Adam K. Jacob, Sandra L. Kopp, and James R. Hebl
INTRODUCTION
Preexisting neurologic disease of the peripheral nervous system, central nervous system, and spinal canal present a unique challenge to both patients and anesthesiologists who desire to use regional anesthesia techniques. Because each of these clinical conditions involves compromise to neural structures, the concern is that further insult from surgical (eg, intraoperative stretch or compression, tourniquet ischemia, hemorrhage) or anesthetic (eg, mechanical trauma, vasoconstrictor-induced ischemia, local anesthetic toxicity) causes may result in new or worsening postoperative neurologic deficits.
Regardless of the underlying etiology, the presence of chronic neural compromise secondary to mechanical (eg, spinal stenosis or compressive radiculopathy), ischemic (eg, peripheral vascular disease), toxic (eg, vincristine or cisplatin chemotherapy), metabolic (eg, diabetes mellitus), or autoimmune (eg, multiple sclerosis) derangements may place patients at increased risk of further neurologic injury. Upton and McComas were the first to describe the “double-crush phenomenon,” which suggests that patients with preexisting neural compromise may be more susceptible to injury at another site when exposed to a secondary insult (Figure 1). Secondary insults may include a variety of acute surgical or anesthetic risk factors, including those of regional anesthetic techniques. Osterman emphasized that not only are two low-grade insults along a peripheral nerve trunk worse than just one at a single site, but that the damage of the dual injury far exceeds the expected additive damage caused by each isolated insult. It may be further postulated that the second insult need not be along the peripheral nerve trunk itself, but rather at any point along the neural transmission pathway. Therefore, the performance of peripheral or neuraxial regional techniques in patients with preexisting neurologic disorders may place them at increased risk of the double-crush phenomenon.
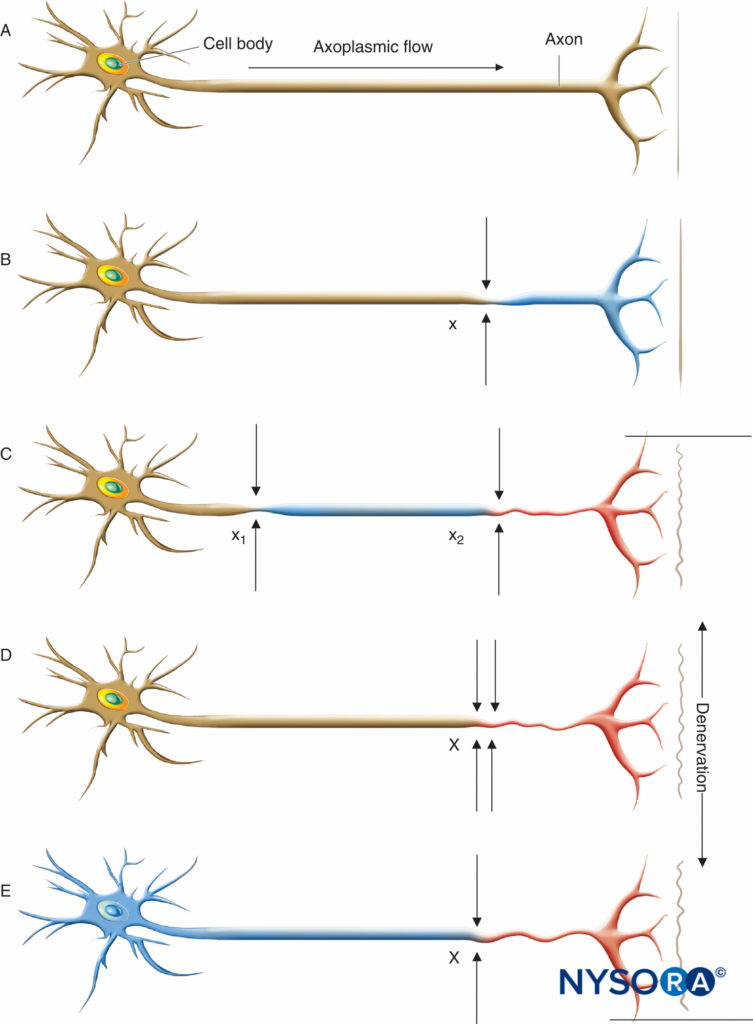
FIGURE 1. Neural lesions resulting in denervation. Axoplasmic flow is indicated by the degree of shading. Complete loss of axoplasmic flow results in denervation (C, D, E). A: Normal neuron. B: Mild neuronal injury at a single site (x) is insufficient to cause denervation distal to the insult. C: Mild neuronal injury at two separate sites (x1 and x2) may cause distal denervation (ie, “double crush”). D: Severe neuronal injury at a single site (X) may also cause distal denervation. E: Axon with a diffuse, preexisting underlying disease process (toxic, metabolic, ischemic) may have impaired axonal flow throughout the neuron, which may or may not be symptomatic but predisposes the axon to distal denervation following a single minor neural insult at x (ie, “double crush”). (Reproduced with permission from Mayo Foundation for Medical Education and Research.)
