled. Quincke used a beveled needle that was sharp and hollow. Bier developed his own sharp needle that did not require an introducer. The needle was larger bore (15 or 17 gauge) with a long, cutting bevel. The main problems with Bier’s needle were pain on insertion and the loss of local anesthetic due to the large hole in the dura after dural puncture. Barker’s needle did not have an inner cannula, was made of nickel, and had a sharp, medium-length bevel with a matching stylet. Labat developed an unbreakable nickel needle that had a sharp, short-length bevel with a matching stylet. Labat believed that the short bevel minimized damage to the tissues when inserted into the back.
Herbert Greene realized that loss of CSF was a major problem in spinal anesthesia and developed a smooth-tip, smaller-gauge needle that resulted in a lower incidence of PDPH. Barnett Greene described the use of a 26-gauge spinal needle in obstetrics with a decreased incidence of PDPH. The Greene needle was popular until the introduction of the Whitacre needle. Hart and Whitacre29 used a pencil-point needle to decrease PDPH from 5%–10% to 2%. Sprotte modified the Whitacre needle and in 1987 published his trial of over 34,000 spinal anesthetics. Modifications of the Sprotte needle occurred the 1990s to produce the needle that is in use today.
Spinal anesthesia has progressed greatly since 1885. Every aspect, from improved equipment and pharmacological agents to greater understanding of physiology and anatomy, have made spinal anesthesia increasingly safer. Changing clinical knowledge has seen shifts in what is considered a contraindication to spinal anesthesia, and the evolution of novel techniques, such as the use of ultrasound, have allowed spinal anesthesia in what would once have been thought impossible situations. Nonetheless, no technique is risk-free, and every effort must be made to prevent complications. Learning how to perform spinal anesthesia is an invaluable skill that all anesthesiologists should have in their armamentarium.
THE RISKS AND BENEFITS OF SPINAL ANESTHESIA
Before offering a patient spinal anesthesia, an anesthesiologist not only must be aware of the indications and contraindications of spinal anesthesia but also must be able to weigh the risks and benefits of performing the procedure. This requires a thorough understanding of the available evidence, in particular how the risk-benefit ratio compares to that of any alternative, and an ability to apply the evidence to a given clinical scenario. Thus, an informed anesthesiologist can facilitate the patient in making an informed decision.
Contraindications and Risks of Spinal Anesthesia
Contraindications to Spinal Anesthesia
There are absolute and relative contraindications to spinal anesthesia (see Table 1). Absolute contraindications include patient refusal; infection at the site of injection; severe, uncorrected hypovolemia; true allergy to any of the drugs; and increased intracranial pressure, except in cases of pseudo–tumor cerebri (idiopathic intracranial hypertension). High intracranial pressure increases the risk of uncal herniation when CSF is lost through the needle. Spinal anesthesia is also contraindicated when the operation is expected to take longer than the duration of the nerve block or result in blood loss such that the development of severe hypovolemia is likely.
TABLE 1. Contraindications to spinal anesthesia.
| Absolute Contraindications | Relative Contraindications |
|---|---|
| • Patient refusal • Infection at the site of injection • Uncorrected hypovolemia • Allergy • Increased intracranial pressure | • Coagulopathy • Sepsis • Fixed cardiac output states • Indeterminate neurological disease |
Coagulopathy, previously considered an absolute contraindication, may be considered depending on the level of derangement. Another relative contraindication of spinal anesthesia is sepsis distinct from the anatomic site of puncture (eg, chorioamnionitis or lower extremity infection). If the patient is on antibiotics and the vital signs are stable, spinal anesthesia may be considered. Spinal anesthesia is relatively contraindicated in cardiac diseases with fixed cardiac output (CO) states. Aortic stenosis, once considered to be an absolute contraindication for spinal anesthesia, does not always preclude a carefully conducted spinal anesthetic.
Indeterminate neurological disease is a relative contraindication. Multiple sclerosis and other demyelinating diseases are challenging. In vitro experiments suggest that demyelinated nerves are more susceptible to local anesthetic toxicity. However, no clinical study has convincingly demonstrated that spinal anesthesia worsens such neurologic diseases. Indeed, with the knowledge that pain, stress, fever, and fatigue exacerbate these diseases, a stress-free central neuraxial nerve block (CNB) may be preferred for surgery.
Spinal anesthesia in the immunocompromised patient also presents a challenge for the anesthesiologist and is the subject of a consensus statement. Although this consensus statement does not provide prescriptive advice for every situation, it does summarize the available evidence. Previous spinal surgery was once thought to be a contraindication. Dural puncture may be difficult, and spread of local anesthetic may be restricted by scar tissue. However, there are case reports of successful spinal anesthesia in this setting, particularly with the assistance of ultrasound. There are theoretical risks in inserting a hollow-body needle through tattoo ink. However, there are no reported complications from inserting a spinal or epidural needle through a tattoo. Stylets may decrease the likelihood of transmitting a core of tissue to the subarachnoid space, and if concerned, a small skin incision may be made prior to needle insertion. Introducers serve to prevent contamination of the CSF with small pieces of epidermis, which could lead to the formation of dermoid spinal cord tumors.
Risks of Spinal Anesthesia: Complications
Complications of spinal block are often divided into major and minor complications. Reassuringly, most major complications are rare. Minor complications, however, are common and therefore should not be dismissed. Minor complications include nausea, vomiting, mild hypotension, shivering, itch, hearing impairment, and urinary retention. PDPH and failed spinal block are significant, and not uncommon, complications of spinal anesthesia. We therefore consider them as moderate complications (see Table 2). Failure of spinal anesthesia has been mentioned as between 1% and 17% and is discussed further in this chapter.
TABLE 2. Complications of spinal anesthesia.
| Minor | Moderate | Major |
|---|---|---|
| • Nausea and vomiting • Mild hypotension • Shivering • Itch • Transient mild hearing impairment • Urinary retention | • Failed spinal • Postdural puncture headache | • Direct needle trauma • Infection (abscess, meningitis) • Vertebral canal hematoma • Spinal cord ischemia • Cauda equina syndrome • Arachnoiditis • Peripheral nerve injury • Total spinal anesthesia • Cardiovascular collapse • Death |
Minor Complications of Spinal Anesthesia
Nausea and Vomiting Nausea and vomiting presenting after spinal anesthesia are distressing for the patient and may impede the surgeon. Incidence of intraoperative nausea and vomiting (IONV) in nonobstetric surgery can be up to 42% and may be as high as 80% in parturients.
Causes are complex and multifactorial. Causes unrelated to the spinal may include patient factors (eg, anxiety, reduced lower esophageal sphincter tone, increased gastric pressure, vagal hyperactivity, hormonal changes); surgical factors (exteriorization of the uterus, peritoneal traction); and other factors (eg, systemic opioids, uterotonic drugs, antibiotics, movement). Spinal anesthesia itself may cause IONV or postoperative nausea and vomiting (PONV) via a variety of mechanisms, including hypotension, intrathecal additives, inadequate nerve block, or high nerve block. Risks factors for IONV under spinal include peak nerve block height greater than T6, baseline heart rate (HR) 60 beats/minute or more, a history of motion sickness, and previous hypotension after spinal nerve block.
Hypotension must be the first consideration when a patient complains of nausea, especially immediately after onset of spinal anesthesia. This has been long known. Evans, in his 1929 textbook on spinal anesthesia, noted that “the sudden fall in blood pressure is followed by nausea.” Mechanisms and management of hypotension are covered in greater detail elsewhere (see section on cardiovascular effects of spinal anesthesia).
A variety of intrathecal additives have been shown to increase IONV or PONV. Intrathecal morphine, diamorphine, clonidine, and neostigmine all increase nausea and vomiting. Intrathecal fentanyl, however, reduces IONV, perhaps by improving nerve block quality, decreasing supplemental opioids, or decreasing hypotension.
While low spinal nerve block can cause nausea from surgical stimulation, high sympathetic spinal nerve block (with relative parasympathetic overactivity) can also result in nausea. Glycopyrrolate was shown to be better than placebo in reducing nausea during cesarean section, although the rate of nausea was still high (42%). However, prophylactic glycopyrrolate can increase hypotension after spinal anesthesia.
A recent meta-analysis suggested metoclopramide (10 mg) was effective and safe for prevention of IONV and PONV in the setting of cesarean delivery under neuraxial nerve block.
Another meta-analysis showed the serotonin 5-HT3 receptor antagonists reduced the incidence of nausea and vomiting and the need for postoperative rescue antiemetic when intrathecal morphine was used for cesarean section.
Despite some studies showing a benefit of P6 (pericardium 6 nei guan point) stimulation, based on Chinese acupuncture, a 2008 systematic review found inconsistent results in preventing IONV and PONV.
Hypotension Mechanisms and management of hypotension are covered elsewhere (see section on cardiovascular effects of spinal anesthesia).
Shivering Crowley et al reviewed shivering and neuraxial anesthesia. Spinal and epidural anesthesia, and indeed general anesthesia, may induce shivering. The incidence of shivering secondary to neuraxial nerve block is difficult to assess given the heterogeneity of studies but is about 55%. In the first 30 minutes after nerve block, spinal anesthesia decreases core body temperature faster than epidural anesthesia. After 30 minutes, both techniques cause temperature to fall at the same rate. Despite this, shivering after spinal anesthesia is no greater than after epidural anesthesia. Indeed, the intensity of shivering seems to be higher with epidurals. Postulated mechanisms for this include an inability to shiver due to more pronounced motor block with spinal anesthesia and a decreased shivering threshold with more dermatomes (and thus thermoregulatory afferents) blocked during spinal anesthesia. Several strategies have been suggested to reduce neuraxial shivering (see Table 3).
TABLE 3. Suggested strategies to prevent and treat neuraxial anesthesia shivering.
| Prevention | Treatment |
|---|---|
| • Prewarm with forced air warmer for 15 minutes • Avoid cold epidural or intravenous fluids • Intrathecal fentanyl 20 μg • Intrathecal meperidine 0.2 mg/kg or 10 mg • Intravenous ondansetron 8 mg • Epidural fentanyl • Epidural meperidine | • Intravenous meperidine 50 mg • Intravenous tramadol 0.25 mg/kg or 0.5 mg/kg or 1 mg/kg • Intravenous clonidine 30, 60, 90, or 150 μg |
Itch Pruritis is a well-known side effect of opiates and is more common with administration via the spinal route (46%) compared with epidural (8.5%) and systemic routes. The severity of pruritis is proportional to intrathecal morphine dose but not epidural morphine dose. Pruritis associated with neuraxial opioids is often distributed around the nose and face. Although symptoms may not be mediated via opioid receptors, pruritis can be treated with the opioid receptor antagonist naloxone.
There are reports of ondansetron being used for opioid-induced pruritis, suggesting a role of serotonin receptors in morphine-induced pruritis. A 2009 meta-analysis of obstetric patients who had received intrathecal morphine showed that 5-HT3 receptor antagonists did not reduce the incidence of pruritis but did reduce the severity of itching and the need to treat pruritis. The 5-HT3 receptor antagonists were useful in treating established pruritis (number needed to treat [NNT] = 3).
Hearing Impairment Hearing loss, particularly in the low-frequency range, has been reported after spinal anesthesia. Quoted incidences vary widely (3%–92%). Otoacoustic emissions, an objective measurement of hearing that reflects outer hair cell function, demonstrated hearing loss to be more common than suspected, but transient, with full recovery occurring in 15 days. Other authors have similarly concluded that hearing loss commonly disappears spontaneously. A comparison of hearing loss after general and spinal anesthesia concluded that hearing loss occurs irrespective of technique. Hearing loss may or may not be associated with PDPH and may improve with an epidural blood patch. Hearing loss after spinal nerve block may be related to needle gauge and may be less common in the obstetric population. Finegold showed that hearing loss did not occur in women having elective cesarean sections when 24-gauge Sprotte needles or 25-gauge Quincke needles were used. It has been suggested that consent for spinal anesthesia should include a discussion for medicolegal reasons of possible hearing loss.
Postoperative Urinary Retention Micturition is the product of a complex interplay of physiology. Postoperative urinary retention (POUR), therefore, is often multifactorial in origin. Patient risk factors for POUR include male sex and previous urologic dysfunction. Surgical risk factors include pelvic or prolonged surgery. Anesthetic factors include anticholinergic drugs, opioids, and fluid administration (>1000 mL). POUR can occur with both neuraxial and general anesthesia.
Occurrence of POUR after neuraxial nerve block is due to neural interruption of the micturition reflex as well as bladder overdistention. Neuraxial opioids exert an effect at the spinal cord and the pontine micturition center. The parasympathetic block induced by spinal anesthesia must end before voiding occurs. This usually corresponds with return of the S2–S4 segments. The type and dose of local anesthetic, as well as the use of neuraxial opioid, influence the return of spontaneous micturition. Time to micturition is quickest with 2-chloroprocaine and slowest with bupivacaine.
A recent systematic review found six studies that compared the effect of neuraxial anesthesia with other techniques. Four studies compared local infiltration with intrathecal anesthesia; three of these found lower incidences of urinary retention with local infiltration. The other two studies found no difference in time to micturition when intrathecal anesthesia was compared with general anesthesia in the first instance and general anesthesia and peripheral nerve block in the second instance.
Postdural Puncture Headache Postdural puncture headache, often classified as a minor (or at least not a major) complication, can be severe and debilitating and has been considered the neurological complication of spinal anesthesia. It is a common cause for medicolegal claims. The incidence of PDPH is influenced by patient demographics and is less common in elderly patients. In a high-risk group, such as obstetric patients, the risk after lumbar puncture with a Whitacre 27-gauge needle is about 1.7%. Needle size and type influence PDPH rate. Other risk factors include lesser body mass index (BMI), female gender, history of recurrent headaches, and previous PDPH.
Postdural puncture headache should be thought of as neither a common “minor” complication nor a rare “major” complication, but as a not uncommon “moderate” complication.
The reader is referred to Postdural Puncture Headache for further detailed information.
Major Complications of Spinal Anesthesia Major complications of spinal anesthesia include direct needle trauma, infection (meningitis or abscess formation), vertebral canal hematoma, spinal cord ischemia, cauda equina syndrome (CES), arachnoiditis, and peripheral nerve injury. The end result of these complications may be permanent neurologic disability. Other major complications include total spinal anesthesia (TSA), cardiovascular collapse, and death.
Direct Needle Trauma Neurologic injury can occur after needle introduction into the spinal cord or nerves. Although the elicitation of paresthesias during spinal anesthesia has been implicated as a risk factor for persistent neurologic injury, it is not known whether an intervention after paresthesia can prevent development of neurologic complications. A retrospective analysis found 298 of 4767 (6.3%) patients experienced paresthesia during spinal needle insertion. Of the 298, four patients had persistent paresthesia postoperatively. A further two patients with postoperative paresthesia did not have paresthesia during needle insertion. All six patients had resolution of symptoms by 24 months. When paresthesia occurs, the spinal needle may be adjacent to or penetrating neural tissue; if the latter is the case, injection of local anesthetic into the spinal nerve may result in permanent neurologic damage. Analogous controversies exist with peripheral nerve block; the implications of paresthesia techniques and extraneural and intraneural injection are the subject of much debate.
Meningitis Meningitis, either bacterial or aseptic, can occur after spinal anesthesia is performed. Sources of infection include contaminated spinal trays and medication, oral flora of the anesthesiologist, and patient infection. Most cases of meningitis after spinal anesthesia in the first half of the 20th century were aseptic and could be traced to chemical contamination and detergents.
Marinac showed that causes of drug- and chemical-induced meningitis include nonsteroidal anti-inflammatory drugs, certain antibiotics, radiographic agents, and muromonab-CD3. There also appears to be an association between the occurrence of the hypersensitivity-type reactions and underlying collagen, vascular, or rheumatologic disease. Carp and Bailey performed lumbar puncture in bacteremic rats, and only those with a circulating Escherichia coli count greater than 50 CFU/mL at the time of lumbar puncture developed meningitis. Although meningitis after lumbar puncture has also been described in bacteremic children, the incidence of meningitis after diagnostic lumbar puncture is not significantly different in bacteremic patients compared with spontaneous incidence of meningitis. Oral flora can contaminate the CSF when a spinal anesthetic is being performed, underlying the importance of wearing a mask. Streptococcus salivarius, Streptococcus viridans, Staphylococcus aureus, Pseudomonas aeruginosa, Acinetobacter, and Mycobacterium tuberculosis have all been isolated in cases of bacterial meningitis after spinal anesthesia or lumbar puncture.
Vertebral Canal Hematoma Vertebral canal hematoma formation is a rare but devastating complication after spinal anesthesia. Although most spinal hematomata occur in the epidural space due to the prominent epidural venous plexus, a few reports have mentioned subarachnoid bleeding as the cause of neurologic deficits. The source of the bleeding can be from either an injured artery or an injured vein. Spinal hematoma and spinal cord ischemia have a poorer prognosis than infective complications. If new or progressive neurologic symptoms develop, an immediate neurosurgery consultation should be obtained, and magnetic resonance imaging (MRI) of the spine should be performed as soon as possible.
Spinal Cord Ischemia The superficial arterial system of the spinal cord consists of three longitudinal arteries (the anterior spinal artery and two posterior spinal arteries) and a pial plexus.
The posterior cord is relatively protected from ischemia by abundant anastomoses. The central area of the anterior spinal cord is reliant on the anterior spinal artery and therefore more prone to ischemia. Proposed mechanisms for spinal cord ischemia secondary to spinal block include prolonged hypotension, the addition of vasoconstrictors to local anesthetics, and compression of arterial supply by vertebral canal hematoma.
Cauda Equina Syndrome Cauda equina syndrome (CES) has been reported with the use of continuous spinal microcatheters. The use of hyperbaric 5% lidocaine for spinal anesthesia is associated with an increased incidence of CES, although other local anesthetics have been implicated.
Other risk factors for CES include lithotomy position, repeated dosing of local anesthetic solution through continuous spinal catheters, and possibly multiple single-injection spinal anesthetics.
Suggestions for prevention of CES from spinal anesthesia include aspiration of CSF before and after local anesthetic injection. Some suggest that when CSF cannot be aspirated after half the dose is injected, a full dose not be administered.
Limiting the amount of local anesthetic given in the subarachnoid space may help prevent CES.
Arachnoiditis Arachnoiditis can occur after spinal injection of local anesthetic solution but is also known to occur after intrathecal steroid injection. Causes of arachnoiditis include infection; myelograms from oil-based dyes; blood in the intrathecal space; neuroirritant, neurotoxic, or neurolytic substances; surgical interventions in the spine; intrathecal corticosteroids; and trauma. Arachnoiditis has been reported after traumatic dural puncture and after unintentional intrathecal injection of local anesthetics, detergents, antiseptics, or other substances.
Peripheral Nerve Injury Spinal anesthesia may indirectly result in peripheral nerve injury. The sensory nerve block induced by spinal anesthesia temporarily abolishes normal protective reflexes. Therefore, care must be taken with appropriate positioning, avoidance of tight plaster casts, and observation of distal circulation. Hence, it is imperative that there is good nursing care of limbs rendered insensate by spinal anesthesia.
Total Spinal Anesthesia Total spinal anesthesia (TSA) results in respiratory depression, cardiovascular compromise, and loss of consciousness. This may or may not be preceded by numbness, paresthesia, or weakness of the upper limb; shortness of breath; nausea; or anxiety. The mechanism of TSA is unclear.
The importance of providing cardiorespiratory support and anxiolysis is illustrated by the management of intentional TSA. Total spinal anesthesia has been used therapeutically for intractable pain. After injection of 20 mL of 1.5% lidocaine at the L3–L4 level, patients were tilted head down. Thiopental was given to prevent unpleasant sensations. After loss of consciousness, paralysis (without muscle relaxant), and pupil dilation, a laryngeal mask airway (LMA) was inserted and positive pressure ventilation applied. Ephedrine and atropine were used for cardiovascular support if required. Mechanical ventilation was required for about an hour, after which the LMA was removed.
Cardiovascular Collapse Cardiovascular collapse can occur after spinal anesthesia, although it is a rare event. Auroy and coworkers reported 9 cardiac arrests in 35,439 spinal anesthetics performed. Refer to the section on Cardiovascular Effects of Spinal Anesthesia.
Estimating the Risks of the Major Complications of Spinal Anesthesia
While minor risks are often thought of as side effects, major complications are of more concern to clinicians and patients. Perception of risk can be influenced by sensational case reports, such as given by Woolley and Roe. Early efforts to assess risk were hampered by lack of good numerator (number of complications) and denominator (number of spinal nerve blocks) data. Vandam and Dripps, in an attempt to redress “unsubstantiated clinical impressions” of mid-20th century anesthesiologists, examined the records of over 10,000 spinal anesthetics. They concluded that objections to spinal anesthesia were undeserved. Retrospective evidence from Finland for the period 1987–1993 estimated the risk of major complication following spinal anesthesia at 1 in 22,000. A no-fault compensation scheme was thought to increase data veracity. Swedish data (Moen) from the period 1990–1999 found a similar risk of 1 in 20,000–30,000. Although good evidence at the time, the Scandinavian evidence was criticized because of retrospective design, which risks underreporting. Moreover, numerator data sourced from administrative databases may not indicate either causation or final outcome.
Auroy attempted to address weaknesses of an earlier study by setting up a telephone hotline, allowing contemporaneous assessment of causality. This prospective study from 1998 to 1999 investigated complications from any type of regional anesthesia. Auroy’s results relied on voluntary contribution by French anesthesiologists (<6% participation rate) and may have been skewed by differing complication rates in those willing to participate. A 2007 review found a much higher incidence of neurological complications after spinal anesthesia in Auroy’s work (3.7–11.8 per 10,000) compared with Moen’s work (0.4 per 10,000). Auroy, unlike Moen, included peripheral neuropathy and radiculopathy in the numerator data.
Designing a prospective study to accurately quantify the risk of spinal anesthesia has been difficult due to the low incidence of major complications. The NAP3 of the Royal College of Anaesthetists is the best evidence to date on major complications after CNB. NAP3 is notable for a variety of reasons: It is the largest prospective audit of CNB to date; it achieved a 100% return rate; and it gathered numerator and denominator data from a variety of sources. It also investigated causality and outcome.
Numerator data in NAP3 pertained to major complications over a 12-month period (2006–2007). Reports came from local hospital reporters and clinicians. Litigation authorities, medical defense organizations, journals, and even Google searches of media reports were reviewed to identify missed complications. Complications were classified as infections, hematomata, nerve injuries, cardiovascular collapses, and wrong-route errors. Notably, PDPH was not included as a major complication. Complications were examined by a panel, and the likelihood of CNB as the cause was established. Denominator data were sourced from a 2-week census and validated by contacting a number of organizations and databases.
The findings of permanent harm were presented optimistically or pessimistically (see Table 4). Optimistic figures excluded complications where recovery was likely or causality tenuous.
TABLE 4. Useful numbers for quoting risk to patients.
| Central Neuraxial block | Risk (Pessimistic) | Risk (Optimistic) |
|---|---|---|
| Permanent harm from major complication | 1 in 25,000 | 1 in 50,000 |
| Death and paraplegia | 1 in 50,000 | 1 in 150,000 |
Permanent harm after any type of CNB was pessimistically 1:23,500 and optimistically 1:50,500. The risk of death or paraplegia after any type of CNB was pessimistically 1:54,500 and optimistically 1:141,500. The incidences of complications of spinals and caudals were at least half that of epidurals and combined spinal-epidural (CSE) nerve blocks. Of approximately 700,000 CNBs, 46% were spinals. Although the authors cautioned against subgroup analysis, the obstetric setting was found to have a low incidence of complications, while the adult perioperative setting had the highest complications. Complete or nearcomplete neurological recovery occurred in 61% of cases.
Importantly, NAP3 did not examine minor complications or major complications without permanent harm. For example, patients may have had cardiovascular collapse requiring intensive care or have had meningitis, but as they made a full recovery were excluded from even the pessimistic calculation. These are complications a patient would consider severe. The authors did acknowledge their figures represent a minimum possible incidence of complications; however, others have speculated that they may have overestimated risk. As there was no control group, NAP3 cannot answer if CNB is safer than other techniques such as general anesthesia.
The NAP3 study reassured us that permanent harm as a result of spinal anesthesia is rare. The large scope and excellent methodology of NAP3 mean a similar audit is unlikely to be repeated soon. Efforts should be made in ameliorating “minor” and “moderate” complications that are more likely to trouble our patients. In particular, PDPH deserves special attention.
Major complications, nonetheless, do happen, and every effort must be made to prevent them. Awareness of the low risk of serious complications should not give rise to complacency.
Indeed, a given complication may become so rare that a single anesthesiologist is unlikely to encounter it in a lifetime of practice. However, given the catastrophic nature of such complications, ongoing vigilance is of paramount importance.
Indications and Benefits of Spinal Anesthesia
Indications
Spinal anesthesia provides excellent operating conditions for surgery below the umbilicus. Thus, it has been used in the fields of urological, gynecological, obstetric, and lower abdominal and perineal general surgery. Likewise, it has been used in lower limb vascular and orthopedic surgery. More recently, spinal anesthesia has been used in surgery above the umbilicus (see section on laparoscopic surgery).
Benefits of Spinal Anesthesia
Although spinal anesthesia is a commonly used technique, with an estimated 324,950 spinal anesthetics each year in the United Kingdom alone, mortality and morbidity benefits are difficult to prove or disprove. It was hypothesized that due to beneficial modulation of the stress response, regional anesthesia would be safer than general anesthesia. However, clinical trials have been contradictory, and debates continue over the superiority of one technique over the other. Evaluations of the benefits of spinal block are troubled by the heterogeneity of studies and arguments about whether analysis should include intention to treat. In addition, much of the evidence for the benefits of neuraxial block pertains to epidurals, and some reviews do not differentiate between spinal and epidural anesthesia. For example, CNB has been shown to reduce blood loss and thromboembolic events. However, the authors of these studies were wise not to analyze spinal and epidural anesthesia individually, as the subgroup sample size would have been inadequate. Further studies are required to elucidate the relative benefits of each technique.
An obvious benefit of spinal anesthesia is the avoidance of the many risks of general anesthesia. However, it must be remembered that there is always the possibility of conversion to general anesthesia, and an emergent general anesthesia may be riskier than a planned general anesthesia.
Spinal anesthesia is advantageous in certain clinical settings. It is now commonplace for women having cesarean delivery to have a neuraxial nerve block. Spinal anesthesia avoids the problems associated with general anesthesia in the pregnant patient, notably risks of difficult airway, awareness, and aspiration. Refer to Obstetric Regional Anesthesia.
Maternal blood loss has been found to be lower with spinal compared with general anesthesia. Falling maternal mortality rates have been attributed to the increase in the practice of regional anesthesia. Moreover, regional anesthesia allows a mother to be awake for childbirth and a partner to be present if desired. However, a Cochrane review found no evidence of the superiority of regional anesthesia over general anesthesia with regard to major maternal or neonatal outcomes Likewise, a 2005 meta-analysis showed cord pH, an indicator of fetal well-being, to be lower with spinal compared with epidural and general anesthesia, although this may have been due to the use of ephedrine in the studies analyzed.
Nonetheless, spinal anesthesia remains the technique of choice for many obstetric anesthesiologists because of safety, reliability, and patient expectation.
A 2005 review of “best practice” for hip fractures found spinal anesthesia to have consistent benefits, and recommended the use of regional anesthesia “whenever possible.” Benefits cited included reduced mortality, deep vein thrombosis (DVT), transfusion requirements, and pulmonary complications. However, these recommendations, based on two reviews, illustrate the shortcomings of the available evidence. The first review had a heterogeneous population and limited power for subgroup analysis; extrapolating the findings to spinal anesthesia for hip fracture surgery is therefore questionable. The second review found only a borderline difference in mortality at 1 month and no difference at 3 months. Moreover, all included studies had methodological flaws.