Unfortunately, the data available regarding any association of pre-existing neurologic disease and post-regional anesthesia dysfunction often conflicting in terms of outcomes and conclusions. As a result, definitive recommendations can rarely be made from the existing scientific literature. However, the following discussion provides a comprehensive review of the available literature on the topic so that patients and clinicians can make an informed decision regarding the potential neurologic risk of performing regional anesthesia in the presence of preexisting neurologic disorders.
PERIPHERAL NERVOUS SYSTEM DISORDERS
The peripheral nervous system is composed of numerous cell types that serve diverse sensory, motor, and autonomic functions. Signs and symptoms of impaired function depend on the distribution and severity of the injury, in addition to the specific element of the nerve that is affected. More than 100 peripheral neuropathies have been identified, each with its own pathophysiology, symptoms, and prognosis.
Hereditary Peripheral Neuropathy
Inherited neuropathies represent a heterogeneous group of diseases that often share the features of insidious onset and indolent course over years to decades. A wide range of genotypes results in phenotypes ranging from mild symptoms and subclinical disease to severe, debilitating conditions. The most common inherited neuropathies are a group of disorders collectively referred to as Charcot–Marie–Tooth (CMT) disease. CMT affects approximately 1 in 2500 people, often beginning in childhood or adolescence. CMT neuropathies are caused from mutations in more than 30 genes responsible for manufacturing neurons or the myelin sheath. Typical signs and symptoms include extreme motor weakness and muscle wasting within the distal lower extremities and feet, gait abnormalities, loss of tendon reflexes, and numbness within the lower limbs. The reported use of peripheral or central regional anesthetic techniques in patients with CMT has been limited to small case series and anecdotal case reports. All patients made uneventful recoveries without worsening of their neurologic conditions. Of note, two cases involving single-injection regional techniques (epidural anesthesia using 18 mL 0.75% ropivacaine and supraclavicular analgesia using 30 mL 0.5% bupivacaine) reported a prolonged effect (12 hours and 30 hours, respectively) of the regional technique compared to the anticipated duration. In both cases, the use of higher local anesthetic concentrations may have contributed to the delayed recovery.
Hereditary neuropathy with liability to pressure palsy (HNPP) is another rare inherited demyelinating peripheral neuropathy in which individuals suffer from repeated motor and sensory neuropathies following brief nerve compression or mild trauma (ie, pressure palsies). First described in the early 1990s, HNPP has been linked to a mutation on the PMP-22 gene resulting in reduced myelin production. Evidence of the use of a regional technique in the setting of HNPP has been limited to a single case report. Lepski and Alderson reported the successful use of an epidural for labor analgesia in a 24-year-old parturient with HNPP. The patient made an uneventful recovery without a worsening of her neurologic condition.
Based upon the lack of clinical evidence, definitive recommendations cannot be made about the safety and use of regional anesthesia in patients with preexisting inherited peripheral neuropathies. However, isolated case reports suggest that peripheral and central regional techniques may be used without worsening a patient’s stable neurologic condition. However, caution should be used to minimize other surgical (eg, tourniquet use) and anesthetic (eg, reduced concentration or dose of local anesthetic) risk factors for perioperative nerve injury when considering the use of regional anesthesia within this patient population.
Acquired Peripheral Neuropathy Diabetic Polyneuropathy
The increasing prevalence of diabetes mellitus (DM) and its associated comorbidities will likely translate to a larger number of diabetic patients presenting for surgery. Despite the clinical benefits and widespread use of regional anesthesia (peripheral and neuraxial block), there remains concern regarding its use in patients with DM. It has been suggested that patients with a history of chronic neural compromise secondary to metabolic conditions such as diabetes may be at an increased risk of worsening neurologic injury following neuraxial or peripheral nerve block.