The stress response to cardiac surgery is reduced by intrathecal bupivacaine in combination with general anesthesia122 and partially attenuated by intrathecal morphine. Low-dose intrathecal morphine (259 ± 53 μg) has been shown to facilitate early extubation after cardiac surgery. A meta analysis of intrathecal morphine in cardiac surgery showed a modest decrease in morphine use and pain scores, although earlier extubation was only seen in a subset of patients receiving less than 500 μg of intrathecal morphine.
As modern anesthesia and perioperative care become safer, it will become increasingly more difficult to prove an advantage of one technique over another. The ideal technique may in fact be a permutation of general anesthesia, neuraxial nerve block, peripheral nerve block, or local infiltration analgesia.
Spinal Anesthesia: The Final Risk-Benefit Analysis
Once armed with the evidence regarding the risks and benefits of spinal anesthesia, the anesthesiologist must decide whether the evidence applies to the individual patient and clinical situation. Although complications can be devastating, NAP3 reassured us that major complications from spinal anesthesia are rare. Compelling benefits are harder to prove, yet there are advantages in certain clinical situations. Furthermore, the risk-benefit ratio must be compared with the risk-benefit ratio of available alternatives. The historical rise in safety of spinal anesthesia has been paralleled by a rise in safety of alternative techniques, including epidural anesthesia, peripheral nerve block, local infiltration analgesia, and of course general anesthesia. This competition between alternate techniques is likely to continue. Moreover, different modalities can be used in conjunction, complicating the final decision. The modern anesthesiologist must consider this matrix of risk-benefit ratios, which is beyond the scope of this chapter.
FUNCTIONAL ANATOMY OF SPINAL block
In reviewing the functional anatomy of spinal block, an intimate knowledge of the spinal column, spinal cord, and spinal nerves must be present. This chapter briefly reviews the anatomy, surface anatomy, and sonoanatomy of the spinal cord.
The vertebral column consists of 33 vertebrae: 7 cervical, 12 thoracic, 5 lumbar, 5 sacral, and 4 coccygeal segments. The vertebral column usually contains three curves. The cervical and lumbar curves are convex anteriorly, and the thoracic curve is convex posteriorly. The vertebral column curves, along with gravity, baricity of local anesthetic, and patient position, influence the spread of local anesthetics in the subarachnoid space. Figure 1 depicts the spinal column, vertebrae, and intervertebral disks and foramina.
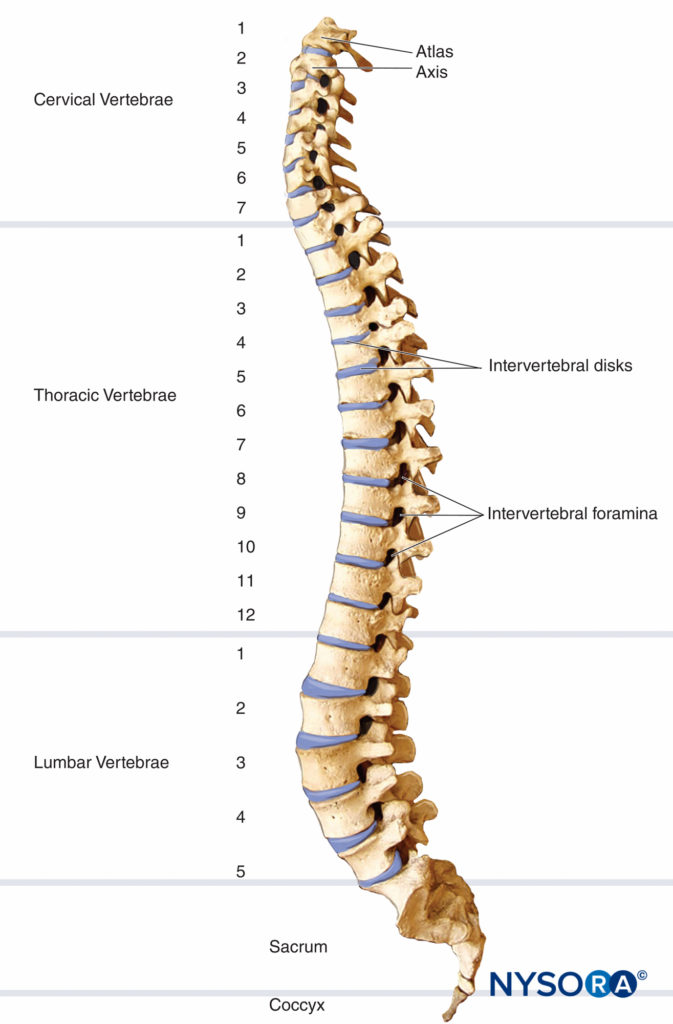
FIGURE 1. Spinal column, vertebrae, and intervertebral disks and foramina.
Five ligaments hold the spinal column together (Figure 2). The supraspinous ligaments connect the apices of the spinous processes from the seventh cervical vertebra (C7) to the sacrum. The supraspinous ligament is known as the ligamentum nuchae in the area above C7. The interspinous ligaments connect the spinous processes together. The ligamentum flavum, or yellow ligament, connects the laminae above and below together. Finally, the posterior and anterior longitudinal ligaments bind the vertebral bodies together.
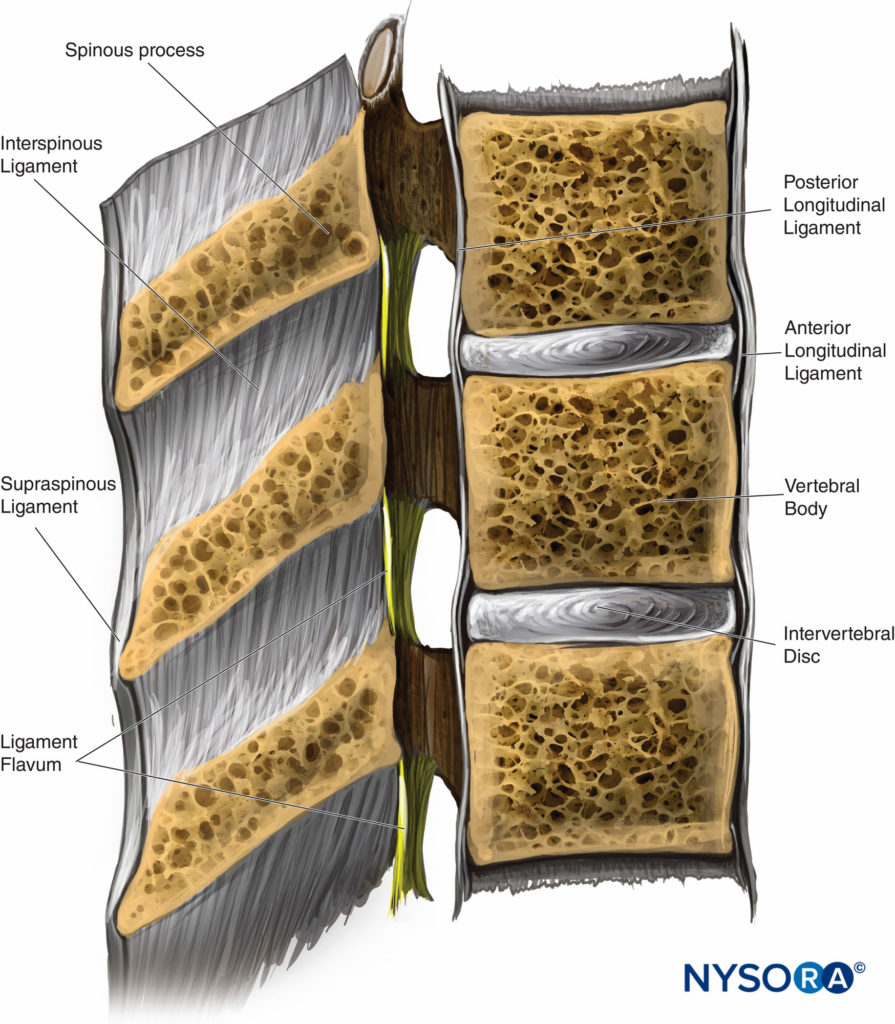
FIGURE 2. Cross section of the spinal canal and adjacent ligaments. (Reproduced with permission from Leffert LR, Schwamm LH: Neuraxial anesthesia in parturients with intracranial pathology: a comprehensive review and reassessment of risk. Anesthesiology. 2013 Sep;119(3):703-718.)
The three membranes that protect the spinal cord are the dura mater, arachnoid mater, and pia mater. The dura mater, or tough mother, is the outermost layer. The dural sac extends to the second sacral vertebra (S2). The arachnoid mater is the middle layer, and the subdural space lies between the dural mater and arachnoid mater. The arachnoid mater, or cobweb mother, also ends at S2, like the dural sac. The pia mater, or soft mother, clings to the surface of the spinal cord and ends in the filum terminale, which helps to hold the spinal cord to the sacrum. The space between the arachnoid and pia mater is known as the subarachnoid space, and spinal nerves run in this space, as does CSF. Figure 3 depicts the spinal cord, dorsal root ganglia and ventral rootlets, spinal nerves, sympathetic trunk, rami communicantes, and pia, arachnoid, and dura maters.
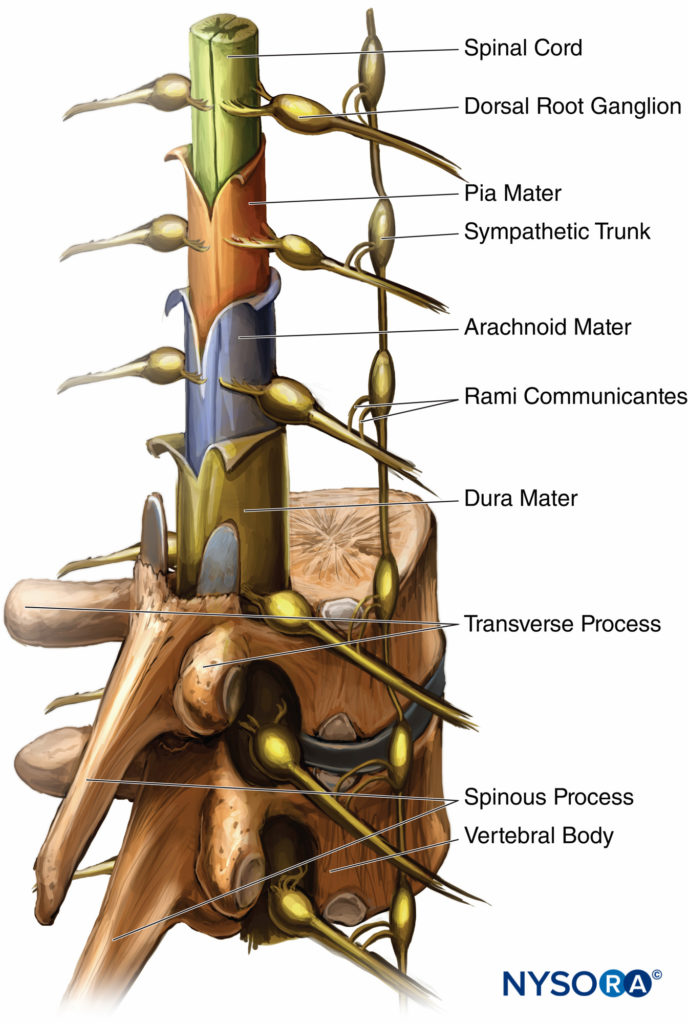
FIGURE 3. Spinal cord with meningeal layers, dorsal root ganglia, and the sympathetic nerve trunk.
When performing a spinal anesthetic using the midline approach, the layers of anatomy that are traversed (from posterior to anterior) are skin, subcutaneous fat, supraspinous ligament, interspinous ligament, ligamentum flavum, dura mater, subdural space, arachnoid mater, and finally the subarachnoid space. When the paramedian technique is applied, the spinal needle should traverse the skin, subcutaneous fat, paraspinous muscle, ligamentum flavum, dura mater, subdural space, and arachnoid mater and then pass into the subarachnoid space.
NYSORA Tips
When performing a spinal anesthetic using the midline approach, the layers of anatomy that are traversed (from posterior to anterior) are
• Skin
• Subcutaneous fat
• Supraspinous ligament
• Interspinous ligament
• Ligamentum flavum
• Dura mater
• Subdural space
• Arachnoid mater
• Subarachnoid space
When performing a spinal anesthetic using the paramedian approach, the spinal needle should traverse
• Skin
• Subcutaneous fat
• Paraspinous muscle
• Ligamentum flavum
• Dura mater
• Subdural space
• Arachnoid mater
• Subarachnoid space
The anatomy of the subdural space requires special attention. The subdural space is a meningeal plane that lies between the dura and the arachnoid mater, extending from the cranial cavity to the second sacral vertebrae. Ultrastructural examination has shown this is an acquired space that only becomes real after tearing of neurothelial cells within the space. The subdural space extends laterally around the dorsal nerve root and ganglion. There is less potential capacity of the subdural space adjacent to the ventral nerve roots. This may explain the sparing of anterior motor and sympathetic fibers during subdural nerve block (SDB) (Figure 4).
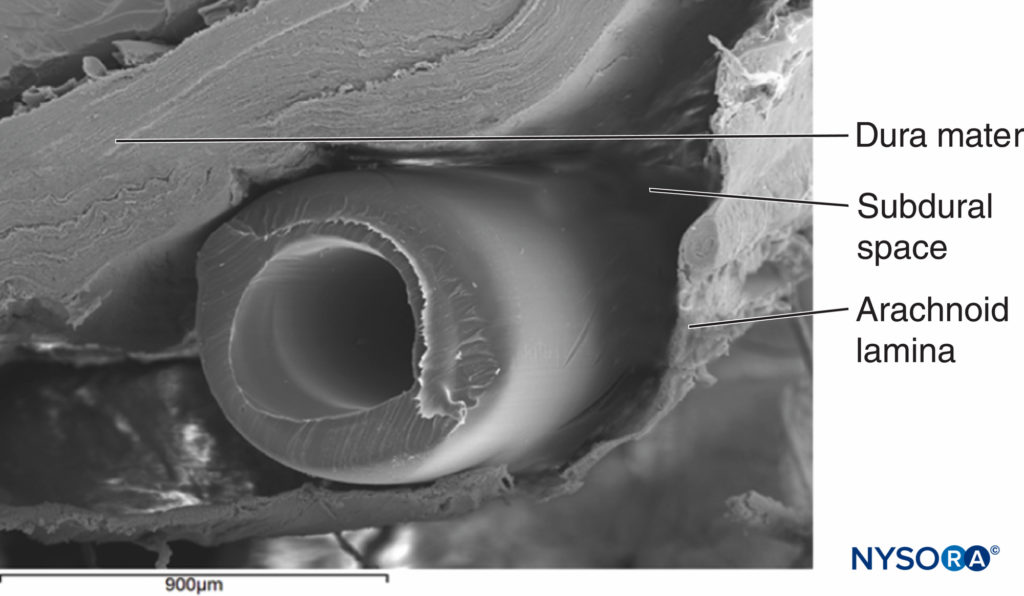
FIGURE 4. Epidural catheter in subdural space. Enhanced view of an epidural catheter inside a subdural space obtained from a cadaver under scanning electron microscopy. Magnification ×20. (Reproduced with permission from Reina MA, Collier CB, Prats-Galino A, et al: Unintentional subdural placement of epidural catheters during attempted epidural anesthesia: an anatomic study of spinal subdural compartment. Reg Anesth Pain Med. 2011 Nov-Dec;36(6):537-541.)
The length of the spinal cord varies according to age. In the first trimester, the spinal cord extends to the end of the spinal column, but as the fetus ages, the vertebral column lengthens more than the spinal cord. At birth, the spinal cord ends at approximately L3. In the adult, the terminal end of the cord, known as the conus edullaris, lies at approximately L1. However, MRI and cadaveric studies have reported a conus medullaris below L1 in 19%–58% and below L2 in 0%–5%. The conus medullaris may lie anywhere between T12 and L3.
Figure 5 Shows a cross section of the lumbar vertebrae and spinal cord. The typical position of the conus medullaris, cauda equina, termination of the dural sac, and filum terminale are shown. A sacral spinal cord in an adult has been reported, although this is extremely rare. The length of the spinal cord must always be kept in mind when a neuraxial anesthetic is performed, as injection into the cord can cause great damage and result in paralysis.
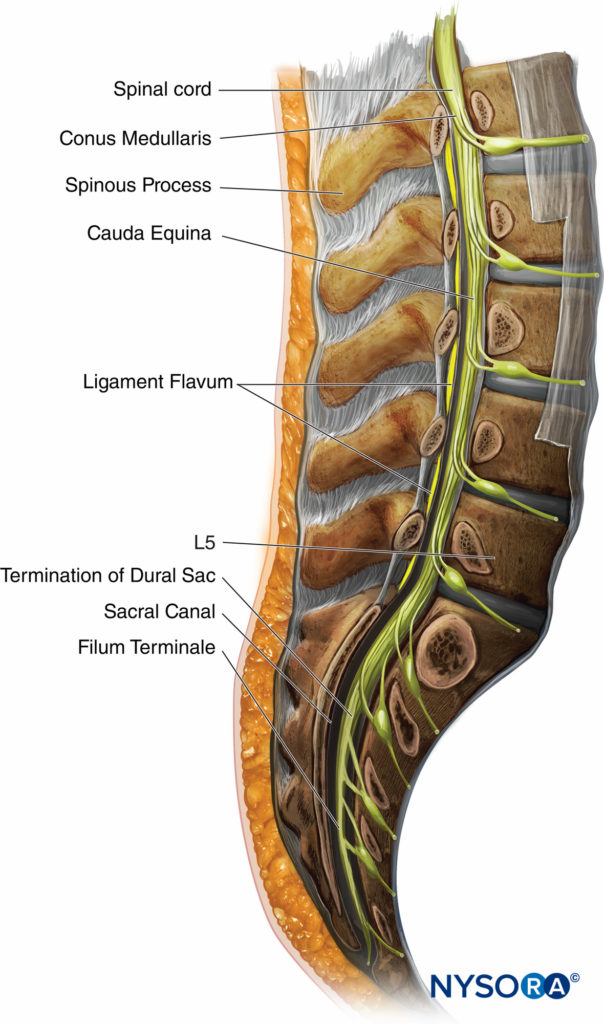
FIGURE 5. Cross section of the lumbar vertebrae.
There are eight cervical spinal nerves and seven cervical vertebrae. Cervical spinal nerves 1 to 7 are numbered according to the vertebral body below. The eighth cervical nerve exits from below the seventh cervical vertebral body. Below this, spinal nerves are numbered according to the vertebral body above. The spinal nerve roots and spinal cord serve as the target sites for spinal anesthesia.
Surface Anatomy
When preparing for spinal anesthetic block, it is important to accurately identify landmarks on the patient.
The midline is identified by palpating the spinous processes. The iliac crests usually are at the same vertical height as the fourth lumbar spinous process or the interspace between the fourth and fifth lumbar vertebrae. An intercristal line can be drawn between the iliac crests to help locate this interspace. Care must be taken to feel for the soft area between the spinous processes to locate the interspace. Depending on the level of anesthesia necessary for the surgery and the ability to feel for the interspace, the L3–L4 interspace or the L4–L5 interspace can be used to introduce the spinal needle. Because the spinal cord commonly ends at the L1-to-L2 level, it is conventional not to attempt spinal anesthesia at or above this level. More recently, segmental thoracic spinal anesthesia has been described.
It would be incomplete to discuss surface anatomy without mentioning the dermatomes that are important for spinal anesthesia. A dermatome is an area of skin innervated by sensory fibers from a single spinal nerve. The tenth thoracic (T10) dermatome corresponds to the umbilicus, the sixth thoracic (T6) dermatome the xiphoid, and the fourth thoracic (T4) dermatome the nipples. Figure 6 illustrates the dermatomes of the human body. To achieve surgical anesthesia for a given procedure, the extent of spinal anesthesia must reach a certain dermatomal level. Dermatomal levels of spinal anesthesia for common surgical procedures are listed in Table 5.
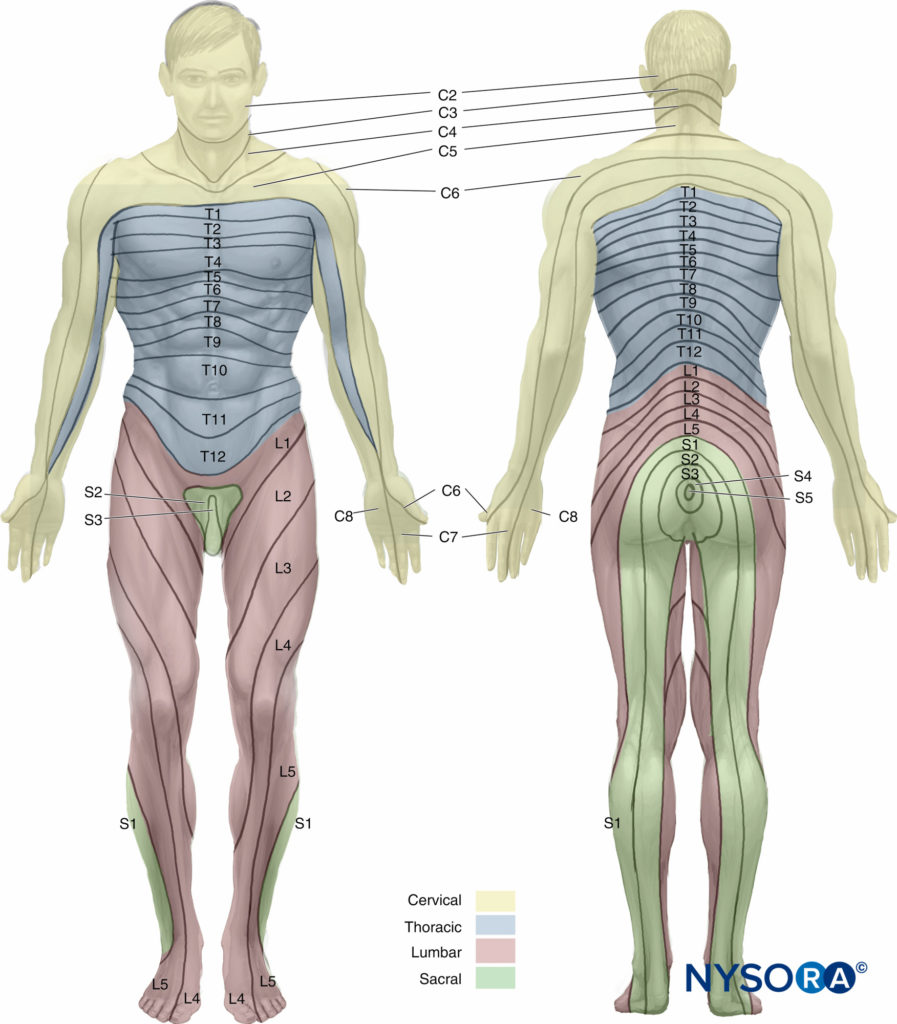
FIGURE 6. Dermatomes of the human body.
TABLE 5. Dermatomal levels of spinal anesthesia for common surgical procedures.
| Procedure | Dermatomal Level |
|---|---|
| Upper abdominal surgery | T4 |
| Intestinal, gynecologic, and urologic surgery | T6 |
| Transurethral resection of the prostate | T10 |
| Vaginal delivery of a fetus and hip surgery | T10 |
| Thigh surgery and lower leg amputations | L1 |
| Foot and ankle surgery | L2 |
| Perineal and anal surgery | S2 to S5 (saddle block) |
NYSORA Tips
• T10 dermatome corresponds to the umbilicus.
• T6 dermatome corresponds to the xiphoid.
• T4 dermatome corresponds to the nipples.
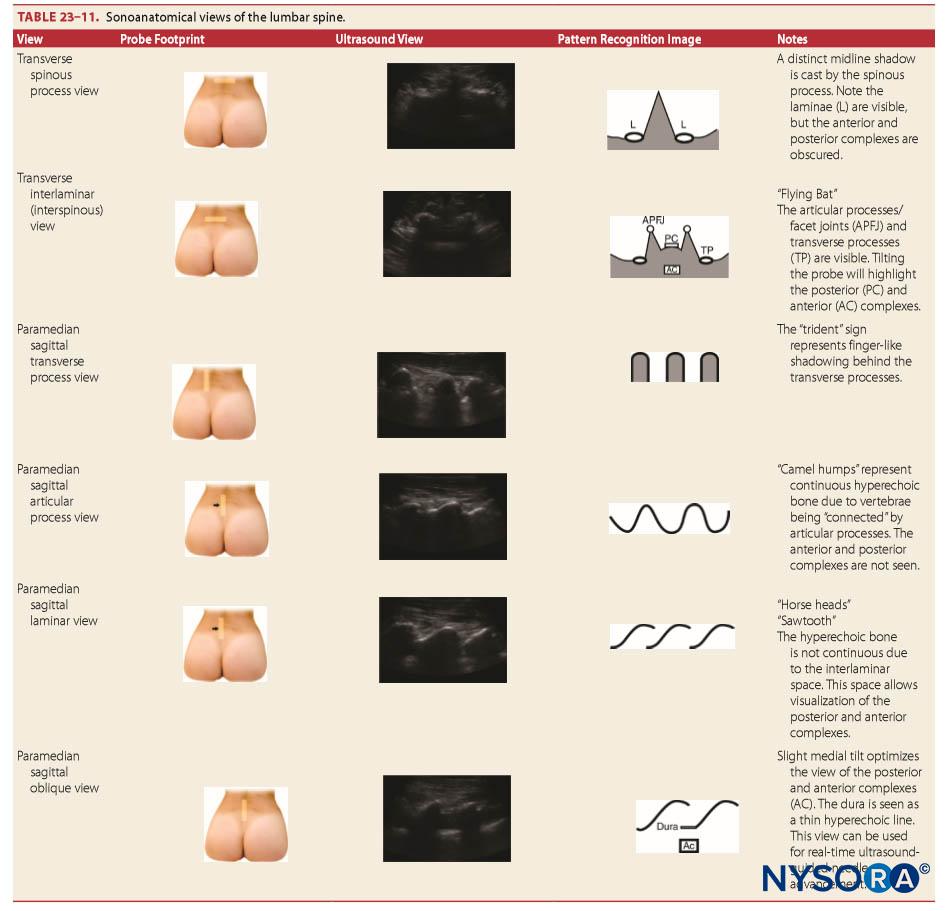
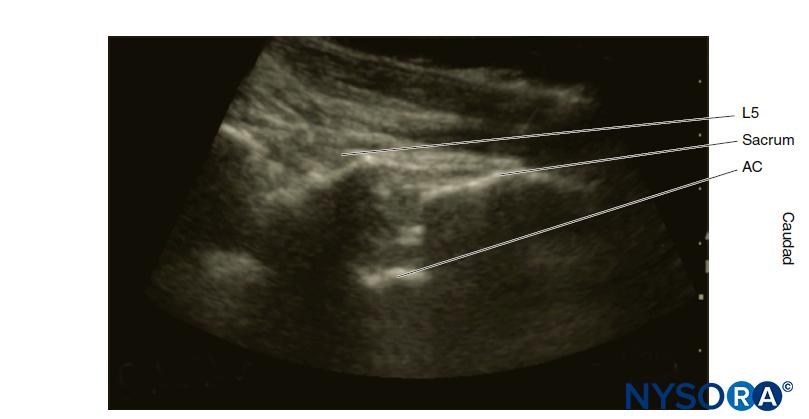
Sonoanatomy
“Surface” anatomy refers to structures close enough to the integument that they are palpable. However, due to body habitus, this may not be possible. Neuraxial ultrasound allows sonoanatomical visualization of these structures and deeper structures. However, as the ultrasound beam cannot penetrate the bony vertebrae, specialized ultrasonic windows are required to visualize the neuraxis. The technique of neuraxial ultrasound is discussed elsewhere (see section on recent developments in spinal anesthesia).
PHARMACOLOGY
The choice of local anesthetic is based on potency of the agent, onset and duration of anesthesia, and side effects of the drug. Two distinct groups of local anesthetics are used in spinal anesthesia, esters and amides, which are characterized by the bond that connects the aromatic portion and the intermediate chain.
Esters contain an ester link between the aromatic portion and the intermediate chain, and examples include procaine, chloroprocaine, and tetracaine. Amides contain an amide link between the aromatic portion and the intermediate chain, and examples include bupivacaine, ropivacaine, etidocaine, lidocaine, mepivacaine, and prilocaine. Although metabolism is important for determining activity of local anesthetics, lipid solubility, protein binding, and pKa also influence activity.
NYSORA Tips
• Potency of local anesthetics is related to lipid solubility.
• The duration of action of a local anesthetic is affected by the protein binding.
• The onset of action is related to the amount of local anesthetic available in the base form.
Lipid solubility relates to the potency of local anesthetics. Low lipid solubility indicates that higher concentrations of local anesthesia must be given to obtain nerve block. Conversely, high lipid solubility produces anesthesia at low concentrations. Protein binding affects the duration of action of a local anesthetic. Higher protein binding results in longer duration of action. The pKa of a local anesthetic is the pH at which ionized and nonionized forms are present equally in solution, which is important because the nonionized form allows the local anesthetic to diffuse across the lipophilic nerve sheath and reach the sodium channels in the nerve membrane. The onset of action relates to the amount of local anesthetic available in the base form. Most local anesthetics follow the rule that the lower the pKa, the faster the onset of action and vice versa. Please refer to Clinical Pharmacology of Local Anesthetics.
Pharmacokinetics of Local Anesthetics in the Subarachnoid Space
Pharmacokinetics of local anesthetics includes uptake and elimination of the drug. Four factors play a role in the uptake of local anesthetics from the subarachnoid space into neuronal tissue: (1) concentration of local anesthetic in CSF, (2) surface area of nerve tissue exposed to CSF, (3) lipid content of nerve tissue, and (4) blood flow to nerve tissue.
The uptake of local anesthetic is greatest at the site of highest concentration in the CSF and is decreased above and below this site. As discussed previously, uptake and spread of local anesthetics after spinal injection are determined by multiple factors, including dose, volume, and baricity of local anesthetic and patient positioning. Both the nerve roots and the spinal cord take up local anesthetics after injection into the subarachnoid space. The more surface area of the nerve root exposed, the greater the uptake of local anesthetic. The spinal cord has two mechanisms for uptake of local anesthetics. The first mechanism is by diffusion from the CSF to the pia mater and into the spinal cord, which is a slow process. Only the most superficial portion of the spinal cord is affected by diffusion of local anesthetics. The second method of local anesthetic uptake is by extension into the spaces of Virchow-Robin, which are the areas of pia mater that surround the blood vessels that penetrate the central nervous system. The spaces of Virchow-Robin connect with the perineuronal clefts that surround nerve cell bodies in the spinal cord and penetrate through to the deeper areas of the spinal cord. Figure 7 is a representation of the periarterial Virchow-Robin spaces around the spinal cord.
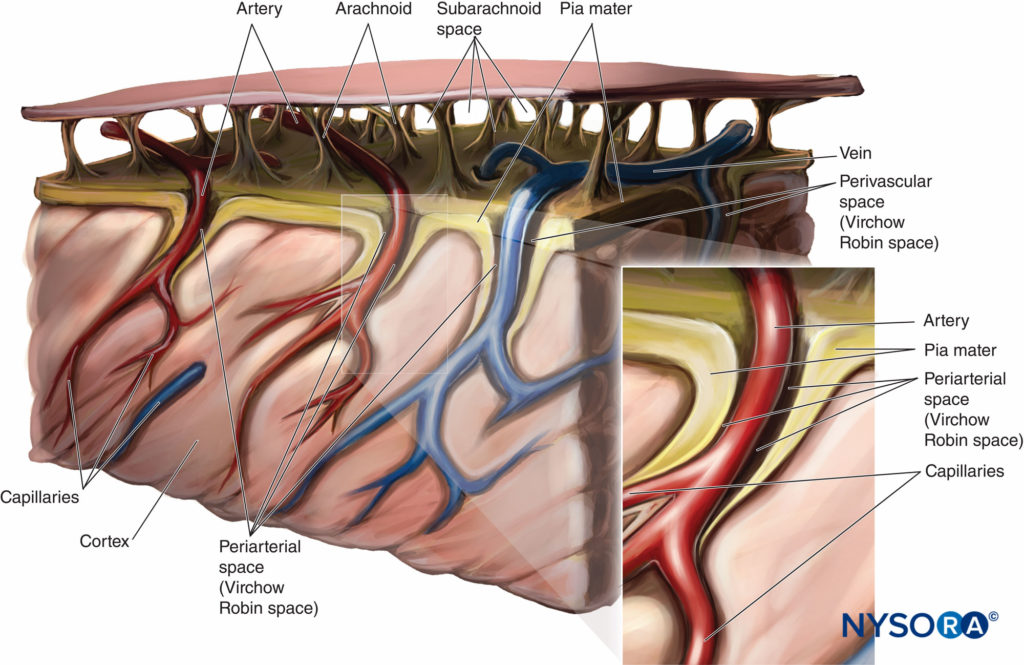
FIGURE 7. Virchow-Robin space.
NYSORA Tips
The three most important modifiable factors in determining distribution of local anesthetics are
• Baricity of the local anesthetic solution
• Position of the patient during and just after injection
• Dose of the anesthetic injected
Lipid content determines uptake of local anesthetics. Heavily myelinated tissues in the subarachnoid space contain higher concentrations of local anesthetics after injection. The higher the degree of myelination, the higher the concentration of local anesthetic, as there is a high lipid content in myelin. If an area of nerve root does not contain myelin, an increased risk of nerve damage occurs in that area.
Blood flow determines the rate of removal of local anesthetics from spinal cord tissue. The faster the blood flows in the spinal cord, the more rapid the anesthetic is washed away. This may partly explain why the concentration of local anesthetics is greater in the posterior spinal cord than in the anterior spinal cord, even though the anterior cord is more readily accessed by the Virchow-Robin spaces. After a spinal anesthetic is administered, blood flow may be increased or decreased to the spinal cord, depending on the particular local anesthetic administered; for example, tetracaine increases cord flow, but lidocaine and bupivacaine decrease it, which affects elimination of the local anesthetic.
Elimination of local anesthetic from the subarachnoid space is by vascular absorption in the epidural space and the subarachnoid space. Local anesthetics travel across the dura in both directions. In the epidural space, vascular absorption can occur, just as in the subarachnoid space. Vascular supply to the spinal cord consists of vessels located on the spinal cord and in the pia mater. Because vascular perfusion to the spinal cord varies, the rate of elimination of local anesthetics varies.
Distribution
The distribution and decrease in concentration of local anesthetics is based on the area of highest concentration, which can be independent of the injection site. Many factors affect the distribution of local anesthetics in the subarachnoid space. Table 6 lists some of these factors.
TABLE 6. Determinants of local anesthetic spread in the subarachnoid space.
| Properties of local anesthetic solution • Baricity • Dose • Volume • Specific gravity |
| Patient characteristics • Position during and after injection • Height (extremely short or tall) • Spinal column anatomy • Decreased cerebrospinal fluid volume (increased intra-abdominal pressure due to increased weight, pregnancy, etc.) |
| Technique • Site of injection • Needle bevel direction |
Baricity plays an important role in determining the spread of local anesthetic in the spinal space and is equal to the density of the local anesthetic divided by the density of the CSF at 37°C. Local anesthetics can be hyperbaric, hypobaric, or isobaric when compared to CSF, and baricity is the main determinant of how the local anesthetic is distributed when injected into the CSF. Table 7 compares the density, specific gravity, and baricity of different substances and local anesthetics.
TABLE 7. Density, specific gravity, and baricity of different substances and local anesthetics.
| Density | Specific Gravity | Baricity | ||
|---|---|---|---|---|
| Water | 0.9933 | 1.0000 | 0.9930 | |
| Cerebrospinal fluid | 1.0003 | 1.0069 | 1.0000 | |
| Hypobaric | ||||
| • Tetracaine | 0.33% in water | 0.9980 | 1.0046 | 0.9977 |
| • Lidocaine | 0.5% in water | N/A | 1.0038 | 0.9985 |
| Isobaric | ||||
| • Tetracaine | 0.5% in 50% CSF | 0.9998 | 1.0064 | 0.9995 |
| • Lidocaine | 2% in water | 1.0003 | 1.0066 | 1.0003 |
| • Bupivacaine | 0.5% in water | 0.9993 | 1.0059 | 0.9990 |
| Hyperbaric | ||||
| • Tetracaine | 0.5% in 5% dextrose | 1.0136 | 1.0203 | 1.0133 |
| • Lidocaine | 5% in 7.5% dextrose | 1.0265 | 1.0333 | 1.0265 |
| • Bupivacaine | 0.5% in 8% dextrose | 1.0210 | 1.0278 | 1.0207 |
| • Bupivacaine | 0.75% in 8% dextrose | 1.0247 | 1.0300 | 1.0227 |
Hypobaric solutions are less dense than CSF and tend to rise against gravity. Isobaric solutions are as dense as CSF and tend to remain at the level at which they are injected. Hyperbaric solutions are more dense than CSF and tend to follow gravity after injection.
Hypobaric solutions have a baricity of less than 1.0 relative to CSF and are usually made by adding distilled sterile water to the local anesthetic. Tetracaine, dibucaine, and bupivacaine have all been used as hypobaric solutions in spinal anesthesia. Patient positioning is important after injection of a hypobaric spinal anesthetic because it is the first few minutes that determine the spread of anesthesia. If the patient is in Trendelenburg position after injection, the anesthetic will spread in the caudal direction and if the patient is in reverse Trendelenburg position, the anesthetic will spread cephalad after injection.
The baricity of isobaric solutions is equal to 1.0. Tetracaine and bupivacaine have both been used with success for isobaric spinal anesthesia. Gravity does not play a role in the spread of isobaric solutions, unlike with hypo- or hyperbaric local anesthetics. Therefore, patient positioning does not affect spread of isobaric solutions. Injection can be made in any position, and then the patient can be placed into the position necessary for surgery.
Hyperbaric solutions have baricity greater than 1.0. A local anesthetic solution can be made hyperbaric by adding dextrose or glucose. Bupivacaine, lidocaine, and tetracaine have all been used as hyperbaric solutions in spinal anesthesia. Patient positioning affects the spread of the anesthetic. A patient in Trendelenburg position would have the anesthetic travel in a cephalad direction and vice versa.
Dose and volume both play a role in the distribution of local anesthetics after spinal injection. For further information, please refer to the section Volume, Concentration, and Dose of Local Anesthetic.
Effects of the Volume of the Lumbar Cistern on Nerve Block Height
Cerebrospinal fluid is produced in the brain at 0.35 mL/min and fills the subarachnoid space. This clear, colorless fluid has an approximate adult volume of 150 mL, half of which is in the cranium and half in the spinal canal. However, CSF volume varies considerably, and decreased CSF volume can result from obesity, pregnancy, or any other cause of increased abdominal pressure. This is partly due to compression of the intervertebral foramen, which displaces the CSF.
Clinical Pearl
Due to the wide variability in CSF volume, the ability to predict the level of the spinal block after local anesthetic injection is very poor, even if BMI is calculated and used.
Multiple factors affect the distribution of local anesthesia after spinal block, one being CSF volume. Carpenter showed that lumbosacral CSF volume correlated with peak sensory nerve block height and duration of surgical anesthesia. The density of CSF is related to peak sensory nerve block level, and lumbosacral CSF volume correlates to peak sensory nerve block level and onset and duration of motor nerve block. However, due to the wide variability in CSF volume, the ability to predict the level of the spinal block after local anesthetic injection is poor, even if BMI is calculated and used.
Local Anesthetics
Cocaine was the first spinal anesthetic used, and procaine and tetracaine soon followed. Lidocaine, 2-chloroprocaine, bupivacaine, mepivacaine, and ropivacaine have also been used intrathecally. In addition, there is a growing interest in medications that produce anesthesia and analgesia while limiting side effects. A variety of medications, including vasoconstrictors, opioids, α2-adrenergic agonists, and acetylcholinesterase inhibitors, have been added to spinal medications to enhance analgesia while reducing the motor block produced by local anesthetics.
Lidocaine was first used as a spinal anesthetic in 1945, and it has been one of the most widely used spinal anesthetics since. Onset of anesthesia occurs in 3 to 5 minutes with a duration of anesthesia that lasts for 1 to 1.5 hour. Lidocaine spinal anesthesia has been used for short-to-intermediate length operating room cases. The major drawback of lidocaine is the association with transient neurologic symptoms (TNSs), which present as low back pain and lower extremity dysesthesias with radiation to the buttocks, thighs, and lower limbs after recovery from spinal anesthesia. TNSs occur in about 14% of patients receiving lidocaine spinal anesthesia. Lithotomy position is associated with a higher incidence of TNSs. Because of the risk of TNSs, lidocaine has mostly been replaced by other local anesthetics.
Intrathecal use of 2-chloroprocaine was described in 1952. In the 1980s, concerns were raised regarding neurotoxicity with the use of 2-chloroprocaine. Studies have suggested that sodium bisulfite, an antioxidant used in combination with 2-chloroprocaine, is responsible. Chronic neurologic deficits have been reported in rabbits when sodium bisulfite was injected into the lumbar subarachnoid space, but when preservative-free 2-chloroprocaine was injected, no permanent neurologic sequelae were noted. Results from clinical trials have shown preservative-free 2-chloroprocaine to be safe, short acting, and acceptable for outpatient surgery. However, addition of epinephrine is not recommended due to an association with flu-like symptoms and back pain. Intrathecal 2-chloroprocaine is not currently approved by the Food and Drug Administration (FDA), although package labeling states it may be used for epidural anesthesia. Onset time is fast, and the duration is around 100 to 120 minutes. The dose ranges from 20 to 60 mg, with 40 mg as a usual dose.
Procaine is a short-acting ester local anesthetic. Procaine has an onset time of 3 to 5 minutes and a duration of 50 to 60 minutes. A dose of 50 to 100 mg has been suggested for perineal and lower extremity surgery. However, there is a 14% incidence of nerve block failure associated with procaine 10%. Concerns about the neurotoxicity of procaine have limited its use. For all these reasons, procaine is currently rarely used for spinal anesthesia.
Bupivacaine is one of the most widely used local anesthetics for spinal anesthesia and provides adequate anesthesia and analgesia for intermediate-to-long-duration operating room cases. Bupivacaine has a low incidence of TNSs. Onset of anesthesia occurs in 5 to 8 minutes, with a duration of anesthesia that lasts from 90 to 150 minutes. For outpatient spinal anesthesia, small doses of bupivacaine are recommended to avoid prolonged discharge time due to duration of nerve block. Bupivacaine is often packaged as 0.75% in 8.25% dextrose. Other forms of spinal bupivacaine include 0.5% with or without dextrose and 0.75% without dextrose.
NYSORA Tips
• Use of intrathecal lidocaine is limited by TNSs.
• Bupivacaine has a very low incidence of TNSs.
• Onset of anesthesia occurs in 5 to 8 minutes with bupivacaine and a duration of anesthesia that lasts from 210 to 240 minutes; thus, it is appropriate for intermediate-to-long operating room cases.
Tetracaine has an onset of anesthesia within 3 to 5 minutes and a duration of 70 to 180 minutes and, like bupivacaine, is used for cases that are intermediate to longer duration. The 1% solution can be mixed with 10% glucose in equal parts to form a hyperbaric spinal anesthetic that is used for perineal and abdominal surgery. With tetracaine, TNSs occur at a lower rate than with lidocaine spinal anesthesia. The addition of phenylephrine may play a role in the development of TNSs.
Mepivacaine is similar to lidocaine and has been used since the 1960s for spinal anesthesia. The incidence of TNSs reported after mepivacaine spinal anesthesia varies widely, with rates from 0% to 30%.
Ropivacaine was introduced in the 1990s. For applications in spinal anesthesia, ropivacaine has been found to be less potent than bupivacaine. Dose range-finding studies have demonstrated the ED95 of spinal ropivacaine in lower limb surgery (11.4 mg), pregnant patients (26.8 mg), and neonates (1.08 mg/kg). Intrathecal use of ropivacaine is not widespread, and large-scale safety data are awaited. An early study identified back pain in 5 of 18 volunteers injected with intrathecal hyperbaric ropivacaine. TNSs have been reported with spinal ropivacaine although the incidence is not as common as seen with lidocaine. Other small studies have not demonstrated any major side effects.
Table 8 shows some of the local anesthetics used for spinal anesthesia and dosage duration and concentration for different levels of spinal block.
TABLE 8. Dose, duration, and onset of local anesthetics used in spinal anesthesia.
| Dose (mg) To T10 | Dose (mg) to T4 | Duration (minutes) Plain | With Epinephrine | Onset (minutes) | |
|---|---|---|---|---|---|
| Commonly used Bupivacaine 0.75% | 8–12 | 14–20 | 90–110 | 100–150 | 5–8 |
| Less commonly used | |||||
| • Lidocaine 5% • Tetracaine 0.5% • Mepivacaine 2% • Ropivacaine 0.75% • Levobupivacaine 0.5% • Chloroprocaine 3% | 50–75 6–10 N/A 15–17 10–15 30 | 75–100 12–16 60–80 18–20 N/A 45 | 60–70 70–90 140–160 140–200 135–170 80–120 | 75–100 120–180 N/A N/A N/A N/A | 3–5 3–5 2–4 3–5 4–8 2–4 |
Additives to Local Anesthesia
Vasoconstrictors have been added to local anesthetics, and both epinephrine and phenylephrine have been studied. Anesthesia is intensified and prolonged with smaller doses of local anesthetics when epinephrine or phenylephrine is added. Tissue vasoconstriction is produced, thus limiting the systemic reabsorption of the local anesthetic and prolonging the duration of action by keeping the local anesthetic in contact with the nerve fibers. However, ischemic complications can occur after the use of vasoconstrictors in spinal anesthesia. In some studies, epinephrine was implicated as the cause of CES because of anterior spinal artery ischemia. Regardless, many studies do not demonstrate an association between the use of vasoconstrictors for spinal anesthesia and the incidence of CES. Phenylephrine has been shown to increase the risk of TNSs and may decrease nerve block height.
Epinephrine is thought to work by decreasing local anesthetic uptake and thus prolonging the spinal block of some local anesthetics. However, vasoconstrictors can cause ischemia, and there is a theoretical concern of spinal cord ischemia when epinephrine is added to spinal anesthetics. Animal models have not shown any decrease in spinal cord blood flow or increase in spinal cord ischemia when epinephrine is given for spinal block, even though some neurologic complications associated with the addition of epinephrine exist.
NYSORA Tips
• Adding 0.1 mL of 1:1000 epinephrine to 10 mL of local anesthetic yields a 1:100,000 concentration of epinephrine.
• Adding 0.1 mL of 1:1000 epinephrine to 20 mL of local anesthetic yields a 1:200,000 concentration and so on (0.1 mL in 30 mL = 1:300,000).
Dilution of epinephrine with local anesthetic is a potential source of drug error, with mistakes potentially incorrect by a factor of 10 or 100. If using epinephrine packaged as 1 mg in 1 mL, which is a 1:1000 solution, a simple rule can be followed. Adding 0.1 mL of epinephrine to 10 mL of local anesthetic yields a 1:100,000 concentration of epinephrine. Adding 0.1 mL of epinephrine to 20 mL of local anesthetic yields a 1:200,000 concentration, and so on (0.1 mL in 30 mL = 1:300,000).
Epinephrine prolongs the duration of spinal anesthesia. In the past, it was thought that epinephrine had no effect on hyperbaric spinal bupivacaine using two-segment regression to test neural block. However, another study showed that epinephrine prolongs the duration of hyperbaric spinal bupivacaine when pinprick, transcutaneous electrical nerve stimulation (TENS) equivalent to surgical stimulation, and tolerance of a pneumatic thigh tourniquet were used to determine neural block. There is controversy regarding prolongation of spinal bupivacaine neural block when epinephrine is added. The same controversy exists about the prolongation of spinal lidocaine with epinephrine.
All four types of opioid receptors are found in the dorsal horn of the spinal cord and serve as the target for intrathecal opioid injection. Receptors are located on spinal cord neurons and terminals of afferents originating in the dorsal root ganglion.
Fentanyl, sufentanil, meperidine, and morphine have all been used intrathecally. Side effects that may be seen include pruritus, nausea and vomiting, and respiratory depression.
The α2-adrenergic agonists can be added to spinal injections of local anesthetics to enhance pain relief and prolong sensory and motor nerve block. Enhanced postoperative analgesia has been demonstrated in cesarean deliveries, fixation of femoral fractures, and knee arthroscopies when clonidine was added to the local anesthetic solution. Clonidine prolongs the sensory and motor block of a local anesthetic after spinal injection.
Sensory block is thought to be mediated by both presynaptic and postsynaptic mechanisms. Clonidine induces hyperpolarization at the ventral horn of the spinal cord and facilitates the action of the local anesthetic, thus prolonging motor block when used as an additive. However, when used alone in intrathecal injections, clonidine does not cause motor nerve block or weakness. Side effects can occur with the use of spinal clonidine and include hypotension, bradycardia, and sedation. Neuraxial clonidine has been used for the treatment for intractable pain.
Acetylcholinesterase inhibitors prevent the breakdown of acetylcholine and produce analgesia when injected intrathecally. The antinociceptive effects are due to increased acetylcholine and generation of nitric oxide. It has been shown in a rat model that diabetic neuropathy can be alleviated after intrathecal neostigmine injection.222 Side effects of intrathecal neostigmine include nausea and vomiting, bradycardia requiring atropine, anxiety, agitation, restlessness, and lower extremity weakness. Although spinal neostigmine provides extended pain control, the side effects that occur do not allow its widespread use.
PHARMACODYNAMICS OF SPINAL ANESTHESIA
The pharmacodynamics of spinal injection of local anesthesia are wide ranging. The cardiovascular, respiratory, gastrointestinal, hepatic, and renal effect consequences of spinal anesthesia are discussed next.
Cardiovascular Effects of Spinal Anesthesia
It is well recognized that spinal anesthesia results in hypotension. In fact, a degree of hypotension often reassures the anesthesiologist that the nerve block is indeed spinal. However, hypotension may cause nausea and vomiting, ischemia of critical organs, cardiovascular collapse, and in the case of the pregnant mother may endanger the fetus. Historically, there have been shifts in the definitions, suggested mechanisms, and management of hypotension.
Defining hypotension is troublesome. One study found 15 different definitions of hypotension in 63 publications. Some definitions used a single criterion (decrease of 80% from baseline), while others used combinations (a fall of 80% from baseline or a systolic blood pressure less than 100 mmHg). The incidence of hypotension in a single cohort of patients varied from 7.4% to 74.1% depending on the definition used.
There have been many suggested mechanisms for spinal anesthesia–induced hypotension, including direct circulatory effects of local anesthetics, relative adrenal insufficiency, skeletal muscle paralysis, ascending medullary vasomotor nerve block, and concurrent respiratory insufficiency. The primary insult, however, is the preganglionic sympathetic nerve block produced by spinal anesthesia. It therefore follows that because the nerve block height determines the extent of sympathetic block, this in turn determines the amount of change in cardiovascular parameters. However, this relationship cannot be predicted. Sympathetic nerve block may be variably between two and six dermatomes above the sensory level and incomplete below this level. The sudden sympathetic nerve block with spinal anesthesia gives little time for cardiovascular compensation, which may account for a similar sympathetic nerve block with epidural anesthesia, but less hypotension.
NYSORA Tips
• Spinal anesthesia nerve block the sympathetic chain, which is the main mechanism of cardiovascular changes.
• The nerve block height determines the level of sympathetic block, which determines the degree of change in cardiovascular parameters.
Sympathetic nerve block causes hypotension via its effects on preload, afterload, contractility, and HR—in other words, the determinants of cardiac output (CO)—and by decreasing systemic vascular resistance (SVR). Preload is decreased by sympathetic nerve block-mediated venodilation, resulting in pooling of blood in the peripheries and decreased venous return. During sympathetic nerve block, the venous system is maximally vasodilated and therefore reliant on gravity to return blood to the heart. Thus, patient positioning, and aortocaval compression in the case of a gravid uterus, markedly influences venous return during spinal anesthesia.
Arterial vasomotor tone can also be decreased by sympathetic nerve block, decreasing SVR, and afterload. Arterial vasodilation, unlike venodilation, is not maximal after spinal block, and vascular smooth muscle continues to retain some autonomic tone after sympathetic denervation. This residual vascular tone can be lost in the presence of hypoxia and acidosis, which may account for cardiovascular collapse after high spinal anesthesia without cardiorespiratory support. Although there is vasodilation below the level of spinal block, there is compensatory vasoconstriction above, mediated by carotid and aortic arch baroreceptors. This is important for two reasons. First, block at higher dermatomal levels may result in less compensation. Second, use of vasodilatory drugs such as glyceryl trinitrate (GTN), sodium nitroprusside, or volatile anesthetics may abolish this compensatory mechanism and worsen hypotension or even result in cardiac arrest.
There may be an initial increase in CO associated with a decreased afterload. Alternatively, CO may fall due to decreased preload. Some studies have shown that CO is unchanged or slightly reduced during onset of spinal anesthesia. Others, in elderly patients, have shown a biphasic change in CO with an initial increase in the first 7 minutes, followed by a fall (Figure 8). This may be attributed to a fall in afterload preceding a fall in preload.
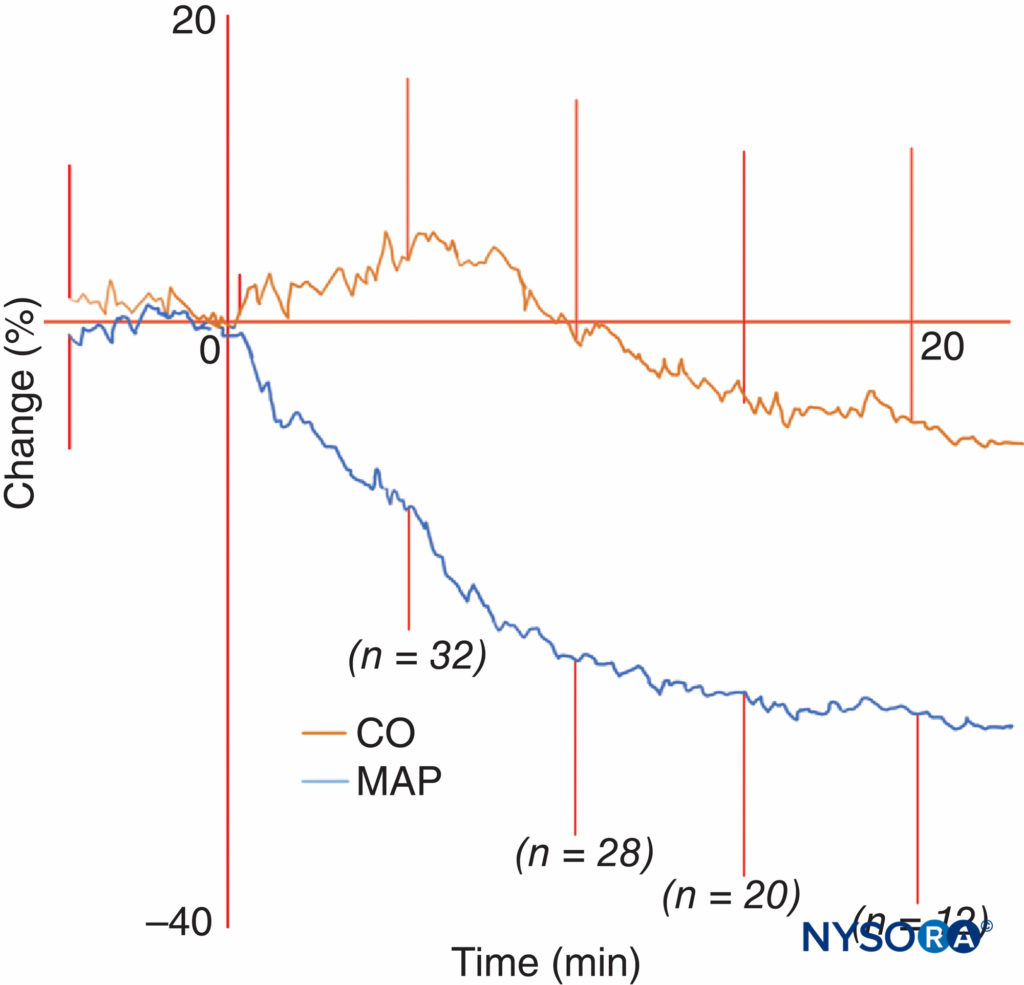
FIGURE 8. Figure from the work of Meyhoff et al showing a fall in mean arterial pressure (MAP) and biphasic cardiac output (CO) after spinal anesthesia. Average CO and MAP changes plus or minus standard deviation during onset of spinal anesthesia in elderly patients. Subarachnoid injection is given at time = 0 minutes. After termination of data collection, the last CO and MAP recording are still represented in the average throughout the rest of the graph. Each line is thus hypothetical as it consists of averages of 32 patients even after data termination; this is done for illustration purposes only. (Reproduced with permission from Meyhoff CS, Hesselbjerg L, Koscielniak-Nielsen Z, et al: Biphasic cardiac output changes during onset of spinal anaesthesia in elderly patients. Eur J Anaesthesiol. 2007 Sep;24(9):770-775.)