Diabetes mellitus is currently the most common cause of systemic polyneuropathy. There are several types of neuropathy associated with DM, but distal symmetric sensorimotor polyneuropathy is the most common form and is generally synonymous with the term diabetic polyneuropathy (DPN). The frequency of DPN ranges from 4%–8% at the time of diagnosis to more than 50% in patients with long-standing diabetes. Despite the fact that patients may be asymptomatic, nearly all will have evidence of abnormal nerve conduction. Furthermore, it is relatively common for patients to present for surgery with either undiagnosed diabetes mellitus or known diabetes with uncontrolled hyperglycemia.
The pathophysiology of DPN is poorly understood and likely multifactorial. Early symptoms, such as numbness, pain, and autonomic dysfunction, are caused by damage to small nerve fibers, which occurs before damage to large fibers becomes apparent. There is pathophysiologic evidence of abnormalities in both large and small neural blood vessels, ultimately contributing to multifocal fiber loss. Axonal degeneration is the most prominent feature of DPN and occurs secondary to the reduced delivery of essential nutrients and other components (oxygen, blood, adenosine triphosphate, glucose) to the axon. Proposed mechanisms include (1) sorbitol deposition in the nerve due to glucose accumulation; (2) local tissue ischemia in sensory and autonomic fibers secondary to endoneurial hypoxia; (3) abnormal tissue repair mechanisms caused by excess glucose; and (4) mitochondrial dysfunction within the dorsal root ganglia.
Currently, there is an abundance of animal data that suggests diabetic nerves may have an increased risk of neurologic injury following regional anesthesia compared to nondiabetic nerves. Kalichman and Calcutt were the first to hypothesize that diabetic nerve fibers may be more susceptible to local anesthetic neurotoxicity for two reasons: (1) the nerve is already stressed due to chronic ischemic hypoxia; and (2) the nerves are exposed to larger concentrations of local anesthetics due to decreased perineural blood flow. More recently, these findings have been supported with both animal and clinical data. Lirk and colleagues used Zucker diabetic fatty rats exposed to hyperglycemia to demonstrate that although the overall neuronal survival difference was low, in vitro local anesthetic neurotoxicity was more pronounced in neurons from diabetic animals. The authors also reported that preexisting subclinical neuropathy led to substantial prolongation of the block duration in vivo. Kroin and colleagues also reported that the duration of sciatic nerve block with lidocaine 1% or ropivacaine 0.5% was longer in streptozotocin-induced diabetic rats compared with nondiabetic rats and that block duration correlated with nerve fiber degeneration. In a subsequent study, the same authors also concluded that, with continuous glycemic control, diabetic rats had a block duration that was similar to nondiabetic rats and 40 minutes shorter than in diabetic rats without glycemic control. Interestingly, acute glycemic control did not lessen the nerve block duration, suggesting that diabetic neuropathy is not rapidly reversed within this animal model. Currently, it is unclear whether the results from animal studies using experimentally induced hyperglycemia can be used to make recommendations for patients with long-standing diabetes mellitus.
Although animal studies have consistently found that diabetic nerves are more sensitive to local anesthetics and potentially more susceptible to neural injury, it is unclear whether diabetic patients experience a higher incidence of neurologic injury after regional anesthesia. There are limited clinical data suggesting that the success of peripheral nerve block (supraclavicular brachial plexus) may be higher in diabetic patients independent of other predictors of success (eg, body mass index) compared to nondiabetic patients. Gebhard and colleagues have proposed several theories for this finding, including (1) a higher sensitivity of diabetic nerve fibers to local anesthetics; (2) possible unknown intraneural penetration before injection; and (3) preexisting DPN with accompanying decreased sensation. Preexisting pathology has long been reported to play a role in the development of postoperative neurologic dysfunction. A recent case report described a persistent postoperative femoral neuropathy after discontinuing a femoral nerve catheter in a patient with a preexisting subclinical diabetic neuropathy that had been undiagnosed preoperatively.