Contractility may be affected by block of the upper thoracic sympathetic nerves. Interestingly, a study investigating the common phenomenon of ST segment depression in healthy women undergoing cesarean section (25-60%) found ST depression to be associated with a hyperkinetic contractile state.
The effect of spinal anesthesia on HR is complex. HR may increase (secondary to hypotension via the baroreceptor reflex) or decrease (either from sympathetic nerve block of cardiac accelerator fibers originating from T1–T4 spinal segments, or via the reverse Bainbridge reflex). The reverse Bainbridge reflex is a decrease in HR due to decreased venous return, detected by stretch receptors in the right atrium, and is weaker than the baroreceptor reflex. The Bezold-Jarisch reflex (BJR) is another reflex that decreases HR. The BJR has been implicated as a cause of bradycardia, hypotension, and cardiovascular collapse after central neuraxial anesthesia, in particular spinal anesthesia.
The BJR is a cardioinhibitory reflex and is usually not a dominant reflex. The association with spinal anesthesia is probably weak. The BJR has been blamed for bradycardia after spinal anesthesia, especially after hemorrhage. Vigorous contractions of an underfilled heart may initiate the BJR. This is more likely with the use of ephedrine rather than phenylephrine.
Young, healthy (American Society of Anesthesiologists class 1) patients have a higher risk of bradycardia. Beta-blocker use also increases the risk of bradycardia. The incidence of bradycardia in the nonpregnant population is about 13%. Even though bradycardia is usually well tolerated, asystole and second- and third-degree heart nerve block can occur, so it is wise to be vigilant when monitoring a patient after spinal anesthesia and treat promptly.
Risk factors associated with hypotension include hypovolemia, preoperative hypertension, high sensory nerve block height, age older than 40 years, obesity, combined general and spinal anesthesia, chronic alcohol consumption, elevated BMI, and urgency of nonobstetric surgery. Hypotension is less likely in women who are in labor compared with those undergoing elective cesarean section. Refer to Regional Anesthesia and Cardiovascular Disease.
Management of Hypotension After Spinal Anesthesia
Changing Beliefs Shifting beliefs in the theoretical basis of spinal-induced hypotension have been echoed by changes in management. For example, if decreased preload is believed to be of primary importance, then positioning and fluid therapy are the treatments of choice, and similarly if vasodilation is the culprit, then a vasoconstrictor should be first line. This has led to vigorous debate. In the 1970s, it was suggested not to give vasopressors until “all other methods of combating hypotension” were utilized, underlining the importance of preload. Evidence to support this was extrapolated from flawed studies on pregnant ewes undergoing general anesthesia, which suggested vasopressors adversely effected the uteroplacental circulation. The title vasopressor of choice has similarly generated much controversy. Ephedrine was traditionally nominated as it preserved uterine blood flow (in the aforementioned animal studies). Work by Ngan Kee, among others, has suggested phenylephrine may be the vasopressor of choice, at least in the elective obstetric setting.
Management Management of hypotension following spinal anesthesia should include frequent (every minute initially) monitoring of blood pressure, in addition to electrocardiogram (ECG), oxygen saturation, and fetal monitoring in the case of a pregnant patient. Consideration should be given to invasive blood pressure monitoring if the patient has significant cardiac comorbidities. Fluid therapy should be used in a dehydrated patient to restore volume prior to commencing spinal anesthesia.
Nonpharmacological methods to treat hypotension include positioning, leg compression, and uterine displacement. Trendelenburg positioning can increase venous return to the heart.
This position should not exceed 20° because extreme Trendelenburg can lead to a decrease in cerebral perfusion and blood flow due to increases in jugular venous pressure. If the level of spinal anesthesia is not fixed, the Trendelenburg position can alter the level of spinal anesthesia and cause a high level of spinal anesthesia in patients receiving hyperbaric local anesthetic solutions.
This can be minimized by raising the upper part of the body with a pillow under the shoulders while keeping the lower part of the body elevated above heart level. A Cochrane review in pregnant women found lower limb compression to have some benefit, although different methods had varying efficacies. Aortocaval compression from a gravid uterus should be avoided. Full lateral positioning results in less hypotension than left lateral tilt, although this may not be practical. A wedge under the right hip, or a tilting table, can be used to achieve left lateral tilt. However, the optimal degree of tilt is unknown, and there may be considerable variability among different patients.
There have been conflicting opinions on appropriate fluid management during spinal anesthesia. Early studies suggested crystalloid “preloading” prior to spinal block was effective. More recent work showed minimal effect of preloading. Colloid preloading does seem to be effective, although this must be balanced against the risk of allergic reactions and increased costs. “Coloading” (rapid administration of fluid immediately after spinal anesthesia) with crystalloid is better than preloading at preventing hypotension.
Hypotension can be limited by lowering the dose of spinal local anesthetic. One review found 5–7 mg of bupivacaine to be sufficient for cesarean section. However, complete motor nerve block was rare, duration was limited, and an epidural catheter for early top-up doses was essential. A meta-analysis in 2011 found lower doses of bupivacaine to be associated with lower anesthetic efficacy but less hypotension and nausea.
Conflicting opinions exist regarding the vasopressor of choice for spinal-induced hypotension. Ephedrine and phenylephrine have been the two main contenders; however, others have been used. Ephedrine is a direct and indirect α- and β-receptor agonist. It was felt to be safer than phenylephrine because it limited vasoconstriction of the uteroplacental circulation in early animal studies. However, ephedrine has a slow onset of action, is subject to tachyphylaxis, and has limited efficacy in treating hypotension. Of more concern is the increased risk of fetal acidosis. Whether this translates to poorer clinical outcomes is uncertain.
Phenylephrine is an direct α1-receptor agonist. It was used successfully in the 1960s for spinal anesthesia in New York, but fell out of favor due to concerns about poor tissue perfusion. In particular, uteroplacental vasoconstriction was noted in (somewhat-flawed) pregnant animal models. Recent work has shown that fetal acidosis does not occur when usual doses are used. In addition, phenylephrine seems superior to ephedrine in reducing hypotension and nausea. Phenylephrine has been used as a bolus or as an infusion and has been used to treat hypotension prophylactically as well as reactively (Table 9).
Optimal dosing regimens are yet to be established. Ngan Kee effectively prevented hypotension in elective obstetric patients by using a combination of crystalloid coload with a prophylactic infusion of phenylephrine.
Phenylephrine is the current vasopressor of choice for spinal hypotension, at least in the elective obstetric setting. There are, however, drawbacks. First, phenylephrine results in decreased CO, although the significance of this is uncertain. Second, intravenous phenylephrine has been shown to decrease spinal nerve block height in pregnant and nonpregnant patients. Third, Cooper referred to two case reports of hypertensive crisis involving phenylephrine and atropine, resulting in significant morbidity. It is suggested that hypertension induced by vasopressors is limited by a reflex decrease in HR. Atropine, in this setting, can therefore result in hypertensive crisis. Finally, the usual presentation of phenylephrine is highly concentrated (10 mg/mL) and needs to be diluted in a 100-mL bag of saline (100 μg/mL). Anesthesiologists more familiar with ephedrine may find this tiresome or, worse still, may commit a drug concentration error. Moreover, as a usual case requires much less than a 100-mL bag of phenylephrine, there is a risk of cross contamination if bags are reused. Cardiovascular collapse can occur after spinal anesthesia, although it is a rare event. Auroy and coworkers reported 9 cardiac arrests in 35,439 spinal anesthetics performed. Bradycardia usually precedes cardiac arrest, and early, aggressive treatment of bradycardia is warranted. Treatment of bradycardia includes intravenous atropine, ephedrine, and epinephrine. In cases of cardiac arrest after spinal anesthesia, epinephrine should be used early, and the Advanced Cardiac Life Support (ACLS) protocol should be initiated. Further work on spinal-induced hypotension is required.
Although treatment is usually aimed at systolic blood pressure, mean blood pressure may be a better target.
Different receptors may also be targeted. For example, prophylactic intravenous ondansetron has been shown to reduce hypotension, perhaps by modulating the BJR. Different patient subpopulations may require different therapies. Most evidence pertains to the elective, healthy obstetric setting, and the extent to which this can be extrapolated to other groups remains to be seen. Last, despite published evidence of the benefits of phenylephrine over ephedrine for elective cesarean section, there is reluctance to change practice. Psychological and institutional barriers to change need to be addressed.
Respiratory Effects of Spinal Anesthesia
In patients with normal lung physiology, spinal anesthesia has little effect on pulmonary function. Lung volumes, resting minute ventilation, dead space, arterial blood gas tensions, and shunt fraction show minimal change after spinal anesthesia. The main respiratory effect of spinal anesthesia occurs during high spinal block when active exhalation is affected due to paralysis of abdominal and intercostal muscles. During high spinal block, expiratory reserve volume, peak expiratory flow, and maximum minute ventilation are reduced. Patients with obstructive pulmonary disease who rely on accessory muscle use for adequate ventilation should be monitored carefully after spinal block. Patients with normal pulmonary function and a high spinal nerve block may complain of dyspnea, but if they are able to speak clearly in a normal voice, ventilation is usually adequate. The dyspnea is usually due to the inability to feel the chest wall move during respiration, and simple assurance is usually effective in allaying the patient’s distress.
NYSORA Tips
• Arterial blood gas measurements do not change during high spinal anesthesia in patients who are spontaneously breathing room air.
• Because a high spinal usually does not affect the cervical area, sparing of the phrenic nerve and normal diaphragmatic function occurs, and inspiration is minimally affected.
Arterial blood gas measurements do not change during high spinal anesthesia in patients who are spontaneously breathing room air. The main effect of high spinal anesthesia is on expiration, as the muscles of exhalation are impaired. Because a high spinal usually does not affect the cervical area, sparing of the phrenic nerve and normal diaphragmatic function occurs, and inspiration is minimally affected. Although Steinbrook and colleagues found that spinal anesthesia was not associated with significant changes in vital capacity, maximal inspiratory pressure, or resting end-tidal PCO2, increased ventilatory responsiveness to CO2 with bupivacaine spinal anesthesia was seen. Refer to Regional Anesthesia and Systemic Disease.
Gastrointestinal Effects of Spinal Anesthesia
The sympathetic innervation to the abdominal organs arises from T6 to L2. Due to sympathetic block and unopposed parasympathetic activity after spinal block, secretions increase, sphincters relax, and the bowel becomes constricted.
Increased vagal activity after sympathetic nerve block causes increased peristalsis of the gastrointestinal tract, which can lead to nausea. Nausea may also result from hypotension-induced gut ischemia, which produces serotonin and other emetogenic substances. The incidence of IONV in nonobstetric surgery can be up to 42% and may be as high as 80% in parturients.
Hepatic and Renal Effects of Spinal Anesthesia
Hepatic blood flow correlates to arterial blood flow. There is no autoregulation of hepatic blood flow; thus, as arterial blood flow decreases after spinal anesthesia, so does hepatic blood flow. If the mean arterial pressure (MAP) after placing a spinal anesthetic is maintained, hepatic blood flow will also be maintained. Patients with hepatic disease must be carefully monitored, and their blood pressure must be controlled during anesthesia to maintain hepatic perfusion. No studies have conclusively shown the superiority of regional or general anesthesia in patients with liver disease. In patients with liver disease, either regional or general anesthesia can be given, as long as the MAP is kept close to baseline.
NYSORA Tips
• If mean blood pressure is maintained after placing a spinal anesthetic, neither hepatic nor renal blood flow will decrease.
• Spinal anesthesia does not alter autoregulation of renal blood flow.
Renal blood flow is autoregulated. The kidneys remain perfused when the MAP remains above 50 mm Hg. Transient decreases in renal blood flow may occur when MAP is less than 50 mm Hg, but even after long decreases in MAP, renal function returns to normal when blood pressure returns to normal.
Again, attention to blood pressure is important after placing a spinal anesthetic, and the MAP should be as close to baseline as possible. Spinal anesthesia does not affect autoregulation of renal blood flow. It has been shown in sheep that renal perfusion changed little after spinal anesthesia.
FACTORS AFFECTING LEVEL OF SPINAL block
Many factors have been suggested as possible determinants of spinal block level. The four main categories of factors are (1) characteristics of the local anesthetic solution, (2) patient characteristics, (3) technique of spinal block, and (4) diffusion. Characteristics of local anesthetic solution include baricity, dose, concentration, and volume injected. Patient characteristics include age, weight, height, gender, intra-abdominal pressure, anatomy of the spinal column, spinal fluid characteristics, and patient position. Techniques of spinal block include site of injection, speed of injection, direction of needle bevel, force of injection, and addition of vasoconstrictors. Although all these factors have been postulated as affecting spinal spread of anesthetic, not many have been shown to change the distribution of block when all other factors that affect block are kept constant.
Site of Injection
The site of injection of local anesthetics for spinal anesthesia can determine the level of block. In some studies, isobaric spinal 0.5% bupivacaine produces sensory block that is reduced by two dermatomes per interspace when injection at L2–L3, L3–L4, and L4–L5 interspaces are compared. However, no difference in nerve block height exists when hyperbaric bupivacaine or dibucaine is injected as a spinal anesthetic in different interspaces.
Age
Some studies have reported changes in nerve block height after spinal anesthesia in the elderly patient as compared with the young patient, but other studies have reported no difference in nerve block height. These studies were performed with both isobaric and hyperbaric 0.5% bupivacaine.
NYSORA Tips
Baricity plays a major role in determining nerve block height after spinal anesthesia in older populations.
Isobaric bupivacaine appears to increase nerve block height, and hyperbaric bupivacaine does not appear to change nerve block height with increasing age. If there is a correlation between increasing age and spinal anesthesia height, it is not strong enough by itself to be a reliable predictor in the clinical setting. Just as with site of injection, it appears that baricity plays a major role in determining nerve block height after spinal anesthesia in older populations, and age is not an independent factor.
Position
Positioning of the patient is important for determining level of block after hyperbaric and hypobaric spinal anesthesia, but not for isobaric solutions. Sitting, Trendelenburg, and prone jackknife positions can greatly change the spread of the local anesthetic due to effect of gravity.
NYSORA Tips
Positioning of the patient is important for determining level of block after hyperbaric and hypobaric spinal anesthesia, but not for isobaric solutions.
The combination of baricity of the local anesthetic solution and patient positioning determines spinal nerve block height. The sitting position in combination with a hyperbaric solution can produce analgesia in the perineum. Trendelenburg positioning will also affect spread of hyperbaric and hypobaric local anesthetics due to the effect of gravity. Prone jackknife positioning is used for rectal, perineal, and lumbar procedures with a hypobaric local anesthetic. This prevents rostral spread of the spinal block after injection.
Flexion of the supine patient’s hips and knees flattens lumbar lordosis and decreases sacral pooling of local anesthetic.
Combined with Trendelenburg positioning, this may help cephalad spread. This position may inadvertently be attained when a urinary catheter is placed after spinal insertion.
Speed of Injectio
Speed of injection has been reported to affect spinal nerve block height, but the data available in the literature are conflicting. In studies using isobaric bupivacaine, there is no difference in spinal nerve block height with different speeds of injection. Even though spinal nerve block height does not change with speed of injection, a smooth, slow injection should be used when giving a spinal anesthetic. If a forceful injection is given and the syringe is not connected tightly to the spinal needle, the needle might disconnect from the syringe with loss of local anesthetic.
NYSORA Tips
Even though spinal nerve block height does not change with speed of injection, use a smooth, slow injection when giving a spinal anesthetic.
Volume, Concentration, and Dose of Local Anesthetic
It is difficult to maintain volume, concentration, or dose of local anesthetic constant without changing any of the other variables; thus, it is difficult to produce high-quality studies that investigate these variables singly. Axelsson and associates showed that volume of local anesthetic can affect spinal nerve block height and duration when equivalent doses are used.
Peng and coworkers showed that concentration of local anesthetic is directly related to dose when determining effective anesthesia. However, dose of local anesthetic plays the greatest role in determining spinal nerve block duration, as neither volume nor concentration of isobaric bupivacaine or tetracaine alter spinal nerve block duration when the dose is held constant. Studies have repeatedly shown that spinal nerve block duration is longer when higher doses of local anesthetic are given. When performing a spinal anesthetic, be cognizant of not only the dose of local anesthetic but also the volume and concentration so the patient is not overdosed or underdosed.
NYSORA Tips
When performing a spinal anesthetic, be cognizant of not only the dose of local anesthetic but also the volume and concentration so the patient is not overdosed or underdosed.
The use of hyperbaric solutions minimizes the importance of dose and volume except when doses of hyperbaric bupivacaine equal to or less than 10 mg are used. In those cases, there is less cephalad spread and a shorter duration of action. A dose of hyperbaric bupivacaine between 10 and 20 mg results in similar nerve block height. When using hyperbaric solutions, it is important to note that patient positioning and baricity are the most influential factors on nerve block height, except when low doses of hyperbaric bupivacaine are used.
EQUIPMENT FOR SPINAL ANESTHESIA
Maintaining Asepsis
No single intervention guarantees asepsis. Therefore, a multiprong approach is advisable.
In the past, most institutions had reusable trays for spinal anesthesia. These trays required preparation by anesthesiologists or anesthesia personnel to ensure that bacterial and chemical contamination would not occur. Currently, commercially prepared, disposable spinal trays are available and are in use by most institutions. These trays are portable, sterile, and easy to use. Figure 9 shows the contents of a standard, commercially prepared spinal anesthetic tray.
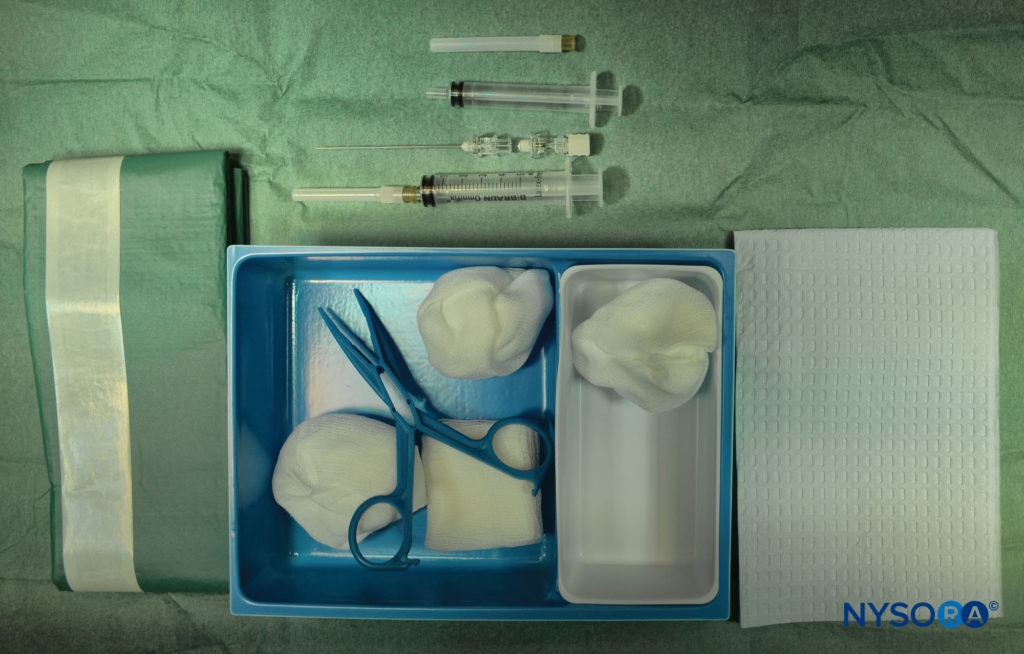
FIGURE 9. Contents of a standard, commercially prepared spinal anesthetic tray.
The ideal skin preparation solution should be bactericidal and have a quick onset and long duration. Chlorhexidine is superior to povidone iodine in all these respects. In addition, the ideal agent should not be neurotoxic. Unfortunately, bactericidal agents are neurotoxic. It is therefore prudent to use the lowest effective concentration and allow the preparation to dry. Although subject to debate, 0.5% chlorhexidine in alcohol 70% is currently recommended by some groups. Contamination of equipment with skin preparation can theoretically lead to the introduction of neurotoxic substances into neural tissue. Of more concern is accidental neuraxial injection of antiseptic solution, possibly from antiseptic solution and local anesthetic being placed in adjacent pots. Therefore, after skin preparation, unused antiseptic should be discarded before commencement of the procedure (and intrathecal drugs should be drawn directly from sterile ampules). Tinted antiseptic solutions may decrease the likelihood of drug error and allow easy identification of missed skin during application.
Proving a benefit of individual infection control measures is difficult due to the rarity of infectious complications. Past evidence has been contradictory. For example, it has been suggested that shedding of skin scales from mask “wiggling” may occur, increasing bacterial contamination. Yet, in 1995 there were calls for routine face mask use after it was unambiguously proven, using polymerase chain reaction (PCR) fingerprinting, that a case of Streptococcus salivarius meningitis originated in the throat of the doctor who had performed a lumbar puncture.
It is our strong belief that face mask wearing should be mandatory when performing spinal anesthesia. A 2006 American Society of Regional Anesthesia and Pain Medicine (ASRA) practice advisory recommended mask wearing in addition to removing jewelry, thorough hand washing, and sterile surgical gloves for all regional anesthesia techniques.
Major components of an aseptic technique also included a surgical hat and sterile draping. Other international professional bodies have similar guidelines.
Prophylactic antibiotics are unnecessary for spinal anesthesia. If, as it happens, antibiotic prophylaxis is required for the prevention of surgical site infection, it may be prudent to administer antibiotics before insertion of a spinal needle.
The reader is referred to Infection Control in Regional Anesthesia for more information.
Resuscitation and Monitoring
Resuscitation equipment must be available whenever a spinal anesthetic is performed. This includes equipment and medication required to secure an airway, provide ventilation, and support cardiac function. All patients receiving spinal anesthesia should have an intravenous line.
The patient must be monitored during the placement of the spinal anesthetic with a pulse oximeter, blood pressure cuff, and ECG. Fetal monitoring should be used in the case of a pregnant patient. Noninvasive blood pressure should be measured at 1-minute intervals initially, as hypotension may be sudden.
Shivering and body habitus may make noninvasive blood pressure measurement difficult. Consideration should be given to invasive blood pressure monitoring if the patient has significant cardiovascular disease.
Needles
Needles of different diameters and shapes have been developed for spinal anesthesia. The ones currently used have a close-fitting, removable stylet, which prevents skin and adipose tissue from plugging the needle and possibly entering the subarachnoid space. Figure 10 shows the different types of needles used along with the type of point at the end of the needle.
The pencil-point needles (Sprotte and Whitacre) have a rounded, noncutting bevel with a solid tip. The opening is located on the side of the needle 2–4 mm proximal to the tip of the needle. The needles with cutting bevels include the Quincke and Pitkin needles. The Quincke needle has a sharp point with a medium-length cutting needle, and the Pitkin has a sharp point and short bevel with cutting edges. Finally, the Greene spinal needle has a rounded point and rounded noncutting bevel. If a continuous spinal catheter is to be placed, a Tuohy needle can be used to find the subarachnoid space before placement of the catheter.
Pencil-point needles provide a better tactile sensation of the layers of ligament encountered but require more force to insert than bevel-tip needles. The bevel of the needle should be directed longitudinally to decrease the incidence of PDPH.
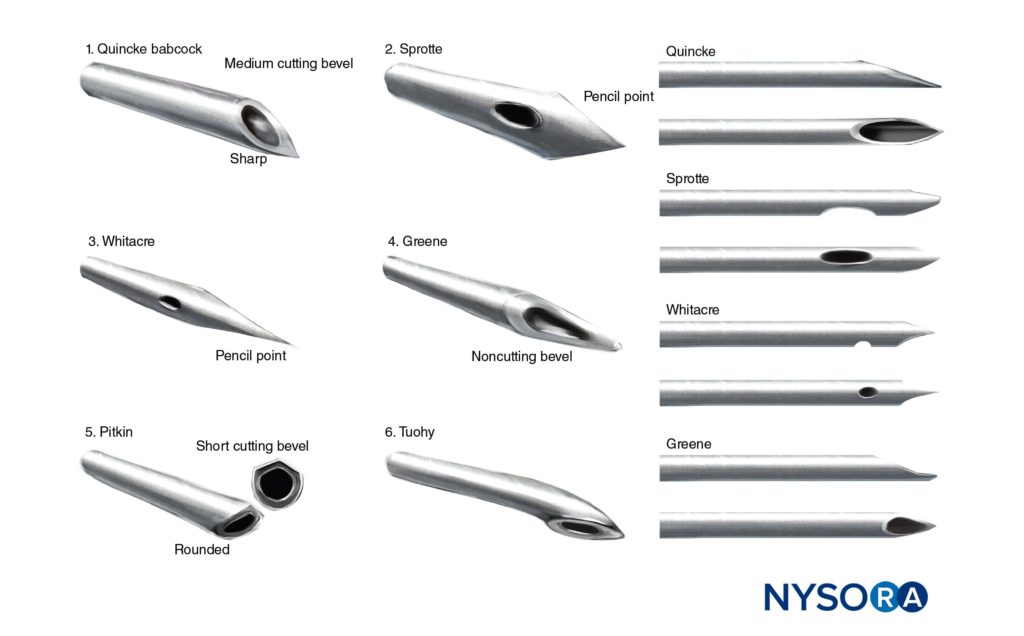
FIGURE 10. Different types of needles.
Small-gauge needles and needles with rounded, noncutting bevels also decrease the incidence of PDPH but are more easily deflected than larger-gauge needles. The reader is referred to Ultrastructural Anatomy of the Spinal Meninges and Related Structures and Postdural Puncture Headache.
NYSORA Tips
• Pencil-point needles provide a better tactile sensation of the layers of ligament encountered but require more force to insert than bevel-tip needles.
• The use of introducers help preventing the passage of epidermic contaminants to the CSF.
Introducers have been designed to assist with the placement of spinal needles into the subarachnoid space due to the difficulty in directing needles of small bore through the tissues. Introducers also serve to prevent contamination of the CSF with small pieces of epidermis, which could lead to the formation of dermoid spinal cord tumors. The introducer is placed into the interspinous ligament in the intended direction of the spinal needle, and the spinal needle is then placed through the introducer.
POSITION OF THE PATIENT
Proper positioning of the patient for spinal anesthesia is essential for a fast, successful nerve block. It has been shown to be an independent predictor for successful first attempt at neuraxial nerve block.316 Many factors come into play for positioning of the patient. Before beginning the procedure, both the patient and the anesthesiologist should be comfortable. This includes positioning the height of the operating room table, providing adequate blankets or covers for the patient, ensuring a comfortable room temperature, and providing sedation for the patient if required. Personnel trained in positioning patients are invaluable, and commercial positioning devices may be useful.
From the Compendium of Regional Anesthesia: Positioning for spinal anesthesia of a patient with a large body mass index.
When providing sedation, it is important to avoid oversedation. The patient should be able to cooperate before, during, and after administration of the spinal anesthetic. There are three main positions for administering a spinal anesthetic: the lateral decubitus, sitting, and prone positions.
Lateral Decubitus Position
A commonly used position for placing a spinal anesthetic is the lateral decubitus position. Ideal positioning consists of having the back of the patient parallel to the edge of the bed closest to the anesthesiologist, with the patient’s knees flexed to the abdomen and neck flexed. Figure 11 shows a patient in the lateral decubitus position.
It is beneficial to have an assistant to help hold and encourage the patient to stay in this position. Depending on the operative site and operative position, a hypo-, iso-, or hyperbaric solution of local anesthetic can be injected.
FIGURE 11. Patient in lateral decubitus position.
NYSORA Tips
• A commonly used position for placing a spinal anesthetic is the lateral decubitus position.
• Ideal positioning consists of having the back of the patient parallel to the edge of the bed closest to the anesthesiologist, knees flexed to the abdomen, and neck flexed.
Sitting Position and “Saddle nerve Block”
Strictly speaking, the sitting position is best utilized for low lumbar or sacral anesthesia and in instances when the patient is obese and there is difficulty in finding the midline. In practice, however, many anesthesiologists prefer the sitting position in all patients who can be positioned this way. The sitting position avoids the potential rotation of the spine that can occur with the lateral decubitus position. Using a stool for a footrest and a pillow for the patient to hold can be valuable in this position. The patient should flex the neck and push out the lower back to open up the lumbar intervertebral spaces. Figure 12 depicts a patient in the sitting position, and the L4–L5 interspace is marked.
When performing a “saddle nerve block,” the patient should remain in the sitting position for at least 5 minutes after a hyperbaric spinal anesthetic is placed to allow the spinal anesthetic to settle into that region. If a higher level of block is necessary, the patient should be placed supine immediately after spinal placement and the table adjusted accordingly.
Figure 12.Patient in sitting position with the L4–L5 interspace marked
NYSORA Tips
• The sitting position is utilized for low lumbar or sacral anesthesia and in instances when the patient is obese and there is difficulty in finding the midline in the lateral position.
• When performing a saddle nerve block, the patient should remain in the sitting position for at least 5 min after a hyperbaric spinal anesthetic is placed to allow the spinal to settle into that region.
Prone Position
The prone position can be utilized for induction of spinal anesthesia if the patient needs to be in this position for the surgery, such as for rectal, perineal, or lumbar procedures. A hypobaric or isobaric solution of local anesthetic is preferred in the prone jackknife position for these procedures. This avoids rostral spread of the local anesthetic and decreases the risk of high spinal anesthesia.
NYSORA Tips
The prone position is utilized for spinal anesthesia if the patient needs to be in this position for the surgery, such as for rectal, perineal, or lumbar procedures.
Another, less-elegant solution is to inject a hyperbaric solution of local anesthetic with the patient in the sitting position and wait until the spinal anesthesia “sets in,” which is typically 15–20 minutes after injection. The patient is then positioned in the prone position with vigilant monitoring, including frequent verbal communication with the patient.
TECHNIQUE OF LUMBAR PUNCTURE
When performing a spinal anesthetic, appropriate monitors should be placed, and airway and resuscitation equipment should be readily available. All equipment for the spinal block should be ready for use, and all necessary medications should be drawn up prior to positioning the patient for spinal anesthesia. Adequate preparation for the spinal reduces the amount of time needed to perform the nerve block and assists with making the patient comfortable.
Proper positioning is the key to making the spinal anesthetic quick and successful. Once the patient is correctly positioned, the midline should be palpated. The iliac crests are palpated, and a line is drawn between them to find the body of L4 or the L4–L5 interspace. Other interspaces can be identified, depending on where the needle is to be inserted.
The skin should be cleaned with skin preparation solution such as 0.5% chlorhexidine, and the area should be draped in a sterile fashion. The skin preparation solution should be allowed to dry, and unused skin preparation solution must be removed from the anesthesiologist’s workspace. A small wheal of local anesthetic is injected into the skin at the planned site of insertion.
More local anesthetic is then administered along the intended path of the spinal needle insertion to the estimated depth of the supraspinous ligament. This serves a dual purpose: additional anesthesia for the spinal needle insertion and identification of the correct path for spinal needle placement. Care must be taken in thin patients to avoid dural puncture, and inadvertent spinal anesthesia, at this stage.
NYSORA Tips
• When performing a spinal anesthetic, appropriate monitors should be placed, and airway and resuscitation equipment should be readily available.
• All equipment for the spinal block should be ready for use, and all necessary medications should be drawn up prior to positioning the patient for spinal anesthesia.
Midline Approach
If the midline approach is used, palpate the desired interspace and inject local anesthetic into the skin and subcutaneous tissue. The introducer needle is placed with a slight cephalad angle of 10° to 15°. Next, the spinal needle is passed through the introducer. The needle passes through the subcutaneous tissue, supraspinous ligament, interspinous ligament, ligamentum flavum, epidural space, dura mater, and subarachnoid mater to reach the subarachnoid space.
From the Compendium of Regional Anesthesia: Spinal anesthesia injection using the midline approach.
Resistance changes as the spinal needle passes through each level on the way to the subarachnoid space. Subcutaneous tissue offers less resistance to the spinal needle than ligaments. When the spinal needle goes though the dura mater, a “pop” is often appreciated. Once this pop is felt, the stylet should be removed from the needle to check for flow of CSF. For spinal needles of higher gauge (26–29 gauge), this usually takes 5–10 seconds, but in some patients, it can take a minute or longer. If there is no flow, some suggest rotating the needle 90° as the needle orifice might be obstructed. Debris can obstruct the orifice of the spinal needle. If necessary, withdraw the needle and clear the orifice before attempting the spinal anesthetic again. A common cause of failure to obtain CSF flow is the spinal needle being off the midline. The midline should be reassessed and the needle repositioned.
If the spinal needle contacts bone, the depth of the needle should be noted and the needle placed more cephalad. If bone is contacted again, the needle depth should be compared with that of the last bone contact to determine what structure is being contacted. For instance, if bone contact is deeper than the first insertion, the needle should be redirected more cephalad to avoid the inferior spinous process. If bone contact is at roughly the same depth as the original insertion, it may be lamina being contacted, and the midline should be reassessed. If bone contact is shallower than the original insertion, the needle should be redirected caudally to avoid the superior spinous process.
NYSORA Tips
• When the spinal needle goes though the dura mater, a “pop” is often appreciated.
• Once this pop is felt, the stylet should be removed from the introducer to check for flow of CSF.
• For spinal needles of a higher gauge (26–29 gauge), this usually takes 5–10 seconds, but in some patients, it can take longer.
• If there is no flow, the needle might be obstructed by a nerve root and rotating it 90° may be helpful.
When the spinal needle needs to be reinserted, it is important to withdraw the needle back to the skin level before redirection. Only make small changes in the angle of direction when reinserting the spinal needle as small changes at the surface lead to large changes in direction when the needle reaches greater depths. Bowing and curving of the spinal needle when inserting through the skin or introducer can also steer the needle off course when attempting to contact the subarachnoid space.
Paresthesias may be elicited when passing a spinal needle. The stylet should be removed from the spinal needle, and if CSF is seen and the paresthesia is no longer present, it is safe to inject the local anesthetic. A cauda equina nerve root may have been encountered. If there is no CSF flow, it is possible that the spinal needle has contacted a spinal nerve root traversing the epidural space. The needle should be removed and redirected toward the side opposite the paresthesia.
After free flow of CSF is established, inject the local anesthetic slowly at a speed of less than 0.5 mL/s. Additional aspiration of CSF at the midpoint and end of injection can be attempted to confirm continued subarachnoid administration but may not always be possible when small needles are used. Once local anesthetic injection is complete, the introducer and spinal needle are removed as one unit from the back of the patient. The patient should then be positioned according to the surgical procedure and baricity of local anesthetic given. The table can be tilted in either the Trendelenburg or the reverse Trendelenburg position as needed to adjust the height of the nerve block after testing the sensory level. The anesthesiologist should carefully monitor and support vital signs.
From the Compendium of Regional Anesthesia: Spinal anesthesia injection showing the local anesthetic spread in the intrathecal space.
Paramedian (Lateral) Approach
If the patient has a calcified interspinous ligament or difficulty in flexing the spine, a paramedian approach to achieve spinal anesthesia can be utilized. The patient can be in any position for this approach: sitting, lateral, or even prone jackknife. After palpating the superior and inferior lumbar spinous processes of the desired interspace, local anesthetic is infiltrated 1 cm lateral to the superior aspect of the inferior spinous process. The needle should be directed slightly medially. A 10° and 15° medial angulation of the needle will reach the midline at a depth of about 5.7 cm (tan 80°) and 3.7 cm (tan 75°), respectively. This demonstrates that small changes in angulation can have pronounced effects on needle-tip placement. Although slight cephalad angulation is also required, a common error is too steep an initial approach. If lamina is contacted, the needle should then be angled cephalad and “walked off” the lamina into the subarachnoid space.
Other methods have been described. All techniques involve a similar vertical axis for the puncture site (1–1.5 cm from the midline). They differ in the horizontal axis (eg, 1 cm lateral to the spinous process, 1 cm lateral to the interspace, 1 cm lateral and 1 cm inferior to the interspace, 1 cm lateral and 1 cm inferior to the inferior aspect of the superior spinous process) and the degree of cephalad angulation required.
Figure Figure 13 shows the landmarks used for a paramedian approach to spinal anesthesia. Figure 14 depicts successful performance of a paramedian spinal anesthetic.

FIGURE 13. Landmarks used in a paramedian approach to spinal anesthesia.
FIGURE 14. Paramedian approach: needle placement.
NYSORA Tips
For the paramedian approach:
• After palpating the superior and inferior lumbar spinous processes of the desired interspace, local anesthetic is infiltrated 1 cm lateral to the superior aspect of the inferior spinous process.
• The needle should be angled in a slight medial and cephalad direction.
• If lamina is contacted, the needle should then be angled cephalad and “walked off ” the lamina into the subarachnoid space.
• The ligamentum flavum is usually the first resistance identified.
Taylor Approach
The Taylor, or lumbosacral, approach to spinal anesthesia is a paramedian approach directed toward the L5–S1 interspace. Because this is the largest interspace, the Taylor approach can be used when other approaches are not successful or cannot be performed. As with the paramedian approach, the patient can be in any position for this approach: sitting, lateral, or prone.
The needle should be inserted at a point 1 cm medial and inferior to the posterior superior iliac spine, then angled cephalad 45°–55° and medially. This angle should be medial enough to reach the midline at the L5–S1 interspace. After needle insertion,
the first significant resistance felt is the ligamentum flavum, and then the dura mater is punctured to allow free flow of CSF as the subarachnoid space is entered. Figure 15 shows the Taylor approach to spinal anesthesia. Real-time ultrasound-guided prone spinal anesthesia via the Taylor approach has been described and may improve patient comfort and compliance during the procedure.
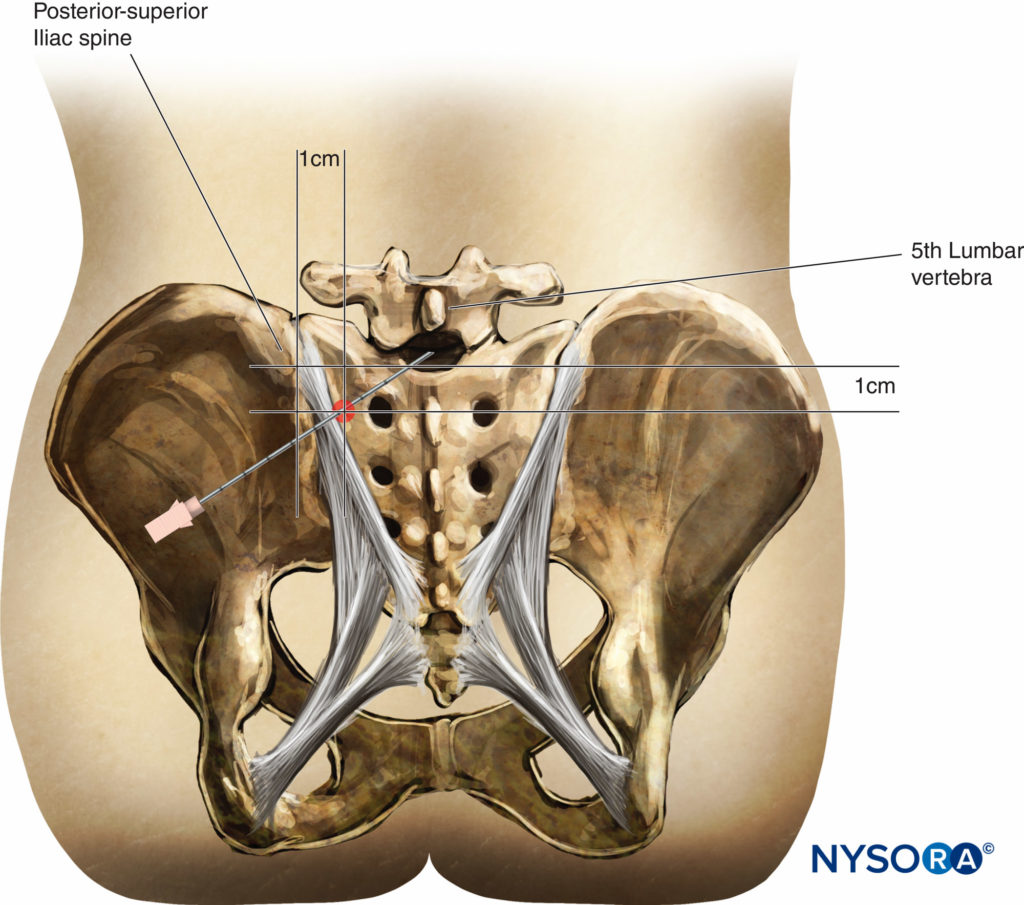
FIGURE 15. Taylor approach to spinal anesthesia.
NYSORA Tips
For the Taylor approach:
• The needle should be inserted at a point 1 cm medial and inferior to the posterior superior iliac spine, then angled cephalad 45°–55° and medially.
• This angle should be medial enough to reach the midline at the L5–S1 interspace.
• After needle insertion, the first significant resistance felt is the ligamentum flavum.
CONTINUOUS CATHETER TECHNIQUES
An indwelling catheter can be placed for continuous spinal anesthesia. Local anesthetics can be dosed repeatedly through the catheter and the level and duration of anesthesia adjusted as necessary for the surgical procedure. Placement of a continuous spinal catheter occurs in a similar fashion as a regular spinal anesthetic except that a larger-gauge needle, such as a Tuohy, is used to enable the passage of the catheter. After insertion of the Tuohy needle, the subarachnoid space is found, and the spinal catheter is passed 2–3 cm into the subarachnoid space. If there is difficulty in passing the catheter, attempt to rotate the Tuohy needle 180°. Never withdraw the catheter back into the needle shaft because there is a risk of shearing the catheter and leaving a piece of it in the subarachnoid space. If the catheter needs to be withdrawn, withdraw the catheter and needle together and attempt the continuous spinal at another interspace. Communication is critical to avoid a spinal catheter being mistaken for the more common epidural catheter. This involves labeling, documentation, handover, and vigilance.
NYSORA Tips
• After insertion of the Tuohy needle, the subarachnoid space is entered and the spinal catheter is passed 2–3 cm into the subarachnoid space.
• If there is difficulty in passing the catheter, attempt to rotate the Tuohy needle 180°.
• Communication is critical to avoid a spinal catheter being mistaken for the more common epidural catheter.
Because the needle used to pass the spinal catheter is a large-bore needle, there is a much higher risk of PDPH, especially in young female patients. Cauda equina syndrome can occur with small spinal catheters, so the FDA has advised against using catheters smaller than 24 gauge for continuous spinal anesthetics.
In 2008, a randomized clinical trial (FDA Investigational Device Exemption) reported on the safety of continuous spinal “microcatheters” in obstetric patients. A 28-gauge catheter was placed in 329 patients; there were no reported permanent neurological outcomes. The trial compared continuous spinal analgesia with epidural analgesia and found lower initial pain scores, higher patient satisfaction, and less motor nerve block in the spinal group, with no difference in neonatal or obstetric outcomes. However, the spinal group had higher pruritis scores and a trend toward more PDPH (9% compared with 4% in the epidural group). Intrathecal catheters were more difficult to remove than epidural catheters. One patient had an intrathecal catheter broken on removal, albeit by an untrained individual, leaving a fragment in the patient’s back.
CLINICAL SITUATIONS ENCOUNTERED IN THE PRACTICE OF SPINAL ANESTHESIA
The Difficult and Failed Spinal
Spinal anesthesia has long been considered a reliable nerve block, with failure rates less than 1%. Conversion to general anesthesia was as low as 0.5% in a prospective cohort study of obstetric patients. However, failure rates as high as 17% have been reported. Failed spinal anesthesia may present as complete absence of nerve block, partial nerve block, or inadequate duration of nerve block.
Although expertise may reduce the chance of a failed spinal, even experienced clinicians will be confronted with failed spinal nerve blocks. After being reassured by the appearance of CSF, a subsequent failed or patchy nerve block can leave an anesthesiologist frustrated and bewildered. A methodical approach is required when managing failed spinal block.
In an excellent review article, Fettes et al classified failure of spinal anesthesia into five groups: failure of lumbar puncture, failure of solution injection, solution spread in the CSF, drug action on the nerve roots and cord, and patient management. Their review is summarized next.
Failed Lumbar Puncture
Whenever there are problems with placing a spinal anesthetic, the anesthesiologist should reassess the position of the patient. A member of the operating room personnel who is trained to assist with patient positioning should be used. Alternatively, positioning of the patient can be enhanced with commercially available positioning devices. These devices can help maintain spinal flexion and create a stable support for the patient, which can be useful if no trained operating room personnel are available to assist with positioning.
If the proposed interspace cannot be found, the interspace above or below the original site of spinal injection can be attempted. When the sitting position cannot be used or is unsuccessful, the lateral decubitus position can be used. Either the midline or the lateral paramedian technique can be attempted. The largest interlaminar space is at L5, and this can be sought via Taylor’s approach, described previously in this chapter.
Three independent predictors of success when performing neuraxial nerve block have been identified: adequate positioning, the anesthesiologist’s experience, and the ability to palpate anatomical landmarks. Improper positioning may be due to patients’ inability to flex the spine rather than anesthesiologists’ failure to encourage flexion. A predictably difficult back should not be used to teach inexperienced trainees. If anatomical landmarks are imperceptible spinal ultrasonography can be used to assist lumbar puncture (see section on neuraxial ultrasound).
Failure of Solution Injection
Because of the small volumes of injectate used in spinal anesthesia, apparently trivial reductions in the volume of solution may result in a less-than-adequate nerve block. Reductions in solution injected may be the result of loss of injectate when the spinal syringe is attached to the needle hub or loss into tissues adjacent to the subarachnoid space due to needle orifice migration or the orifice straddling a number of potential spaces (eg, the subarachnoid and subdural or epidural spaces). Intentional reductions in dose, usually to decrease side effects, may also result in decreased efficacy.
Failure of Solution Spread Within the CSF
Failure of solution spread within the CSF may be due to spinal deformities such as kyphosis or scoliosis, previous surgery, transverse or longitudinal spinal septae, spinal stenosis, or extradural cysts. Tarlov cysts are a type of extradural cyst seen incidentally on MRI scans and have an incidence as high as 9%.
Although usually asymptomatic, they contain CSF and may account for positive aspiration of CSF yet failure of complete nerve block. Lumbar CSF volume is an important determinant of spread.
Failure of Drug Action
Failure of drug action may result from the incorrect drug being administered. The correct drug may be inactive as the result of physicochemical instability (less likely with modern agents) or may be impaired due to chemical incompatibilities when two or more agents are used. The phenomenon of local anesthetic resistance has been questioned in the literature.
Failure of Patient Management
Descartes’s classic 17th-century picture of pain showing a connection between a boy’s burning foot and his brain via the middle of his back—“just as when you pull one end of a string, you cause a bell hanging at the other end to ring”—could lead one to believe spinal anesthesia can cure all pain. However, pain perception is far more complex, and despite perfect spinal block, a patient may experience discomfort or pain. Patients should be counseled preoperatively about expected “normal” sensations such as pulling, pushing, and stretching. Preoperative testing of spinal block to reassure both the patient and the anesthesiologist may paradoxically distress the patient if performed too early. Intraoperatively, a patient may require supplemental anxiolysis and analgesia or general anesthesia.
Management of a failed spinal nerve block will depend on whether it occurs preoperatively or intraoperatively and the nature of the failure. Options to optimize spinal anesthesia include changing the patient’s position to improve spread and repeating t he spinal block. Two important principles must be remembered when repeating a spinal nerve block. First, the second attempt must not be identical to the first. This is not only to avoid a repeat failure but also perhaps to prevent a second dose of local anesthetic accumulating in a restricted space, which can lead to neurological injury. Second, a repeat dose may result in excessive spread of local anesthetic. Alternatives such as epidural anesthesia, peripheral nerve block, local infiltration, systemic analgesia, and general anesthesia should be considered based on the merits of the case and are beyond the scope of this chapter.
Inadvertent Subdural Nerve Block
Failed subarachnoid nerve block may be the result of inadvertent subdural injection and deserves special attention. The subdural space is a potential space that only becomes real after tearing of neurothelial cells within the space as a result of iatrogenic needle insertion and fluid injection (see Figure 4). Characteristic features of a SDB are a high sensory level with motor and sympathetic sparing. This may be the result of the limited ventral capacity of the space, which results in sparing of the anterior motor and sympathetic fibers. However, a SDB may also present in a number of different ways: failed nerve block, unilateral nerve block, Horner syndrome, trigeminal nerve palsy, respiratory insufficiency, or unconsciousness due to brainstem involvement. Onset of nerve block is slower than subarachnoid nerve block but faster than epidural nerve block and usually resolves after 2 hours.
The incidence of subdural injection after contrast myelography ranges between 1% and 13%. The incidence of SDB after attempted spinal anesthesia is unknown. Because the dura is intentionally breached during attempted spinal anesthesia, the incidence of SDB may be higher compared with epidural nerve block (variously quoted as between 0.024% and 0.82%). The size of the acquired subdural space is probably proportional to the volume of fluid injected. Therefore, typical volumes used with spinal anesthesia may not be as significant as volumes used with epidural anesthesia
Outpatient Spinal Anesthesia
Each year, the number of surgeries increase, and more are performed on an outpatient basis. As anesthesiologists, we are always looking for new ways to provide efficient anesthetic care that is safe, controls pain, allows the patient to be discharged home in a timely fashion as per postanesthesia care unit protocol, and is easily performed and reproducible. It has previously been suggested that spinal anaesthesia may be incorporated into the outpatient surgery model.
Unilateral Spinal Nerve Block
Use of a unilateral spinal nerve block for elderly patients and outpatient surgery has undergone a resurgence. Unilateral spinal anesthesia was described in 1950 by Ruben and Kamsler. Their report concerned 116 patients for surgical reduction of hip fracture performed under unilateral spinal block. No deaths were reported, and no increase in the hazard of operation was found. Recently, attention has returned to the use of unilateral spinal anesthesia in elderly patients326 and for outpatient surgery.
Use of unilateral spinal anesthesia results in decreased changes in systolic, mean and diastolic pressures, or oxygen saturation in elderly trauma patients (eg, hip fracture). Keeping the operative side up and using a hypobaric spinal solution in a low dose for these cases results in excellent anesthesia and remarkable hemostability when the patient is kept in the lateral position for 5–10 minutes before repositioning supine. When using hyperbaric solutions, the operative side should be dependent.
Outpatient surgery using hyperbaric 0.5% bupivacaine takes about 16 minutes for development of surgical anesthesia from time of injection with unilateral spinal anesthesia and 13 minutes with traditional bilateral spinal anesthesia. Less hemodynamic changes are found in the unilateral spinal anesthesia group, with quicker regression of the nerve block and equal time to discharge home.
Compared with other outpatient surgery, less motor nerve block is required for knee arthroscopy. Doses of hyperbaric bupivacaine as low as 4–5 mg are effective when combined with unilateral positioning. Higher doses delay recovery. Addition of intrathecal opioids improves analgesia but increases opioid-related side effects. Ropivacaine does not improve recovery time.
In performing unilateral spinal anesthesia, use of a pencil-point 25-gauge or 27-gauge needle with the orifice directed at the operative side is suggested. Low-dose bupivacaine should be used, with hyperbaric bupivacaine (operative side down) in outpatient surgery and hypobaric bupivacaine (operative side up) in the elderly trauma patient. A slow injection rate should be used to produce laminar flow that will assist in producing a unilateral block. There is little evidence that keeping a patient in the lateral position for more than 15 minutes is helpful.
The Obstetric Patient
In 1901, Kreis described the first spinal anesthetic for vaginal delivery. The following year, Hopkins performed the first successful spinal anesthetic for cesarean section in a woman with placenta previa. Spinal anesthesia for labor and delivery has progressed greatly since that time. Although many arguments are made against general anesthesia in the pregnant woman due to increased risk of aspiration and difficult intubation, the anesthesiologist must be prepared to induce general anesthesia in the face of a failed or total spinal anesthetic.
Obstetric regional anesthesia is a topic in itself, and as such is covered in Obstetric Regional Anesthesia. Examples of how spinal anesthesia differs in the obstetric population are listed in Table 10.