In patients with diabetes mellitus, a decreased sensitivity to electrical stimulation combined with diminished sensory function and an increased sensitivity to local anesthetic toxicity may increase the risk of intraneural injection during peripheral nerve block using a peripheral nerve stimulator. Currently, there is a lack of clinical evidence suggesting that the use of ultrasound guidance is safer than peripheral nerve stimulation within the general population. However, this lack of established clinical benefit is less clear for diabetic patients. For example, there are a limited number of animal and clinical studies that suggest that ultrasound guidance may be a more desirable method of neural localization in diabetic patients. Animal studies have shown that low-threshold electrical stimulation may not offer protection from intraneural injection in the presence of hyperglycemia. Rigaud and colleagues demonstrated that all needle insertions within a hyperglycemic dog model resulted in intraneural injection (6/6), whereas only one (1/18) intraneural injection occurred in control dogs. Sites and colleagues also concluded that ultrasound guidance may be a preferred method of neural localization in diabetic patients after failing to elicit a motor response or paresthesia in two patients undergoing sciatic nerve block using peripheral nerve stimulation. The authors described a very weak motor response in both diabetic patients with a stimulating current of more than 2.4 mA despite perineural placement of the stimulating needle using ultrasound guidance. Another potential application of ultrasound technology is the ability to use the cross-sectional area of a peripheral nerve to identify a clinical or subclinical peripheral neuropathy: a diagnosis that historically would have required complex nerve conduction studies.
Findings of spinal cord involvement in diabetic patients suggest that the same or similar mechanism of injury may affect not only peripheral nerves but neural elements within the central neuraxis as well. Using magnetic resonance imaging, Selvarahah and colleagues described early central nervous system involvement consisting of a significant reduction in the spinal cord cross-sectional area in patients with both subclinical and clinically detectable diabetic peripheral neuropathy. A case report of a diabetic patient experiencing a persistent lower extremity neuropathy after what appeared to be uneventful epidural analgesia reinforces concerns that diabetic patients may be at an increased risk of neurologic injury following neuraxial anesthesia. A retrospective review also evaluated neurologic complications in patients with preexisting peripheral sensorimotor neuropathy or diabetic polyneuropathy who subsequently underwent neuraxial anesthesia or analgesia. Of the 567 patients studied, two (0.4%; 95% CI 0.1%–1.3%) experienced new or progressive postoperative neurologic deficits when compared to preoperative findings. The authors concluded that although the risk of severe postoperative neurologic injury among diabetic patients is rare, it appears to be higher than that reported in the general population. Although the neuraxial technique could not be definitively implicated as the primary cause of the neurologic insult, it may have been a contributing factor among patients with preexisting neural compromise.
In summary, patients with DPN likely have neural elements that are more sensitive to the effects of local anesthetic. As a result, diabetic peripheral nerves may be more susceptible to subsequent injury from local anesthetic toxicity or ischemic insults. Ultimately, the decision to use regional anesthesia in diabetic patients should be made on an individual basis after a thorough discussion with the patient regarding the potential risks and benefits of the technique. Consideration should be given to decreasing the concentration or total dose of local anesthetic for both peripheral and neuraxial techniques—particularly in profoundly symptomatic patients. Furthermore, the use of ultrasound guidance may facilitate perineural needle placement and the use of lower local anesthetic volumes in diabetic patients, although definitive data ensuring increased safety with ultrasound guidance are currently lacking. Decreasing the concentration or dose of local anesthetic and eliminating epinephrine additives should also be considered given that diabetic nerves are already at risk of neural ischemia and infarction due to changes within the endoneural microvasculature.
Chemotherapy-Induced Neuropathy
Chemotherapy-induced peripheral neuropathy (CIPN) is a frequent side effect of several commonly used chemotherapeutic agents. It is a dose-limiting side effect that occurs in approximately 30%–40% of patients. The exact mechanism of injury is unclear, although damage to microtubules, interference with microtubule-based axonal transport, mitochondrial disruption, and cytotoxic effects on DNA are all possible mechanisms. The degree of neurotoxicity depends on the agent used, the duration of administration, and the cumulative dose received. Cisplatin, oxaliplatin, and carboplatin characteristically induce a pure sensory, painful peripheral neuropathy, whereas vincristine, paclitaxel, and suramin tend to induce a mixed sensorimotor neuropathy with or without involvement of the autonomic nervous system. Symptoms are often in the “glove-and-stocking” distribution and consist of pain or paresthesias. Patients at risk of developing CIPN include those with preexisting neural damage secondary to diabetes mellitus, excessive alcohol use, or inherited peripheral neuropathy. In general, a prolonged period of regeneration is required to restore neurologic function with incomplete recovery being the most common outcome. However, patients who recover from CIPN have an increased risk of developing progressive neuropathic symptoms if exposed to additional neurotoxic agents. Local anesthetics are potentially neurotoxic, and caution should be used when deciding whether to perform regional anesthesia in patients who have received chemotherapeutic agents known to cause CIPN. It is common for patients to have a subclinical neuropathy that presents only following a second neurologic insult, such as a peripheral or neuraxial block.