TABLE 10. Spinal anesthesia in the obstetric patient.
| Consent | • May be difficult to obtain truly informed consent in a laboring patient. |
| Risks | • Lower risk of major permanent complications compared with neuraxial block for nonobstetric surgery.26 • Higher risk of postdural puncture headache. • A 2005 meta-analysis showed cord pH, an indicator of fetal well-being, to be lower with spinal compared with epidural and general anesthesia, although this may be attributed to the use of ephedrine in the studies analyzed.118 |
| Benefits | • Avoidance of maternal risks of general anesthesia, in particular the three As: aspiration, awareness, and difficult airway. • Avoidance of fetal exposure to general anesthesia drugs. • Early maternal bonding with the newborn. • Partner or support person may be present. |
| Indications | • Spinal anesthesia may be used for labor analgesia, forceps delivery, cesarean delivery, manual removal of placenta, perineal repair, or nonobstetric surgery in the obstetric patient. |
| Anatomy | • Exaggerated lumbar lordosis during pregnancy can increase the height of the intercristal line such that 6% of term women have an intercristal line at or above L3.352 • The pronounced lumbar flexion required to perform spinal anesthesia may be difficult due to the gravid uterus. |
| Physiology | • Aortocaval compression from a gravid uterus may worsen spinal-induced hypotension, posing risks for both mother and fetus. |
| Pharmacology | • Pregnant women require less local anesthetic to achieve the same level of anesthesia as nonpregnant women. This observation is likely due to both hormonal and mechanical factors. |
| Technique | • Be prepared to convert to general anesthesia. Prior to placement of a spinal anesthetic, the pregnant patient should receive 30 mL of 0.3 M sodium citrate orally to decrease stomach acidity. Equipment and drugs necessary to administer general anesthesia should be readily available. • After the spinal anesthetic is given, the patient should be in the supine position with left uterine displacement. Fetal heart rate should be monitored by Doppler or fetal scalp electrocardiogram (ECG). • A T4-level block is usually required for a cesarean section due to traction on the peritoneum and uterine exteriorization. • Some patients complain of dyspnea due to abdominal and intercostal motor block, but if the patient is able to speak clearly, reassurance and monitoring is usually all that is required. Sensory loss in the upper limb or inability to extend the forearm (C7/C8) should warn the clinician regarding impending diaphragmatic paralysis (C3/C4/C5). • If the mother wants to nurse the newborn, an assessment of upper limb strength should be made. Adequate staffing should allow someone other than the anesthesiologist to be responsible for the well-being of the newborn. • Hypotension and nausea are common, especially in the elective setting (see section on management of hypotension after spinal anesthesia). Prophylactic phenylephrine and “coloading” with fluid effectively prevents hypotension and nausea. Table 23–9 provides a suggested regimen for managing hypotension during elective cesarean section. |
The Anticoagulated Patient
As the population ages, more patients are presenting for surgery with pre-, intra-, or postoperative requirements for antiplatelet, anticoagulant, or thrombolytic therapy. Novel agents continue to be developed, giving rise to concerns in patients undergoing spinal anesthesia. These concerns led to the evolution of the ASRA evidence-based guidelines on regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy, now up to its third edition
The reader is referred to Neuraxial Anesthesia and Peripheral Nerve Blocks in Patients on Anticoagulants for an in-depth discussion on the use of neuraxial anesthesia in the anticoagulated patient.
Other Clinical Situations
Spinal anesthesia in the pediatric patient and the patient with preexisting neurology are covered in Pediatric Epidural and Spinal Anesthesia & Analgesia and Regional Anesthesia in the Patient with Preexisting Neurologic Disease, respectively.
RECENT DEVELOPMENTS IN SPINAL ANESTHESIA
Neuraxial Ultrasound
Conventional palpation of surface anatomy has been shown to be unreliable. Neuraxial ultrasound aims to overcome the inaccuracies of surface anatomy with sonoanatomy. The first description of ultrasound-assisted lumbar puncture was in 1971. More recently, neuraxial ultrasound has been used as a preprocedure scan and for real-time needle placement. Much of the evidence regarding neuraxial ultrasound pertains to preprocedural scanning prior to epidural insertion, especially in the setting of obstetric anesthesia, and has been produced by a limited number of specialized centers. This evidence shows that scanning decreases needle attempts, accurately predicts depth to the epidural space, and may improve the success rate of junior trainees.
Spinal ultrasonography in the setting of single-shot spinal anesthesia is less well studied. Ultrasonography allows increased accuracy at identifying lumbar interspaces. This is important as palpation of the lumbar spine is likely to generate a higher interspace than expected, and the conus medullaris has been shown to be at times lower than the conventionally taught L1 level. These two facts not only pose a theoretical risk but also have resulted in persistent neurological injury. An observational study in orthopedic patients demonstrated accurate ultrasonographic prediction of the depth to the dura prior to spinal insertion. Preprocedural ultrasonography has been used to achieve spinal anesthesia in clinically difficult situations such as obesity, kyphoscoliosis, and previous spinal surgery, including Harrington rods. Real-time ultrasound-guided spinal anesthesia has been described in technically difficult patients and in the prone position via Taylor’s approach. A randomized trial comparing preprocedural scanning with standard palpation for spinal anesthesia in patients with difficult surface anatomical landmarks showed a twofold difference in first attempt success (62% ultrasound vs. 32% control).
Ultrasound scanning of the neuraxis is best learned in tailored workshops and simulations. Real-time ultrasound advancement of a spinal needle into the subarachnoid space is an expert skill, and practitioners should possess considerable probe and needle skills. Preprocedure scanning and marking of a patient’s back require less hand-eye coordination but may also be difficult to learn. Competence at identifying designated spinous processes has been achieved after scanning 22–36 patients. Here, we outline six sonoanatomical views of the lumbar spine and a simplified method for performing a neuraxial preprocedure scan and outline common beginner pitfalls.
Sonoanatomy
Different researchers have described varying numbers of necessary sonographic views, often associated with fanciful monikers. Karmakar refers to “horse heads,” “camel humps,” and “trident” signs (longitudinal paramedian views), whereas Carvalho refers to a “saw” (longitudinal view) and a “flying bat” (transverse view). The novice should not become bewildered by the varying nomenclature, as they are simply tools of pattern recognition.
Specialized ultrasonic windows are required to visualize the neuraxis due to the bony structures that encase it. Six basic views are shown in Table 11.
The anterior and posterior complexes are useful terms for identifying structures. The anterior complex represents the anterior dura, posterior longitudinal ligament, and posterior vertebral body. The posterior complex represents the ligamentum flavum, epidural space, and posterior dura.
While the “target” of spinal anesthesia is the posterior complex, the visualization of the anterior complex denotes a clear ultrasonographic window through the interlaminar space.
Neuraxial Ultrasound Preprocedure Scan
- After positioning the patient in a conventional manner, apply a low-frequency (2- to 5-MHz) curved array probe to the middle of the patient’s lower back in a transverse orientation.
- Optimize the image for depth, frequency, and time-gain compensation.
- Mark the midline. This is done by simply aligning the transversely oriented probe such that there is symmetry of the ultrasound appearance (left side of screen being a mirror of the right side). This will correspond to either the transverse spinous process or transverse interlaminar view. Sliding the transversely applied probe in a cephalad direction, a marking pen is used at intervals to mark the skin adjacent to the middle of the long edge of the probe.Practically, it helps to start low and mark above the probe on skin free of ultrasound gel. This technique assumes there is actual symmetry in the patient’s anatomy (no scoliosis, rotation, or metalwork).
- Identify the lumbosacral junction. The probe is oriented to obtain a paramedian sagittal laminar view. After identifying the lamina, the probe is slid caudally until a continuous hyperechoic line (sacrum) is seen. An anterior complex should be seen between the sacrum and fifth lumbar lamina (see Figure 16 ).
- Mark the lamina of L1–L5. The probe, maintaining a paramedian orientation, can then be moved cephalad as a marking pen, again at the midpoint of the long edge of the probe, and is used to mark the lamina or interlaminar spaces.
- Obtain a transverse interlaminar view at the desired level. The probe is rotated transversely at the desired level (eg, L3–L4). Slight cephalad-caudal tilting and sliding are necessary to optimize the appearance of the posterior and anterior complex.
- Identify the dura (posterior complex) and mark the depth with calipers.
- Note the tilt of the probe (usually slightly cephalad). This indicates the required angulation of the needle once inserted at the optimal insertion point.
- Mark the optimal needle insertion point. A pen is used to mark the four midpoints of the long and short edges of the probe. The probe is put down, and a horizontal and vertical line is constructed. Where they intersect is the optimal needle insertion point. The vertical line should correspond with the previously marked midline.
- Check the optimal insertion point by reapplying probe and ensuring a good view of the anterior complex.


FIGURE 16. Ultrasound image of the lumbosacral junction. A continuous hyperechoic line (sacrum) is seen. An anterior complex (AC) should be seen between the sacrum and fifth lumbar lamina.
Additional views of the spine can be obtained by placing the probe in a paramedian sagittal orientation and sliding laterally through the paramedian laminar, articular process, and transverse process views. The paramedian oblique view is obtained by tilting the probe medially, aiming to highlight the posterior and anterior complexes through the interlaminar space. This view can be used for real-time ultrasound-guided spinal anesthesia.
Pitfalls
The most significant pitfall is, after initial training, waiting for the difficult patient before attempting neuraxial ultrasound. Ultrasound scanning requires pattern recognition, and skills need to be attained by scanning “easy” backs. Imprecise skin marking has been postulated as a reason for failure. Care should be taken to ensure the curved array probe is perpendicular to the skin when using a marking pen. Confusing the anterior complex for the posterior complex risks gross overestimation of the depth to the (posterior) dura. When measuring dural depth, the probe may indent the skin, thereby underestimating depth. Misidentification of the lumbosacral junction or failing to recognize anomalies of the junction, present in 12% of the population, will result in incorrect labeling of the interlaminar spaces. Last, ultrasound gel should be cleaned from the skin prior to performing neuraxial nerve block.
Laparoscopic Surgery With Lumbar Spinal Anesthesia
Lumbar spinal anesthesia has been used in the settings of laparoscopic extraperitoneal and intraperitoneal inguinal hernia repair, outpatient gynecological laparoscopy, laparoscopic cholecystectomy, and laparoscopic ventral hernia repair. Laparoscopic surgery with an awake patient requires some special considerations. First, patient selection and education are paramount. Caution should be used when interpreting general anesthesia conversion rates in clinical trials as patients who consent to the trial may be more likely to tolerate an awake procedure. Anxiolysis should be offered, and patients should be counseled about expected sensations. Pneumoperitoneum can be perceived as a weight on the abdomen. The possibility of conversion to general anesthesia, which is often due to shoulder tip pain, should be discussed.
Surgical technique and trocar sites may need to be modified. Pneumoperitoneum with nitrous oxide insufflation has been used to avoid peritoneal irritation and pain thought to be associated with conventional carbon dioxide insufflation.
However, carbon dioxide insufflation has subsequently been used. Avoidance of head-up left lateral tilt that is associated with diaphragmatic irritation has been suggested. Some studies have limited insufflation to less than 11 mmHg and used a nasogastric tube to decompress the stomach and reduce aspiration risk. Others did not modify surgical technique except for low-flow insufflation (nasogastric tubes were avoided and maintained carbon dioxide insufflation at 15 mmHg).
Addition of intrathecal fentanyl or clonidine may decrease shoulder tip pain.
The main two drawbacks of spinal anesthesia for laparoscopic cholecystectomy seemed to be shoulder tip pain resulting in patient dissatisfaction or conversion to general anesthesia and a high rate of PDPH (up to 10%). Due to the small numbers and heterogeneous techniques in previous studies, it has been difficult to establish the ideal technique.
Tzovaras and colleagues in 2008 published an interim analysis of a randomized trial. One hundred patients were randomized to either general or spinal anesthesia for laparoscopic cholecystectomy. Both arms of the study had nasogastric tubes and carbon dioxide insufflation to a maximum of 10 mmHg. The spinal group had 3 mL of 0.5% hyperbaric bupivacaine, 250 μg morphine, and 20 μg fentanyl injected at the L2–L3 level via a 25-gauge pencil-point needle in the right lateral decubitus position. The patient was then placed in the Trendelenburg position for 3 minutes. Despite intraoperative shoulder tip discomfort or pain in 43% patients of the spinal group, only half of these patients required fentanyl, and no patient required conversion to general anesthesia.
Of those in the spinal and general anesthesia groups, 96% and 94%, respectively, were highly or fairly satisfied with their procedure. Moreover, postoperative pain was less in the spinal group compared with the general anesthesia group. The trial was discontinued as the primary endpoint (pain) was reached with the first 100 patients. No patient in the spinal group had a classic PDPH (G. Tzovaras, personal communication, 2012).
Thoracic Spinal Anesthesia
Thoracic spinal anesthesia was described in the early 1900s by Professor Thomas Jonnesco, although he was criticized by his contemporaries, including Professor Bier. He called his technique “general spinal analgesia” and described two puncture sites, the T1–T2 and T12–L1 interspaces, depending on the surgery required. In his article, he made astounding claims of being able to perform head and neck surgery, including total laryngotomy, under high-thoracic analgesia and incorrectly predicted in 1909 that his technique would “in a short time be universally accepted.”
In 2006, thoracic spinal anesthetic for a patient requiring laparoscopic cholecystectomy was reported. Segmental thoracic spinal anesthesia for laparoscopic cholecystectomy was shown to be effective in a small number of healthy patients, although the authors cautioned that the technique, still in its infancy, should not be used in routine practice.
Spinal anesthesia is traditionally performed in the lumbar region below the level of the conus medullaris to avoid injury to the spinal cord. However, MRI images, albeit in a supine position, have shown that the mid- to lower thoracic segment of the cord lies anteriorly, such that there is a CSF-filled space between the dura and the cord (see Figure 17).
FIGURE 17. Midline MRI of the spinal column. In the thoracic segments, the spinal cord is positioned anteriorly leaving a significant space (*) between the posterior dura and the spinal cord. At the lumbar level, the space disappears almost completely. (Reproduced with permission from van Zundert AA, Stultiens G, Jakimowicz JJ, et al: Segmental spinal anaesthesia for cholecystectomy in a patient with severe lung disease. Br J Anaesth. 2006 Apr;96(4):464-466.)
SUMMARY
Spinal anesthesia is a reliable, safe, and effective form of anesthesia. Much has changed since its beginnings in the late 19th century. Mastery of spinal anesthesia comes with practice, diligence, and knowledge of physiology, pharmacology, and anatomy.
Patient safety must always be at the forefront when considering performing a spinal anesthetic. Spinal anesthesia is an indispensable technique in the practice of modern anesthesia. Supplementary video related to spinal anesthesia can be found at NYSORA Students Educational Videos: Spinal Anesthesia.
This text is a sample of content from the Compendium of Regional Anesthesia on the NYSORA LMS.
NYSORA’s Compendium of Regional Anesthesia is simply the most comprehensive, and practical curriculum on Regional Anesthesia from A to Z, featuring NYSORA’s Premium content. As opposed to textbooks and e-books, the Compendium is continuously updated and features NYSORA’s newest videos, animations, and visual content.
The Compendium is one of several gold-standard educational courses on NYSORA’s Learning System (the NYSORA LMS), and registration to NYSORALMS.com is free. The FULL access to the Compendium, however, is based on an annual subscription, as it requires an army of illustrators, video editors, and an educational team to continue making it the BEST tool for education on everything regional anesthesia. While you can think of the compendium as an ebook on steroids, a quick test drive will give you a real-time feel of how incredible the Compendium really is. Your subscription will transform the way you read about regional anesthesia:
- Learn visually: Everything regional, including spinal, epidural, and nerve block procedures and management protocols
- Review step-by-step techniques instructions for over 60 nerve blocks
- Access NYSORA’s fabled illustrations, animations, and videos (such as Reverse Ultrasound Anatomy)
- Access RA info on any device via the desktop platform and mobile app
- Get real-time updates
- Review infographics for exam preparation (e.g. EDRA)
- Use the Community feed with real case discussions, images and videos are posted and discussed by subscribers and world’s top experts alike.
Even if you do not wish to subscribe to the Compendium, do register to the NYSORA LMS, be the first to know what’s new in regional anesthesia, and get involved in case discussions.
Here’s what the activity feed on NYSORA LMS looks like:
We are convinced that once you experience the Compendium on the NYSORA LMS, and you’ll never go back to your old books, and your subscription will support keeping NYSORA.com free for the rest of the world.
Additional Reading
- Koller C: Vorlaufige Mittheilung über locale Anasthesirung am Auge. Klin Mbl Augenheilk 1884;22:60–63.
- Halsted WS: Practical comments on the use and abuse of cocaine; suggested by its invariably successful employment in more than a thousand minor surgical operations. NY Med J 1885;42:294.
- Corning JL: Spinal anaesthesia and local medication of the cord. NY Med J 1885;42:483–485.
- Gorelick PB, Zych D: James Leonard Corning and the early history of spinal puncture. Neurology 1987;37(4):672–674.
- Arendt K, Demaerschalk BM, Wingerchuk DM, Camann W: Atraumatic lumbar puncture needles: After all these years, are we still missing the point? Neurologist 2009;15(1):17–20.
- Wynter E: Four cases of tubercular meningitis in which paracentesis of the theca vertebralis was performed for relief of fluid pressure. Lancet 1891:981–982.
- Ball C, Westhorpe R: Local anaesthesia—early spinal anaesthesia. Anaesth Intensive Care 2003;31(5):493.
- Bier A: Experiments regarding the cocainization of the spinal cord. Dtsch Z Chir 1899;51:361–369.
- Marx GF: The first spinal anesthesia. Who deserves the laurels? Reg Anesth 1994;19(6):429–430.
- Wulf HF: The centennial of spinal anesthesia. Anesthesiology 1998;89(2):500–506.Larson MD: Tait and Caglieri. The first spinal anesthetic in America. Anesthesiology 1996;85(4):913–919.
- Matas R: Local and regional anesthesia with cocaine and other analgesic drugs, including the subarachnoid method, as applied in the general surgical practice. Phil Med J 1900;6:820–843.
- Vandam LD: On the origins of intrathecal anesthesia. Reg Anesth Pain Med 1998;23(4):335–339; discussion 84–87.
- Tuffier T: Anesthesie medullaire chirurgicale par injection sous-arachnoidienne lombaire de cocaine; technique et resultats. Semin Med 1900;20:167.
- Barker AE: A report on clinical experiences with spinal analgesia in 100 cases and some reflections on the procedure. BMJ 1907;1:665–674.
- Labat G: Circulatory disturbances associated with subarachnoid nerve block. Long Island Med J 1927;21:573.
- Pitkin G: Controllable spinal anesthesia. Am J Surg 1928;5:537.
- Sise LF: Spinal anesthesia for upper and lower abdominal operations. N Engl J Med 1928;199:61.Sise LF: Pontocainglucose for spinal anesthesia. Surg Clin North Am 1935;15:1501.
- Brown DL, Fink BR: The history of neural block and pain management. In Cousins MJ, Bridenbaugh PO (eds): Neural block, 3rd ed. Lippincott-Raven, 1998, pp 3–27.
- Adriani J, Roman-Vega D: Saddle nerve block anesthesia. Am J Surg 1946;71:12.
- Maltby JR, Hutter CD, Clayton KC: The Woolley and Roe case. Br J Anaesth 2000;84(1):121–126.
- Kennedy F, Effron AS, Perry G: The grave spinal cord paralyses caused by spinal anesthesia. Surg Gynecol Obstet 1950;91(4):385–398.
- Dripps RD, Vandam LD: Long-term follow-up of patients who received 10,098 spinal anesthetics: Failure to discover major neurological sequelae. JAMA 1954;156(16):1486–1491.
- Morgan P: Spinal anaesthesia in obstetrics. Can J Anaesth 1995;42(12): 1145–1163.
- Cook TM, Counsell D, Wildsmith JA: Major complications of central neuraxial nerve block: Report on the Third National Audit Project of the Royal College of Anaesthetists. Br J Anaesth 2009;102(2):179–190.
- Greene HM: Lumbar puncture and the prevention of postdural puncture headache. JAMA 1926;86:391–392.
- Greene BA: A 26 gauge lumbar puncture needle: Its value in the prophylaxis of headache following spinal analgesia for vaginal delivery. Anesthesiology 1950;11:464–469.
- Hart JR, Whitacre RJ: Pencil-point needle in prevention of postspinal headache. JAMA 1951;147(7):657–658.
- Sprotte G, Schedel R, Pajunk H: [An “atraumatic” universal needle for single-shot regional anesthesia: clinical results and a 6 year trial in over 30,000 regional anesthesias]. Reg Anaesth 1987;10(3):104–108.
- Calthorpe N: The history of spinal needles: Getting to the point. Anaesthesia 2004;59(12):1231–1241.
- McDonald SB: Is neuraxial block contraindicated in the patient with aortic stenosis? Reg Anesth Pain Med 2004;29(5):496–502.
- O’Keefe JH Jr, Shub C, Rettke SR: Risk of noncardiac surgical procedures in patients with aortic stenosis. Mayo Clin Proc 1989;64(4): 400–405.
- Collard CD, Eappen S, Lynch EP, Concepcion M: Continuous spinal anesthesia with invasive hemodynamic monitoring for surgical repair of the hip in two patients with severe aortic stenosis. Anesth Analg 1995; 81(1):195–198.
- Bamford C, Sibley W, Laguna J: Anesthesia in multiple sclerosis. Can J Neurol Sci 1978;5(1):41–44.
- Kytta J, Rosenberg PH: Anaesthesia for patients with multiple sclerosis. Ann Chir Gynaecol 1984;73(5):299–303.
- Bouchard P, Caillet JB, Monnet F, Banssillon V: [Spinal anesthesia and multiple sclerosis]. Ann Fr Anesth Reanim 1984;3(3):194–198.
- Levesque P, Marsepoil T, Ho P, Venutolo F, Lesouef JM: [Multiple sclerosis disclosed by spinal anesthesia]. Ann Fr Anesth Reanim 1988; 7(1):68–70.
- Vadalouca A, Moka E, Sykiotis C: Combined spinal-epidural technique for total hysterectomy in a patient with advanced, progressive multiple sclerosis. Reg Anesth Pain Med 2002;27(5):540–541.
- Horlocker TT, Wedel DJ: Regional anesthesia in the mmunocompromised patient. Reg Anesth Pain Med 2006;31(4):334–345.
- Chin KJ, Macfarlane AJ, Chan V, Brull R: The use of ultrasound to facilitate spinal anesthesia in a patient with previous lumbar laminectomy and fusion: A case report. J Clin Ultrasound 2009;37(8):482–485.
- Prasad GA, Tumber PS, Lupu CM: Ultrasound guided spinal anesthesia. Can J Anaesth 2008;55(10):716–717.
- Costello JF, Balki M: Cesarean delivery under ultrasound-guided spinal anesthesia [corrected] in a parturient with poliomyelitis and Harrington instrumentation. Can J Anaesth 2008;55(9):606–611.
- Douglas MJ, Swenerton JE: Epidural anesthesia in three parturients with lumbar tattoos: A review of possible implications. Can J Anaesth 2002; 49(10):1057–1060.
- Mavropoulos A, Camann W: Use of a lumbar tattoo to aid spinal anesthesia for cesarean delivery. Int J Obstet Anesth 2009;18(1):98–99.
- Balki M, Carvalho JC: Intraoperative nausea and vomiting during cesarean section under regional anesthesia. Int J Obstet Anesth 2005; 14(3):230–241.
- Mishriky BM, Habib AS: Metoclopramide for nausea and vomiting prophylaxis during and after caesarean delivery: A systematic review and meta-analysis. Br J Anaesth 2012;108(3):374–383.
- Carpenter RL, Caplan RA, Brown DL, Stephenson C, Wu R: Incidence and risk factors for side effects of spinal anesthesia. Anesthesiology 1992;76(6):906–916.
- Evans CH: Spinal Anesthesia Principles and Technique. Hoeber, 1929.
- Ure D, James KS, McNeill M, Booth JV: Glycopyrrolate reduces nausea during spinal anaesthesia for caesarean section without affecting neonatal outcome. Br J Anaesth 1999;82(2):277–279.
- George RB, Allen TK, Habib AS: Serotonin receptor antagonists for the prevention and treatment of pruritus, nausea, and vomiting in women undergoing cesarean delivery with intrathecal morphine: A systematic review and meta-analysis. Anesth Analg 2009;109(1):174–182.
- Allen TK, Habib AS: P6 stimulation for the prevention of nausea and vomiting associated with cesarean delivery under neuraxial anesthesia: A systematic review of randomized controlled trials. Anesth Analg 2008;107(4):1308–1312.
- Crowley LJ, Buggy DJ: Shivering and neuraxial anesthesia. Reg Anesth Pain Med 2008;33(3):241–252.
- Saito T, Sessler DI, Fujita K, Ooi Y, Jeffrey R: Thermoregulatory effects of spinal and epidural anesthesia during cesarean delivery. Reg Anesth Pain Med 1998;23(4):418–423.
- Ballantyne JC, Loach AB, Carr DB: Itching after epidural and spinal opiates. Pain 1988;33(2):149–160.Bauchat JR: Focused review: Neuraxial morphine and oral herpes reactivation in the obstetric population. Anesth Analg 2010;111(5): 1238–1241.
- Crighton IM, Hobbs GJ, Reid MF: Ondansetron for the treatment of pruritus after spinal opioids. Anaesthesia 1996;51(2):199–200.
- Schaffartzik W, Hirsch J, Frickmann F, Kuhly P, Ernst A: Hearing loss after spinal and general anesthesia: A comparative study. Anesth Analg 2000;91(6):1466–1472.
- Karatas E, Goksu S, Durucu C, Isik Y, Kanlikama M: Evaluation of hearing loss after spinal anesthesia with otoacoustic emissions. Eur Arch Otorhinolaryngol 2006;263(8):705–710.
- Lee CM, Peachman FA: Unilateral hearing loss after spinal anesthesia treated with epidural blood patch. Anesth Analg 1986;65(3):312.
- Fog J, Wang LP, Sundberg A, Mucchiano C: Hearing loss after spinal anesthesia is related to needle size. Anesth Analg 1990;70(5):517–522.
- Finegold H, Mandell G, Vallejo M, Ramanathan S: Does spinal anesthesia cause hearing loss in the obstetric population? Anesth Analg
2002;95(1):198–203; table of contents. - Michel O, Brusis T: Hearing loss as a sequel of lumbar puncture. Ann Otol Rhinol Laryngol 1992;101(5):390–394.
- Mulroy MF, Alley EA: Management of bladder volumes when using neuraxial anesthesia. Int Anesthesiol Clin 2012;50(1):101–110.
- Choi S, Mahon P, Awad IT: Neuraxial anesthesia and bladder dysfunction in the perioperative period: A systematic review. Can J Anaesth 2012;59(7):681–703.
- Lambert DH: Complications of spinal anesthesia. Int Anesthesiol Clin 1989;27(1):51–55.
- Harrington BE: Postdural puncture headache and the development of the epidural blood patch. Reg Anesth Pain Med 2004;29(2):136–163; discussion 35.
- Vandam LD, Dripps RD: Long-term follow-up of patients who received 10,098 spinal anesthetics; syndrome of decreased intracranial pressure (headache and ocular and auditory difficulties). JAMA 1956;161(7): 586–591.
- Choi PT, Galinski SE, Takeuchi L, Lucas S, Tamayo C, Jadad AR: PDPH is a common complication of neuraxial block in parturients: A meta-analysis of obstetrical studies. Can J Anaesth 2003;50(5): 460–469.
- Horlocker TT: Complications of spinal and epidural anesthesia. Anesthesiol Clin North Am 2000;18(2):461–485.
- Horlocker TT, McGregor DG, Matsushige DK, Schroeder DR, Besse JA: A retrospective review of 4767 consecutive spinal anesthetics: central
nervous system complications. Perioperative Outcomes Group. Anesth Analg 1997;84(3):578–584. - Reiss W, Shariat AN, Kurapati S, Hadzic A: Intraneural injections. Reg Anesth Pain Med 2011;36(1):97–98.
- Burke D, Wildsmith JA: Meningitis after spinal anaesthesia. Br J Anaesth 1997;78(6):635–636.
- Goldman WW Jr, Sanford JP: An “epidemic” of chemical meningitis. Am J Med 1960;29:94–101.
- Hurst EW: Adhesive arachnoiditis and vascular blockage caused by detergents and other chemical irritants: An experimental study. J Pathol
Bacteriol 1955;70(1):167–178.Marinac JS: Drug- and chemical-induced aseptic meningitis: A review of the literature. Ann Pharmacother 1992;26(6):813–822. - Carp H, Bailey S: The association between meningitis and dural puncture in bacteremic rats. Anesthesiology 1992;76(5):739–742.
- Teele DW, Dashefsky B, Rakusan T, Klein JO: Meningitis after lumbar puncture in children with bacteremia. N Engl J Med 1981;305(18):1079–1081.
- Eng RH, Seligman SJ: Lumbar puncture-induced meningitis. JAMA 1981;245(14):1456–1459.
- Conangla G, Rodriguez L, Alonso-Tarres C, Avila A, de la Campa AG:[Streptococcus salivarius meningitis after spinal anesthesia]. Neurologia
2004;19(6):331–333. - Pandian JD, Sarada C, Radhakrishnan VV, Kishore A: Iatrogenic meningitis after lumbar puncture—A preventable health hazard. J Hosp Infect 2004;56(2):119–124.
- Kocamanoglu IS, Sener EB, Tur A, Ustun E, Sahinoglu H: Streptococcal meningitis after spinal anesthesia: Report of a case. Can J Anaesth 2003; 50(3):314–315.
- Yaniv LG, Potasman I: Iatrogenic meningitis: An increasing role for resistant viridans streptococci? Case report and review of the last 20 years. Scand J Infect Dis 2000;32(6):693–696.
- Santillan A, Nacarino V, Greenberg E, Riina HA, Gobin YP, Patsalides A: Vascular anatomy of the spinal cord. J Neurointerv Surg 2012;4(1):
67–74. - Ilias WK, Klimscha W, Skrbensky G, Weinstabl R, Widhalm A: Continuous microspinal anaesthesia: Another perspective on mechanisms
inducing cauda equina syndrome. Anaesthesia 1998;53(7):618–623. - Rigler ML, Drasner K, Krejcie TC, et al: Cauda equina syndrome after continuous spinal anesthesia. Anesth Analg 1991;72(3):275–281.
- Benson JS: US Food and Drug Administration safety alert: Cauda equina syndrome associated with use of small-bore catheters in continuous spinal anesthesia. AANA J 1992;60(3):223.
- Mollmann M, Holst D, Lubbesmeyer H, Lawin P: Continuous spinal anesthesia: Mechanical and technical problems of catheter placement. Reg Anesth 1993;18(6 Suppl):469–472.
- Loo CC, Irestedt L: Cauda equina syndrome after spinal anaesthesia with hyperbaric 5% lignocaine: A review of six cases of cauda equina syndrome reported to the Swedish Pharmaceutical Insurance 1993–1997. Acta Anaesthesiol Scand 1999;43(4):371–379.
- Panadero A, Monedero P, Fernandez-Liesa JI, Percaz J, Olavide I, Iribarren MJ: Repeated transient neurological symptoms after spinal anaesthesia with hyperbaric 5% lidocaine. Br J Anaesth 1998;81(3): 471–472.
- Pavon A, Anadon Senac P: [Neurotoxicity of intrathecal lidocaine]. Rev Esp Anestesiol Reanim 2001;48(7):326–336.
- Moen V, Dahlgren N, Irestedt L: Severe neurological complications after central neuraxial blocks in Sweden 1990–1999. Anesthesiology 2004;101(4):950–959.
- Akioka K, Torigoe K, Maruta H, et al: A case of cauda equina syndrome following spinal anesthesia with hyperbaric dibucaine. J Anesth 2001;
15(2):106–107. - Lopez-Soriano F, Lajarin B, Verdu JM, Rivas F, Lopez-Robles J: [Cauda equina hemisyndrome after intradural anesthesia with bupivacaine for
hip surgery]. Rev Esp Anestesiol Reanim 2002;49(9):494–496. - Vianna PT, Resende LA, Ganem EM, Gabarra RC, Yamashita S, Barreira AA: Cauda equina syndrome after spinal tetracaine: Electromyographic evaluation—20 years follow-up. Anesthesiology 2001;95(5): 1290–1291.
- Woods WW, Franklin RG: Progressive adhesive arachnoiditis following spinal anesthesia. Calif Med 1951;75(3):196–198.
- Joseph SI, Denson JS: Spinal anesthesia, arachnoiditis, and paraplegia. JAMA 1958;168(10):1330–1333.
- Parnass SM, Schmidt KJ: Adverse effects of spinal and epidural anaesthesia. Drug Saf 1990;5(3):179–194.
- Roche J: Steroid-induced arachnoiditis. Med J Aust 1984;140(5):281–284.
- Aldrete JA: Neurologic deficits and arachnoiditis following neuroaxial anesthesia. Acta Anaesthesiol Scand 2003;47(1):3–12.
- Yokoyama M, Itano Y, Kusume Y, Oe K, Mizobuchi S, Morita K: Total spinal anesthesia provides transient relief of intractable pain. Can J Anaesth 2002;49(8):810–813.
- Auroy Y, Benhamou D, Bargues L, et al: Major complications of regional anesthesia in France: The SOS Regional Anesthesia Hotline Service.
Anesthesiology 2002;97(5):1274–1280. - Vandam LD, Dripps RD: Long-term follow-up of patients who received 10,098 spinal anesthetics. IV. Neurological disease incident to traumatic
lumbar puncture during spinal anesthesia. JAMA 1960;172:1483–1487. - Aromaa U, Lahdensuu M, Cozanitis DA: Severe complications associated with epidural and spinal anaesthesias in Finland 1987–1993. A study
based on patient insurance claims [see comment]. Acta Anaesthesiol Scand 1997;41(4):445–452. - Auroy Y, Narchi P, Messiah A, Litt L, Rouvier B, Samii K: Serious complications related to regional anesthesia: Results of a prospective survey in France. Anesthesiology 1997;87(3):479–486.
- Brull R, McCartney CJ, Chan VW, El-Beheiry H: Neurological complications after regional anesthesia: Contemporary estimates of risk.
Anesth Analg 2007;104(4):965–974. - Buggy DJ: Central neuraxial nerve block: Defining risk more clearly. Br J Anaesth 2009;102(2):151–153.
- van Zundert AA, Stultiens G, Jakimowicz JJ, et al: Laparoscopic cholecystectomy under segmental thoracic spinal anaesthesia: A feasibility
study. Br J Anaesth 2007;98(5):682–686. - van Zundert AA, Stultiens G, Jakimowicz JJ, van den Borne BE, van der Ham WG, Wildsmith JA: Segmental spinal anaesthesia for cholecystectomy in a patient with severe lung disease. Br J Anaesth 2006; 96(4):464–466.
- Hamad MA, El-Khattary OA: Laparoscopic cholecystectomy under spinal anesthesia with nitrous oxide pneumoperitoneum: A feasibility study. Surg Endosc 2003;17(9):1426–1428.
- Tzovaras G, Fafoulakis F, Pratsas K, Georgopoulou S, Stamatiou G, Hatzitheofilou C: Laparoscopic cholecystectomy under spinal anesthesia: A pilot study. Surg Endosc 2006;20(4):580–582.
- Tzovaras G, Fafoulakis F, Pratsas K, Georgopoulou S, Stamatiou G,Hatzitheofilou C: Spinal versus general anesthesia for laparoscopic cholecystectomy: Interim analysis of a controlled randomized trial. Arch Surg 2008;143(5):497–501.
- Yuksek YN, Akat AZ, Gozalan U, et al: Laparoscopic cholecystectomy under spinal anesthesia. Am J Surg 2008;195(4):533–536.
- Cook TM: Report and findings of the 3rd National Audit Project of the Royal College of Anaesthetists. 2009:17–26.
- Guay J: The effect of neuraxial nerve blocks on surgical blood loss and blood transfusion requirements: A meta-analysis. J Clin Anesth 2006;18(2):
124–128. - Mauermann WJ, Shilling AM, Zuo Z: A comparison of neuraxial nerve block versus general anesthesia for elective total hip replacement: A meta-analysis. Anesth Analg 2006;103(4):1018–1025.
- Afolabi BB, Lesi FE, Merah NA: Regional versus general anaesthesia for caesarean section. Cochrane Database Syst Rev 2006(4):CD004350.
- Reynolds F, Seed PT: Anaesthesia for Caesarean section and neonatal acid-base status: A meta-analysis. Anaesthesia 2005;60(7):636–653.
- Beaupre LA, Jones CA, Saunders LD, Johnston DW, Buckingham J, Majumdar SR: Best practices for elderly hip fracture patients. A systematic overview of the evidence. J Gen Intern Med 2005;20(11): 1019–1025.
- Rodgers A, Walker N, Schug S, et al: Reduction of postoperative mortality and morbidity with epidural or spinal anaesthesia: Results from overview of randomised trials. BMJ 2000;321(7275):1493.
- Parker MJ, Handoll HH, Griffiths R: Anaesthesia for hip fracture surgery in adults. Cochrane Database Syst Rev 2004(4):CD000521.
- Lee TW, Grocott HP, Schwinn D, Jacobsohn E: High spinal anesthesia for cardiac surgery: Effects on beta-adrenergic receptor function, stress response, and hemodynamics. Anesthesiology 2003;98(2):499–510.
- Hall R, Adderley N, MacLaren C, et al: Does intrathecal morphine alter the stress response following coronary artery bypass grafting surgery? Can J Anaesth 2000;47(5):463–466.
- Parlow JL, Steele RG, O’Reilly D: Low dose intrathecal morphine facilitates early extubation after cardiac surgery: results of a retrospective continuous quality improvement audit. Can J Anaesth 2005;52(1):94–99.
- Liu SS, Nerve Block BM, Wu CL: Effects of perioperative central neuraxial analgesia on outcome after coronary artery bypass surgery: A meta-analysis. Anesthesiology 2004;101(1):153–161.
- Agarwal D, Mohta M, Tyagi A, Sethi AK: Subdural nerve block and the anaesthetist. Anaesth Intensive Care 2010;38(1):20–26.
- Reina MA, De Leon Casasola O, Lopez A, De Andres JA, Mora M, Fernandez A: The origin of the spinal subdural space: Ultrastructure findings. Anesth Analg 2002;94(4):991–995; table of contents.
- Broadbent CR, Maxwell WB, Ferrie R, Wilson DJ, Gawne-Cain M, Russell R: Ability of anaesthetists to identify a marked lumbar interspace. Anaesthesia 2000;55(11):1122–1126.
- Saifuddin A, Burnett SJ, White J: The variation of position of the conus medullaris in an adult population. A magnetic resonance imaging study. Spine (Phila Pa 1976) 1998;23(13):1452–1456.
- Reiman A, Anson B: Vertebral termination of the spinal cord with report of a case of sacral cord. Anat Rec 1944;88:127.
- Bromage PR: Neurological complications of subarachnoid and epidural anaesthesia. Acta Anaesthesiol Scand 1997;41(4):439–444.
- Reynolds F: Damage to the conus medullaris following spinal anaesthesia. Anaesthesia 2001;56(3):238–247.
- Covino BG: Pharmacology of local anaesthetic agents. Br J Anaesth 1986;58(7):701–716.
- Greene NM: Uptake and elimination of local anesthetics during spinal anesthesia. Anesth Analg 1983;62(11):1013–1024.
- Stienstra R, Greene NM: Factors affecting the subarachnoid spread of local anesthetic solutions. Reg Anesth 1991;16(1):1–6.
- Cohen EN: Distribution of local anesthetic agents in the neuraxis of the dog. Anesthesiology 1968;29(5):1002–1005.
- Schell RM, Brauer FS, Cole DJ, Applegate RL 2nd: Persistent sacral nerve root deficits after continuous spinal anaesthesia. Can J Anaesth 1991;38(7):908–911.
- Hogan Q: Size of human lower thoracic and lumbosacral nerve roots. Anesthesiology 1996;85(1):37–42.
- Kaneko S, Matsumoto M, Tsuruta S, Hirata T, Gondo T, Sakabe T: The nerve root entry zone is highly vulnerable to intrathecal tetracaine in rabbits. Anesth Analg 2005;101(1):107–114; table of contents.
- Takenami T, Yagishita S, Asato F, Hoka S: Neurotoxicity of intrathecally administered tetracaine commences at the posterior roots near entry into the spinal cord. Reg Anesth Pain Med 2000;25(4):372–379.
- Kristensen JD, Karlsten R, Gordh T: Spinal cord blood flow after intrathecal injection of ropivacaine and bupivacaine with or without epinephrine in rats. Acta Anaesthesiol Scand 1998;42(6):685–690.
- Dohi S, Matsumiya N, Takeshima R, Naito H: The effects of subarachnoid lidocaine and phenylephrine on spinal cord and cerebral blood flow in dogs. Anesthesiology 1984;61(3):238–244.
- Kozody R, Palahniuk RJ, Cumming MO: Spinal cord blood flow following subarachnoid tetracaine. Can Anaesth Soc J 1985;32(1):23–29.
- Greene NM: Distribution of local anesthetic solutions within the subarachnoid space. Anesth Analg 1985;64(7):715–730.
- Horlocker TT, Wedel DJ: Density, specific gravity, and baricity of spinal anesthetic solutions at body temperature. Anesth Analg 1993;76(5): 1015–1018.
- Hallworth SP, Fernando R, Columb MO, Stocks GM: The effect of posture and baricity on the spread of intrathecal bupivacaine for elective cesarean delivery. Anesth Analg 2005;100(4):1159–1165.
- McLeod GA: Density of spinal anaesthetic solutions of bupivacaine, levobupivacaine, and ropivacaine with and without dextrose. Br J Anaesth 2004;92(4):547–551.
- Brown DT, Wildsmith JA, Covino BG, Scott DB: Effect of baricity on spinal anaesthesia with amethocaine. Br J Anaesth 1980;52(6):589–596.
- Siker ES, Wolfson B, Stewart WD, Pavilack P, Pappas MT: Mepivacaine for spinal anesthesia: Effects of changes in concentration and baricity. Anesth Analg 1966;45(2):191–196.
- Chambers WA, Edstrom HH, Scott DB: Effect of baricity on spinal anaesthesia with bupivacaine. Br J Anaesth 1981;53(3):279–282.
- Denson DD, Bridenbaugh PO, Turner PA, Phero JC: Comparison of neural block and pharmacokinetics after subarachnoid lidocaine in the rhesus monkey. II: Effects of volume, osmolality, and baricity. Anesth Analg 1983;62(11):995–1001.
- Hare GM, Ngan JC: Density determination of local anaesthetic opioid mixtures for spinal anaesthesia. Can J Anaesth 1998;45(4):341–346.
- Bodily MN, Carpenter RL, Owens BD: Lidocaine 0.5% spinal anaesthesia: a hypobaric solution for short-stay perirectal surgery. Can J Anaesth 1992;39(8):770–773.
- Lui AC, Polis TZ, Cicutti NJ: Densities of cerebrospinal fluid and spinal anaesthetic solutions in surgical patients at body temperature. Can J Anaesth 1998;45(4):297–303.
- Hogan QH, Prost R, Kulier A, Taylor ML, Liu S, Mark L: Magnetic resonance imaging of cerebrospinal fluid volume and the influence of body habitus and abdominal pressure. Anesthesiology 1996;84(6): 1341–1349.
- Carpenter RL, Hogan QH, Liu SS, Crane B, Moore J: Lumbosacral cerebrospinal fluid volume is the primary determinant of sensory nerve block extent and duration during spinal anesthesia. Anesthesiology 1998;89(1): 24–29.
- Higuchi H, Hirata J, Adachi Y, Kazama T: Influence of lumbosacral cerebrospinal fluid density, velocity, and volume on extent and duration of plain bupivacaine spinal anesthesia. Anesthesiology 2004;100(1): 106–114.
- Schneider M, Ettlin T, Kaufmann M, et al: Transient neurologic toxicity after hyperbaric subarachnoid anesthesia with 5% lidocaine. Anesth Analg 1993;76(5):1154–1157.
- Zaric D, Christiansen C, Pace NL, Punjasawadwong Y: Transient neurologic symptoms after spinal anesthesia with lidocaine versus other local anesthetics: A systematic review of randomized, controlled trials. Anesth Analg 2005;100(6):1811–1816.
- Wang BC, Hillman DE, Spielholz NI, Turndorf H: Chronic neurological deficits and Nesacaine-CE—an effect of the anesthetic, 2-chloroprocaine, or the antioxidant, sodium bisulfite? Anesth Analg 1984;63(4):445–447.
- Smith KN, Kopacz DJ, McDonald SB: Spinal 2-chloroprocaine: a dose-ranging study and the effect of added epinephrine. Anesth Analg 2004;98(1):81–88; table of contents.
- Hejtmanek MR, Pollock JE: Chloroprocaine for spinal anesthesia: A retrospective analysis. Acta Anaesthesiol Scand 2011;55(3):267–272.
- Pollock JE: Intrathecal chloroprocaine—not yet “safe” by US FDA parameters. Int Anesthesiol Clin 2012;50(1):93–100.
- Le Truong HH, Girard M, Drolet P, Grenier Y, Boucher C, Bergeron L: Spinal anesthesia: A comparison of procaine and lidocaine. Can J Anaesth 2001;48(5):470–473.
- Johnson ME: Neurotoxicity of spinal procaine—a caution. Reg Anesth Pain Med 2001;26(3):288.
- Hampl KF, Schneider MC, Ummenhofer W, Drewe J: Transient neurologic symptoms after spinal anesthesia. Anesth Analg 1995; 81(6):1148–1153.
- Hampl KF, Heinzmann-Wiedmer S, Luginbuehl I, et al: Transient neurologic symptoms after spinal anesthesia: a lower incidence with prilocaine and bupivacaine than with lidocaine. Anesthesiology 1998; 88(3):629–633.
- Keld DB, Hein L, Dalgaard M, Krogh L, Rodt SA: The incidence of transient neurologic symptoms (TNS) after spinal anaesthesia in patients undergoing surgery in the supine position. Hyperbaric lidocaine 5% versus hyperbaric bupivacaine 0.5%. Acta Anaesthesiol Scand 2000; 44(3):285–290.
- Freedman JM, Li DK, Drasner K, Jaskela MC, Larsen B, Wi S: Transient neurologic symptoms after spinal anesthesia: An epidemiologic study of 1863 patients. Anesthesiology 1998;89(3):633–641.
- Sumi M, Sakura S, Kosaka Y: Intrathecal hyperbaric 0.5% tetracaine as a possible cause of transient neurologic toxicity. Anesth Analg 1996; 82(5):1076–1077.
- Sakura S, Sumi M, Sakaguchi Y, Saito Y, Kosaka Y, Drasner K: The addition of phenylephrine contributes to the development of transient
neurologic symptoms after spinal anesthesia with 0.5% tetracaine. Anesthesiology 1997;87(4):771–778. - Liguori GA, Zayas VM, Chisholm MF: Transient neurologic symptoms after spinal anesthesia with mepivacaine and lidocaine. Anesthesiology
1998;88(3):619–623. - Salazar F, Bogdanovich A, Adalia R, Chabas E, Gomar C: Transient neurologic symptoms after spinal anaesthesia using isobaric 2%
mepivacaine and isobaric 2% lidocaine. Acta Anaesthesiol Scand 2001; 45(2):240–245. - Eberhart LH, Morin AM, Kranke P, Geldner G, Wulf H: [Transient neurologic symptoms after spinal anesthesia. A quantitative systematic
overview (meta-analysis) of randomized controlled studies]. Anaesthesist 2002;51(7):539–546. - Ganapathy S, Sandhu HB, Stockall CA, Hurley D: Transient neurologic symptom (TNS) following intrathecal ropivacaine. Anesthesiology 2000;93(6):1537–1539.
- Lee YY, Ngan Kee WD, Chang HK, So CL, Gin T: Spinal ropivacaine for lower limb surgery: A dose response study. Anesth Analg 2007;105(2):520–523.
- Khaw KS, Ngan Kee WD, Wong EL, Liu JY, Chung R: Spinal ropivacaine for cesarean section: a dose-finding study. Anesthesiology 2001;95(6):1346–1350.
- Frawley G, Skinner A, Thomas J, Smith S: Ropivacaine spinal anesthesia in neonates: A dose range finding study. Paediatr Anaesth
2007;17(2):126–132. - McDonald SB, Liu SS, Kopacz DJ, Stephenson CA: Hyperbaric spinal ropivacaine: A comparison to bupivacaine in volunteers. Anesthesiology
1999;90(4):971–977. - Wahedi W, Nolte H, Klein P: [Ropivacaine for spinal anesthesia. A dosefinding study]. Anaesthesist 1996;45(8):737–744.
- van Kleef JW, Veering BT, Burm AG: Spinal anesthesia with ropivacaine: A double-blind study on the efficacy and safety of 0.5% and 0.75%
solutions in patients undergoing minor lower limb surgery. Anesth Analg 1994;78(6):1125–1130. - Hughes D, Hill D, Fee JP: Intrathecal ropivacaine or bupivacaine with fentanyl for labour. Br J Anaesth 2001;87(5):733–737.
- Breebaart MB, Vercauteren MP, Hoffmann VL, Adriaensen HA: Urinary bladder scanning after day-case arthroscopy under spinal anaesthesia: Comparison between lidocaine, ropivacaine, and levobupivacaine. Br J Anaesth 2003;90(3):309–313.
- Brown DL: Spinal, epidural, and caudal anesthesia. In Miller RD (ed). Anesthesia, 4th ed. Churchill Livingston, 1994, pp 1505–1533.
- Pawlowski J, Sukhani R, Pappas AL, et al: The anesthetic and recovery profile of two doses (60 and 80 mg) of plain mepivacaine for ambulatory
spinal anesthesia. Anesth Analg 2000;91(3):580–584. - Kallio H, Snall EV, Kero MP, Rosenberg PH: A comparison of intrathecal plain solutions containing ropivacaine 20 or 15 mg versus bupivacaine 10 mg. Anesth Analg 2004;99(3):713–717; table of contents.
- McNamee DA, Parks L, McClelland AM, et al: Intrathecal ropivacaine for total hip arthroplasty: Double-blind comparative study with isobaric
7.5 mg ml(-1) and 10 mg ml(-1) solutions. Br J Anaesth 2001;87(5):743–747. - Burke D, Kennedy S, Bannister J: Spinal anesthesia with 0.5% S(-)-bupivacaine for elective lower limb surgery. Reg Anesth Pain Med 1999;24(6):519–523.
- Glaser C, Marhofer P, Zimpfer G, et al: Levobupivacaine versus racemic bupivacaine for spinal anesthesia. Anesth Analg 2002;94(1):194–198;
table of contents. - Alley EA, Kopacz DJ, McDonald SB, Liu SS: Hyperbaric spinal levobupivacaine: A comparison to racemic bupivacaine in volunteers. Anesth Analg 2002;94(1):188–193; table of contents.
- Bridenbaugh PO, Greene NM, Brull SJ: Spinal (subarachoid) neural block. In Cousins MJ, Bridenbaugh PO (eds). Neural block, 3rd ed. Lippincott-Raven, 1998.
- Tetzlaff JE, Dilger J, Yap E, Smith MP, Schoenwald PK: Cauda equina syndrome after spinal anaesthesia in a patient with severe vascular disease. Can J Anaesth 1998;45(7):667–669.
- Lee DS, Bui T, Ferrarese J, Richardson PK: Cauda equina syndrome after incidental total spinal anesthesia with 2% lidocaine. J Clin Anesth 1998;10(1):66–69.
- Maehara Y, Kusunoki S, Kawamoto M, et al: A prospective multicenter trial to determine the incidence of transient neurologic symptoms after spinal anesthesia with phenylephrine added to 0.5% tetracaine. Hiroshima J Med Sci 2001;50(2):47–51.
- Kozody R, Palahniuk RJ, Wade JG, Cumming MO, Pucci WR: The effect of subarachnoid epinephrine and phenylephrine on spinal cord blood flow. Can Anaesth Soc J 1984;31(5):503–508.
- Porter SS, Albin MS, Watson WA, Bunegin L, Pantoja G: Spinal cord and cerebral blood flow responses to subarachnoid injection of local anesthetics with and without epinephrine. Acta Anaesthesiol Scand 1985;29(3):330–338.
- Concepcion M, Maddi R, Francis D, Rocco AG, Murray E, Covino BG:Vasoconstrictors in spinal anesthesia with tetracaine—A comparison of
epinephrine and phenylephrine. Anesth Analg 1984;63(2):134–138. - Armstrong IR, Littlewood DG, Chambers WA: Spinal anesthesia with tetracaine—effect of added vasoconstrictors. Anesth Analg 1983;62(9):
793–795. - Rice LJ, DeMars PD, Whalen TV, Crooms JC, Parkinson SK: Duration of spinal anesthesia in infants less than one year of age. Comparison of three hyperbaric techniques. Reg Anesth 1994;19(5):325–329.
- Chambers WA, Littlewood DG, Scott DB: Spinal anesthesia with hyperbaric bupivacaine: effect of added vasoconstrictors. Anesth Analg
1982;61(1):49–52. - Moore JM, Liu SS, Pollock JE, Neal JM, Knab JH: The effect of epinephrine on small-dose hyperbaric bupivacaine spinal anesthesia: clinical
implications for ambulatory surgery. Anesth Analg 1998;86(5):973–977. - Racle JP, Poy JY, Benkhadra A, Jourdren L, Fockenier F: [Prolongation of spinal anesthesia with hyperbaric bupivacaine by adrenaline and
clonidine in the elderly]. Ann Fr Anesth Reanim 1988;7(2):139–144. - Vercauteren MP, Jacobs S, Jacquemyn Y, Adriaensen HA: Intrathecal labor analgesia with bupivacaine and sufentanil: The effect of adding 2.25 microg epinephrine. Reg Anesth Pain Med 2001;26(5):473–477.
- Goodman SR, Kim-Lo SH, Ciliberto CF, Ridley DM, Smiley RM: Epinephrine is not a useful addition to intrathecal fentanyl or fentanylbupivacaine for labor analgesia. Reg Anesth Pain Med 2002;27(4):374–379.
- Gautier PE, Debry F, Fanard L, Van Steenberge A, Hody JL: Ambulatory combined spinal-epidural analgesia for labor. Influence of epinephrine on bupivacaine-sufentanil combination. Reg Anesth 1997;22(2):143–149.
- Chambers WA, Littlewood DG, Logan MR, Scott DB: Effect of added epinephrine on spinal anesthesia with lidocaine. Anesth Analg 1981;60(6):417–420.
- Chiu AA, Liu S, Carpenter RL, Kasman GS, Pollock JE, Neal JM: The effects of epinephrine on lidocaine spinal anesthesia: A cross-over study.
Anesth Analg 1995;80(4):735–739. - Racle JP, Benkhadra A, Poy JY: Subarachnoid anaesthesia produced by hyperbaric lignocaine in elderly patients. Prolongation of effect with
adrenaline. Br J Anaesth 1988;60(7):831–835. - Spivey DL: Epinephrine does not prolong lidocaine spinal anesthesia in term parturients. Anesth Analg 1985;64(5):468–470.
- Moore DC, Chadwick HS, Ready LB: Epinephrine prolongs lidocaine spinal: Pain in the operative site the most accurate method of determining
local anesthetic duration. Anesthesiology 1987;67(3):416–418. - Glynn CJ, Mather LE, Cousins MJ, Wilson PR, Graham JR: Spinal narcotics and respiratory depression. Lancet 1979;2(8138):356–357.
- Cunningham AJ, McKenna JA, Skene DS: Single injection spinal anaesthesia with amethocaine and morphine for transurethral prostatectomy. Br J Anaesth 1983;55(5):423–427.
- Nordberg G, Hedner T, Mellstrand T, Dahlstrom B: Pharmacokinetic aspects of intrathecal morphine analgesia. Anesthesiology 1984;60(5): 448–454.
- Abouleish E, Rawal N, Rashad MN: The addition of 0.2 mg subarachnoid morphine to hyperbaric bupivacaine for cesarean delivery: A prospective study of 856 cases. Reg Anesth 1991;16(3):137–140.
- Borgeat A, Singer T: Nausea and vomiting after spinal anaesthesia with morphine. Acta Anaesthesiol Scand 1998;42(10):1231.
- Bonnet F, Buisson VB, Francois Y, Catoire P, Saada M: Effects of oral and subarachnoid clonidine on spinal anesthesia with bupivacaine. Reg
Anesth 1990;15(4):211–214. - Dobrydnjov I, Axelsson K, Thorn SE, et al: Clonidine combined with small-dose bupivacaine during spinal anesthesia for inguinal herniorrhaphy: A randomized double-blinded study. Anesth Analg 2003;96(5):1496–1503; table of contents.
- Strebel S, Gurzeler JA, Schneider MC, Aeschbach A, Kindler CH: Smalldose intrathecal clonidine and isobaric bupivacaine for orthopedic
surgery: a dose-response study. Anesth Analg 2004;99(4):1231–1238;table of contents. - Filos KS, Goudas LC, Patroni O, Polyzou V: Hemodynamic and analgesic profile after intrathecal clonidine in humans. A dose-response
study. Anesthesiology 1994;81(3):591–601; discussion 27A–28A. - Hassenbusch SJ, Gunes S, Wachsman S, Willis KD: Intrathecal clonidine in the treatment of intractable pain: A phase I/II study. Pain Med 2002;3(2):85–91.
- Ackerman LL, Follett KA, Rosenquist RW: Long-term outcomes during treatment of chronic pain with intrathecal clonidine or clonidine/opioid combinations. J Pain Symptom Manage 2003;26(1):668–677.
- Chen SR, Khan GM, Pan HL: Antiallodynic effect of intrathecal neostigmine is mediated by spinal nitric oxide in a rat model of diabetic neuropathic pain. Anesthesiology 2001;95(4):1007–1012.
- Ho KM, Ismail H, Lee KC, Branch R: Use of intrathecal neostigmine as an adjunct to other spinal medications in perioperative and peripartum analgesia: A meta-analysis. Anaesth Intensive Care 2005;33(1):41–53.
- Yegin A, Yilmaz M, Karsli B, Erman M: Analgesic effects of intrathecal neostigmine in perianal surgery. Eur J Anaesthesiol 2003;20(5): 404–408.
- Tan PH, Kuo JH, Liu K, Hung CC, Tsai TC, Deng TY: Efficacy of intrathecal neostigmine for the relief of postinguinal hemiorrhaphy pain. Acta Anaesthesiol Scand 2000;44(9):1056–1060.
- Klohr S, Roth R, Hofmann T, Rossaint R, Heesen M: Definitions of hypotension after spinal anaesthesia for caesarean section: Literature search and application to parturients. Acta Anaesthesiol Scand 2010; 54(8):909–921.
- Mark JB, Steele SM: Cardiovascular effects of spinal anesthesia. Int Anesthesiol Clin 1989;27(1):31–39.
- Cooper DW: Caesarean delivery vasopressor management. Curr Opin Anaesthesiol 2012;25(3):300–308.
- Khaw KS, Ngan Kee WD, Lee SW: Hypotension during spinal anaesthesia for Caesarean section: Implications, detection, prevention and treatment. Fetal Matern Med Rev 2006;17(2):157–183.
- Shimosato S, Etsten BE: The role of the venous system in cardiocirculatory dynamics during spinal and epidural anesthesia in man. Anesthesiology 1969;30(6):619–628.
- Cooper J: Cardiac arrest during spinal anesthesia. Anesth Analg 2001; 93(1):245.
- Salinas FV, Sueda LA, Liu SS: Physiology of spinal anaesthesia and practical suggestions for successful spinal anaesthesia. Best Pract Res Clin Anaesthesiol 2003;17(3):289–303.
- Meyhoff CS, Hesselbjerg L, Koscielniak-Nielsen Z, Rasmussen LS: Biphasic cardiac output changes during onset of spinal anaesthesia in elderly patients. Eur J Anaesthesiol 2007;24(9):770–775.
- Roy L, Ramanathan S: ST-segment depression and myocardial contractility during cesarean section under spinal anesthesia. Can J Anaesth 1999;46(1):52–55.
- Ou CH, Tsou MY, Ting CK, Chiou CS, Chan KH, Tsai SK: Occurrence of the Bezold-Jarisch reflex during Cesarean section under spinal anesthesia—A case report. Acta Anaesthesiol Taiwan 2004;42(3):175–178.
- Mackey DC, Carpenter RL, Thompson GE, Brown DL, Bodily MN: Bradycardia and asystole during spinal anesthesia: A report of three cases without morbidity. Anesthesiology 1989;70(5):866–868.
- Campagna JA, Carter C: Clinical relevance of the Bezold-Jarisch reflex. Anesthesiology 2003;98(5):1250–1260.
- Lesser JB, Sanborn KV, Valskys R, Kuroda M: Severe bradycardia during spinal and epidural anesthesia recorded by an anesthesia information management system. Anesthesiology 2003;99(4):859–866.
- Kinsella SM, Tuckey JP: Perioperative bradycardia and asystole: Relationship to vasovagal syncope and the Bezold-Jarisch reflex. Br J Anaesth 2001;86(6):859–868.
- Bernards CM, Hymas NJ: Progression of first degree heart nerve block to high-grade second degree nerve block during spinal anaesthesia. Can J Anaesth 1992;39(2):173–175.
- Tarkkila P, Isola J: A regression model for identifying patients at high risk of hypotension, bradycardia and nausea during spinal anesthesia. Acta Anaesthesiol Scand 1992;36(6):554–558.
- Klasen J, Junger A, Hartmann B, et al: Differing incidences of relevant hypotension with combined spinal-epidural anesthesia and spinal anesthesia. Anesth Analg 2003;96(5):1491–1495; table of contents.
- Hartmann B, Junger A, Klasen J, et al: The incidence and risk factors for hypotension after spinal anesthesia induction: an analysis with automated data collection. Anesth Analg 2002;94(6):1521–1529; table of contents.
- Ngan Kee WD, Khaw KS, Lau TK, Ng FF, Chui K, Ng KL: Randomised double-blinded comparison of phenylephrine versus ephedrine for maintaining blood pressure during spinal anaesthesia for non-elective Caesarean section. Anaesthesia 2008;63(12):1319–1326.
- James FM 3rd, Greiss FC Jr, Kemp RA: An evaluation of vasopressor therapy for maternal hypotension during spinal anesthesia. Anesthesiology 1970;33(1):25–34.
- Wildsmith JA: Management of hypotension during spinal anesthesia. Reg Anesth Pain Med 2000;25(3):322.
- Sinclair CJ, Scott DB, Edstrom HH: Effect of the trendelenberg position on spinal anaesthesia with hyperbaric bupivacaine. Br J Anaesth 1982;54(5):497–500.
- Cyna AM, Andrew M, Emmett RS, Middleton P, Simmons SW: Techniques for preventing hypotension during spinal anaesthesia for caesarean section. Cochrane Database Syst Rev 2006(4):CD002251.
- Husaini SW, Russell IF: Volume preload: Lack of effect in the prevention of spinal-induced hypotension at caesarean section. Int J Obstet Anesth 1998;7(2):76–81.
- Jackson R, Reid JA, Thorburn J: Volume preloading is not essential to prevent spinal-induced hypotension at caesarean section. Br J Anaesth 1995;75(3):262–265.
- Dahlgren G, Granath F, Pregner K, Rosblad PG, Wessel H, Irestedt L: Colloid vs. crystalloid preloading to prevent maternal hypotension during spinal anesthesia for elective cesarean section. Acta Anaesthesiol Scand 2005;49(8):1200–1206.
- Dyer RA, Farina Z, Joubert IA, et al: Crystalloid preload versus rapid crystalloid administration after induction of spinal anaesthesia (coload) for elective caesarean section. Anaesth Intensive Care 2004;32(3): 351–357.
- Roofthooft E, Van de Velde M: Low-dose spinal anaesthesia for Caesarean section to prevent spinal-induced hypotension. Curr Opin Anaesthesiol 2008;21(3):259–262.
- Arzola C, Wieczorek PM: Efficacy of low-dose bupivacaine in spinal anaesthesia for Caesarean delivery: Systematic review and meta-analysis. Br J Anaesth 2011;107(3):308–318.
- Ngan Kee WD, Lee A: Multivariate analysis of factors associated with umbilical arterial pH and standard base excess after Caesarean section under spinal anaesthesia. Anaesthesia 2003;58(2):125–130.
- Labartino L, Mojdehi E, Mauro AL: Management of hypotension following spinal anesthesia for cesarean section. Anesth Analg 1966;45(2):179–182.
- Ngan Kee WD, Khaw KS, Ng FF: Comparison of phenylephrine infusion regimens for maintaining maternal blood pressure during spinal anaesthesia for caesarean section. Br J Anaesth 2004;92(4): 469–474.
- Ngan Kee WD, Khaw KS, Ng FF: Prevention of hypotension during spinal anesthesia for cesarean delivery: An effective technique using combination phenylephrine infusion and crystalloid cohydration. Anesthesiology 2005;103(4):744–750.
- Habib AS: A review of the impact of phenylephrine administration on maternal hemodynamics and maternal and neonatal outcomes in women undergoing cesarean delivery under spinal anesthesia. Anesth Analg 2012;114(2):377–390.
- Park YH, Ryu T, Hong SW, Kwak KH, Kim SO: The effect of the intravenous phenylephrine on the level of spinal anesthesia. Korean J Anesthesiol 2011;61(5):372–376.
- Caplan RA, Ward RJ, Posner K, Cheney FW: Unexpected cardiac arrest during spinal anesthesia: A closed claims analysis of predisposing factors. Anesthesiology 1988;68(1):5–11.
- Lovstad RZ, Granhus G, Hetland S: Bradycardia and asystolic cardiac arrest during spinal anaesthesia: A report of five cases. Acta Anaesthesiol Scand 2000;44(1):48–52.
- Pan PH, Moore CH, Ross VH: Severe maternal bradycardia and asystole after combined spinal-epidural labor analgesia in a morbidly obese parturient. J Clin Anesth 2004;16(6):461–464.
- Pollard JB: Cardiac arrest during spinal anesthesia: common mechanisms and strategies for prevention. Anesth Analg 2001;92(1):252–256.
- Cooper DW: Effect of vasopressors on systolic, mean and diastolic arterial pressure during spinal anaesthesia in pregnancy. Int J Obstet Anesth 2008;17(1):90–92.
- Sahoo T, SenDasgupta C, Goswami A, Hazra A: Reduction in spinal-induced hypotension with ondansetron in parturients undergoing caesarean section: A double-blind randomised, placebo-controlled study. Int J Obstet Anesth 2012;21(1):24–28.
- Smiley RM: Burden of proof. Anesthesiology 2009;111(3):470–472.
- Greene NM, Brull SJ: Physiology of Spinal Anesthesia, 4th ed. Williams & Wilkins, 1981.
- Steinbrook RA, Concepcion M, Topulos GP: Ventilatory responses to hypercapnia during bupivacaine spinal anesthesia. Anesth Analg 1988;67(3):247–252.
- Nakayama M, Kanaya N, Fujita S, Namiki A: Effects of ephedrine on indocyanine green clearance during spinal anesthesia: Evaluation by the finger piece method. Anesth Analg 1993;77(5):947–949.
- Zinn SE, Fairley HB, Glenn JD: Liver function in patients with mild alcoholic hepatitis, after enflurane, nitrous oxide-narcotic, and spinal
anesthesia. Anesth Analg 1985;64(5):487–490. - Igarashi M, Kawana S, Iwasaki H, Namiki A: [Anesthetic management for a patient with citrullinemia and liver cirrhosis]. Masui 1995;
44(1):96–99. - Fukuda T, Okutani R, Kono K, Yoshimura Y, Ochiai N: [Anesthetic management for cesarean section of a patient with transient diabetes
insipidus and acute severe liver dysfunction]. Masui 1993;42(10):1511–1516. - McNeill MJ, Bennet A: Use of regional anaesthesia in a patient with acute porphyria. Br J Anaesth 1990;64(3):371–373.
- Consolo D, Ouardirhi Y, Wessels C, Girard C: [Obstetrical anaesthesia and porphyrias]. Ann Fr Anesth Reanim 2005;24(4):428–431.
- Runciman WB, Mather LE, Ilsley AH, Carapetis RJ, Upton RN: A sheep preparation for studying interactions between blood flow and drug
disposition. III: Effects of general and spinal anaesthesia on regional blood flow and oxygen tensions. Br J Anaesth 1984;56(11):1247–1258. - Runciman WB, Mather LE, Ilsley AH, Carapetis RJ, Upton RN: A sheep preparation for studying interactions between blood flow and drug
disposition. IV: The effects of general and spinal anaesthesia on blood flow and cefoxitin disposition. Br J Anaesth 1985;57(12):1239–1247. - Runciman WB, Mather LE, Ilsley AH, Carapetis RJ, Upton RN: A sheep preparation for studying interactions between blood flow and drug
disposition. VI: Effects of general or subarachnoid anaesthesia on blood flow and chlormethiazole disposition. Br J Anaesth 1986;58(11):1308–1316. - Mather LE, Runciman WB, Ilsley AH, Carapetis RJ, Upton RN: A sheep preparation for studying interactions between blood flow and drug disposition. V: The effects of general and subarachnoid anaesthesia on blood flow and pethidine disposition. Br J Anaesth 1986;58(8): 888–896.
- Taivainen T, Tuominen M, Rosenberg PH: Influence of obesity on the spread of spinal analgesia after injection of plain 0.5% bupivacaine at the
L3–4 or L4–5 interspace. Br J Anaesth 1990;64(5):542–546. - Tuominen M, Taivainen T, Rosenberg PH: Spread of spinal anaesthesia with plain 0.5% bupivacaine: Influence of the vertebral interspace used
for injection. Br J Anaesth 1989;62(4):358–361. - Tuominen M, Kuulasmaa K, Taivainen T, Rosenberg PH: Individual predictability of repeated spinal anaesthesia with isobaric bupivacaine. Acta Anaesthesiol Scand 1989;33(1):13–14.
- Sundnes KO, Vaagenes P, Skretting P, Lind B, Edstrom HH: Spinal analgesia with hyperbaric bupivacaine: Effects of volume of solution. Br
J Anaesth 1982;54(1):69–74. - Konishi R, Mitsuhata H, Saitoh J, Hirabayashi Y, Shimizu R: [The spread of subarachnoid hyperbaric dibucaine in the term parturient]. Masui 1997;46(2):184–187.
- Veering BT, Ter Riet PM, Burm AG, Stienstra R, Van Kleef JW: Spinal anaesthesia with 0.5% hyperbaric bupivacaine in elderly patients: Effect
of site of injection on spread of analgesia. Br J Anaesth 1996;77(3):343–346. - Cameron AE, Arnold RW, Ghorisa MW, Jamieson V: Spinal analgesia using bupivacaine 0.5% plain. Variation in the extent of the nerve block with
patient age. Anaesthesia 1981;36(3):318–322. - Pitkanen M, Haapaniemi L, Tuominen M, Rosenberg PH: Influence of age on spinal anaesthesia with isobaric 0.5% bupivacaine. Br J Anaesth 1984;56(3):279–284.
- Veering BT, Burm AG, Vletter AA, van den Hoeven RA, Spierdijk J: The effect of age on systemic absorption and systemic disposition of bupivacaine after subarachnoid administration. Anesthesiology 1991;74(2):250–257.
- Racle JP, Benkhadra A, Poy JY, Gleizal B: Spinal analgesia with hyperbaric bupivacaine: Influence of age. Br J Anaesth 1988;60(5): 508–514.
- Schiffer E, Van Gessel E, Gamulin Z: Influence of sex on cerebrospinal fluid density in adults. Br J Anaesth 1999;83(6):943–944.
- Pargger H, Hampl KF, Aeschbach A, Paganoni R, Schneider MC: Combined effect of patient variables on sensory level after spinal 0.5%
plain bupivacaine. Acta Anaesthesiol Scand 1998;42(4):430–434. - Povey HM, Jacobsen J, Westergaard-Nielsen J: Subarachnoid analgesia with hyperbaric 0.5% bupivacaine: Effect of a 60-min period of sitting. Acta Anaesthesiol Scand 1989;33(4):295–297
- Alston RP, Littlewood DG, Meek R, Edstrom HH: Spinal anaesthesia with hyperbaric bupivacaine: Effects of concentration and volume when
administered in the sitting position. Br J Anaesth 1988;61(2):144–148. - Alston RP: Spinal anaesthesia with 0.5% bupivacaine 3 mL: Comparison of plain and hyperbaric solutions administered to seated patients. Br J Anaesth 1988;61(4):385–389.
- Mitchell RW, Bowler GM, Scott DB, Edstrom HH: Effects of posture and baricity on spinal anaesthesia with 0.5% bupivacaine 5 ml. A double-blind study. Br J Anaesth 1988;61(2):139–143.
- Povey HM, Olsen PA, Pihl H: Spinal analgesia with hyperbaric 0.5% bupivacaine: Effects of different patient positions. Acta Anaesthesiol Scand 1987;31(7):616–619.
- Maroof M, Khan RM, Siddique M, Tariq M: Hypobaric spinal anaesthesia with bupivacaine (0.1%) gives selective sensory nerve block for ano-rectal surgery. Can J Anaesth 1995;42(8):691–694.
- Fettes PD, Jansson JR, Wildsmith JA: Failed spinal anaesthesia: mechanisms, management, and prevention. Br J Anaesth 2009;102(6):739–748.
- Tuominen M, Pitkanen M, Rosenberg PH: Effect of speed of injection of 0.5% plain bupivacaine on the spread of spinal anaesthesia. Br J Anaesth 1992;69(2):148–149.
- Van Gessel EF, Praplan J, Fuchs T, Forster A, Gamulin Z: Influence of injection speed on the subarachnoid distribution of isobaric bupivacaine
0.5%. Anesth Analg 1993;77(3):483–487. - Stienstra R, Van Poorten F: Speed of injection does not affect subarachnoid distribution of plain bupivacaine 0.5%. Reg Anesth 1990;
15(4):208–210. - Bucx MJ, Kroon JW, Stienstra R: Effect of speed of injection on the maximum sensory level for spinal anesthesia using plain bupivacaine
0.5% at room temperature. Reg Anesth 1993;18(2):103–105. - Axelsson KH, Edstrom HH, Sundberg AE, Widman GB: Spinal anaesthesia with hyperbaric 0.5% bupivacaine: Effects of volume. Acta
Anaesthesiol Scand 1982;26(5):439–445. - Peng PW, Chan VW, Perlas A: Minimum effective anaesthetic concentration of hyperbaric lidocaine for spinal anaesthesia. Can J Anaesth 1998;45(2):122–129.
- Alfonsi P, Brusset A, Levy R, Gauneau P, Chauvin M: [Spinal anesthesia with bupivacaine without glucose in the elderly: Effect of concentration and volume on the hemodynamic profile]. Ann Fr Anesth Reanim 1991;10(6):543–547.
- Pflug EA, Aasheim GM, Beck HA: Spinal anesthesia: bupivacaine versus tetracaine. Anesth Analg 1976;55(4):489–492.
- Sheskey MC, Rocco AG, Bizzarri-Schmid M, Francis DM, Edstrom H, Covino BG: A dose-response study of bupivacaine for spinal anesthesia. Anesth Analg 1983;62(10):931–935.
- Kopacz DJ: Spinal 2-chloroprocaine: Minimum effective dose. Reg Anesth Pain Med 2005;30(1):36–42.
- Van Zundert AA, Grouls RJ, Korsten HH, Lambert DH: Spinal anesthesia. Volume or concentration—What matters? Reg Anesth 1996;21(2):112–118.
- Cook TM, Fischer B, Bogod D, et al: Antiseptic solutions for central neuraxial block: Which concentration of chlorhexidine in alcohol should we use? Br J Anaesth 2009;103:456–457.Schweizer RT: Mask wiggling as a potential cause of wound
contamination. Lancet 1976;2(7995):1129–1130. - Veringa E, van Belkum A, Schellekens H: Iatrogenic meningitis by Streptococcus salivarius following lumbar puncture. J Hosp Infect 1995;29(4):316–318.
- Hebl JR: The importance and implications of aseptic techniques during regional anesthesia. Reg Anesth Pain Med 2006;31(4):311–323.
- Association of Anaesthetists of Great Britain and Ireland. Infection control in anaesthesia. Anaesthesia 2008;63(9):1027–1036.
- Ross BK, Chadwick HS, Mancuso JJ, Benedetti C: Sprotte needle for obstetric anesthesia: Decreased incidence of post dural puncture
headache. Reg Anesth 1992;17(1):29–33. - de Filho GR, Gomes HP, da Fonseca MH, Hoffman JC, Pederneiras SG, Garcia JH: Predictors of successful neuraxial nerve block: A prospective study. Eur J Anaesthesiol 2002;19(6):447–451.
- Lee PJ, Tang R, Sawka A, Krebs C, Vaghadia H: Brief report: Real-time ultrasound-guided spinal anesthesia using Taylor’s approach. Anesth
Analg 2011;112(5):1236–1238. - Palmer CM: Continuous spinal anesthesia and analgesia in obstetrics. Anesth Analg 2010;111(6):1476–1479.
- Arkoosh VA, Palmer CM, Yun EM, et al: A randomized, double-masked, multicenter comparison of the safety of continuous intrathecal labor analgesia using a 28-gauge catheter versus continuous epidural labor analgesia. Anesthesiology 2008;108(2):286–298.
- Sng BL, Lim Y, Sia AT: An observational prospective cohort study of incidence and characteristics of failed spinal anaesthesia for caesarean
section. Int J Obstet Anesth 2009;18(3):237–241. - Levy JH, Islas JA, Ghia JN, Turnbull C: A retrospective study of the incidence and causes of failed spinal anesthetics in a university hospital.
Anesth Analg 1985;64(7):705–710. - Hoppe J, Popham P: Complete failure of spinal anaesthesia in obstetrics. Int J Obstet Anesth 2007;16(3):250–255.
- Reina MA, Collier CB, Prats-Galino A, Puigdellivol-Sanchez A, Maches F, De Andres JA: Unintentional subdural placement of epidural catheters during attempted epidural anesthesia: An anatomic study of spinal subdural compartment. Reg Anesth Pain Med 2011;36(6):537–541.
- Capdevila X, Dadure C: Perioperative management for one day hospital admission: Regional anesthesia is better than general anesthesia. Acta Anaesthesiol Belg 2004;55(Suppl):33–36.
- Ruben JE, Kamsler PM: Unilateral spinal anesthesia for surgical reduction of hip fractures. Am J Surg 1950;79(2):312–317.
- Khatouf M, Loughnane F, Boini S, et al: [Unilateral spinal anaesthesia in elderly patient for hip trauma: A pilot study]. Ann Fr Anesth Reanim 2005;24(3):249–254.
- Cappelleri G, Aldegheri G, Danelli G, et al: Spinal anesthesia with hyperbaric levobupivacaine and ropivacaine for outpatient knee arthroscopy: A prospective, randomized, double-blind study. Anesth Analg 2005;101(1):77–82; table of contents.
- Fanelli G, Borghi B, Casati A, Bertini L, Montebugnoli M, Torri G: Unilateral bupivacaine spinal anesthesia for outpatient knee arthroscopy. Italian Study Group on Unilateral Spinal Anesthesia. Can J Anaesth 2000;47(8):746–751.
- Nair GS, Abrishami A, Lermitte J, Chung F: Systematic review of spinal anaesthesia using bupivacaine for ambulatory knee arthroscopy. Br J Anaesth 2009;102(3):307–315.
- Casati A, Fanelli G: Unilateral spinal anesthesia. State of the art. Minerva Anestesiol 2001;67(12):855–862.
- Schneider MC, Holzgreve W: [100 years ago: Oskar Kreis, a pioneer in spinal obstetric analgesia at the University Women’s Clinic of Basel]. Anaesthesist 2001;50(7):525–528.
- Horlocker TT, Wedel DJ, Rowlingson JC, et al: Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine evidence-based guidelines (third edition). Reg Anesth Pain Med 2010;35(1):64–101.
- Bogin IN, Stulin ID: [Application of the method of 2-dimensional echospondylography for determining landmarks in lumbar punctures]. Zh Nevropatol Psikhiatr Im S S Korsakova 1971;71(12):1810–1811.Perlas A: Evidence for the use of ultrasound in neuraxial nerve blocks. Reg Anesth Pain Med 2010;35(2 Suppl):S43–S46.
- Chin KJ, Karmakar MK, Peng P: Ultrasonography of the adult thoracic and lumbar spine for central neuraxial block. Anesthesiology 2011;114(6):1459–1485.
- Chin KJ, Perlas A: Ultrasonography of the lumbar spine for neuraxial and lumbar plexus nerve blocks. Curr Opin Anaesthesiol 2011;24(5):567–572.
- Chin KJ, Perlas A, Singh M, et al: An ultrasound-assisted approach facilitates spinal anesthesia for total joint arthroplasty. Can J Anaesth 2009;56(9):643–650.
- O’Donnell D, Prasad A, Perlas A: Ultrasound-assisted spinal anesthesia in obese patients. Can J Anaesth 2009;56(12):982–983.
- Chin KJ, Chan VW, Ramlogan R, Perlas A: Real-time ultrasound-guided spinal anesthesia in patients with a challenging spinal anatomy: Two case reports. Acta Anaesthesiol Scand 2010;54(2):252–255.
- Chin KJ, Perlas A, Chan V, Brown-Shreves D, Koshkin A, Vaishnav V: Ultrasound imaging facilitates spinal anesthesia in adults with difficult surface anatomic landmarks. Anesthesiology 2011;115(1):94–101.
- Margarido CB, Arzola C, Balki M, Carvalho JC: Anesthesiologists’ learning curves for ultrasound assessment of the lumbar spine. Can J Anaesth 2010;57(2):120–126.
- Halpern SH, Banerjee A, Stocche R, Glanc P: The use of ultrasound for lumbar spinous process identification: A pilot study. Can J Anaesth 2010;57(9):817–822.
- Carvalho JC: Ultrasound-facilitated epidurals and spinals in obstetrics. Anesthesiol Clin 2008;26(1):145–158, vii–viii.
- Bron JL, van Royen BJ, Wuisman PI: The clinical significance of lumbosacral transitional anomalies. Acta Orthop Belg 2007;73(6):687–695.
- Spivak H, Nudelman I, Fuco V, et al: Laparoscopic extraperitoneal inguinal hernia repair with spinal anesthesia and nitrous oxide insufflation. Surg Endosc 1999;13(10):1026–1029.
- Schmidt J, Carbajo MA, Lampert R, Zirngibl H: Laparoscopic intraperitoneal onlay polytetrafluoroethylene mesh repair (IPOM) for inguinal hernia during spinal anesthesia in patients with severe medical conditions. Surg Laparosc Endosc Percutan Tech 2001;11(1):34–37.
- Vaghadia H, Viskari D, Mitchell GW, Berrill A: Selective spinal anesthesia for outpatient laparoscopy. I: Characteristics of three hypobaric solutions. Can J Anaesth 2001;48(3):256–260.
- Tzovaras G, Zacharoulis D, Georgopoulou S, Pratsas K, Stamatiou G, Hatzitheofilou C: Laparoscopic ventral hernia repair under spinal anesthesia: A feasibility study. Am J Surg 2008;196(2):191–194.
- Chilvers CR, Vaghadia H, Mitchell GW, Merrick PM: Small-dose hypobaric lidocaine-fentanyl spinal anesthesia for short duration outpatient laparoscopy. II. Optimal fentanyl dose. Anesth Analg 1997; 84(1):65–70.
- Ghodki PS, Sardesai SP, Thombre SK: Evaluation of the effect of intrathecal clonidine to decrease shoulder tip pain in laparoscopy under spinal anaesthesia. Indian J Anaesth 2010;54(3):231–234.
- Jonnesco T. Remarks ON GENERAL SPINAL ANALGESIA. Br Med J 1909;2(2550):1396–1401.
- Lee AJ, Ranasinghe JS, Chehade JM, Arheart K, Saltzman BS, Penning DH, et al: Ultrasound assessment of the vertebral level of the intercristal line in pregnancy. Anesth Analg 2011;113(3):559–564.