Entrapment Neuropathy
Entrapment neuropathy, one of the most prevalent disorders of the peripheral nervous system, occurs when a single nerve is chronically compressed or mechanically injured at a specific location. Ulnar nerve entrapment at the elbow, referred to as “cubital entrapment syndrome,” is the second most frequent upper extremity compression neuropathy. The ulnar nerve is at increased risk because of its superficial location in the region of the medial elbow. Injury to the nerve may occur as a result of acute trauma, compression, repetitive traction, subluxation of the nerve, osteoarthritis, or gout or following an upper extremity surgical procedure. Initial symptoms include hyperesthesia in the ulnar nerve distribution, elbow pain, and paresthesias in the ring and small fingers. These symptoms are often intermit-tent and may progress over the course of months to years. In the later stages of the disease, weakness of the intrinsic muscles of the hand with or without visible atrophy may be observed. At present, the most common practice is to conservatively treat patients with mild symptoms without weakness or atrophy, whereas surgery is indicated for patients who do not improve after conservative management or present with severe neurological signs and symptoms (eg, persistent paraesthesia, objective weakness, muscular atrophy).
General, regional, or local anesthesia can be used for surgical decompression of an entrapped ulnar nerve. The choice of anesthetic depends on the surgical procedure, whether nerve function will be tested intraoperatively, and the extent of damage accompanying the nerve injury. In a 2001 cohort study of 360 patients with preexisting ulnar neuropathy undergoing ulnar nerve transposition, Hebl and colleagues found that anesthetic technique (general anesthesia versus regional anesthesia) did not affect the neurologic outcome immediately after surgery or two to six weeks postoperatively. A preoperative discussion with the surgeon to determine the intraoperative plan and address specific concerns related to the patient’s disease process will assist the anesthesiologist in choosing the most appropriate anesthetic technique.
Inflammatory Neuropathy Guillain–Barré Syndrome
Guillain–Barré syndrome (GBS) is an acute, inflammatory demyelinating polyneuropathy characterized by areflexia and diffuse ascending neuromuscular paralysis. The etiology of GBS is unclear, although infection, pregnancy, vaccinations, immunosuppression, systemic illnesses, and transfusion have all been proposed as potential triggers. The degree and distribution of paralysis is variable and can include sensory nerve, cranial nerve, and autonomic nervous system involvement. Symptoms peak approximately two to four weeks after the initial onset with most patients experiencing a prolonged recovery. Unfortunately, many patients experience moderate to severe neurological impairment for years after the initial diagnosis.
There are several reports of GBS occurring in the postoperative period following a variety of surgical procedures using various types of anesthetic. However, case reports of regional anesthesia use in patients with GBS are generally limited to the obstetric population. Some patients with GBS may have autonomic instability and will subsequently experience an exaggerated response to neuraxial block, whereas other GBS patients will exhibit a normal response to neuraxial anesthesia. Although there have been reports of successful neuraxial anesthesia in parturients with GBS, the potential for local anesthetics to interact with peripheral myelin or cause direct nerve trauma cannot be ignored. There is some evidence to suggest that epidural anesthesia may precipitate or reactivate GBS hours to weeks following surgery. However, it is difficult to determine if this is due to the effects of the epidural, the natural progression of the disease, the surgical procedure, or the stress response related to surgery. Although it has been suggested that acute neuronal inflammation may be a relative contraindication to regional anesthesia, existing data provide little information regarding the safety of neuraxial anesthesia or peripheral nerve block in patients with GBS. Ultimately, the decision to perform regional anesthesia should be made on an individual basis after a thorough discussion with the patient regarding the potential risks and benefits.
Postsurgical Inflammatory Neuropathy
Postsurgical inflammatory neuropathy (PSIN) is a recently described autoimmune or inflammatory process that may be the cause of severe postoperative neurologic deficits. Staff and colleagues recently described a series of 33 patients who developed PSIN within 30 days of surgery. The diagnosis was confirmed in most patients following a peripheral nerve biopsy. PSIN is believed to be an idiopathic, immune-mediated response to a physiologic stress such as an infectious process, a vaccination, or a surgical procedure. The condition may present as focal, multifocal, or diffuse neurologic deficits in the setting of negative radiographic imaging. Complicating the diagnosis, the onset of neurologic deficits may not be apparent during the immediate postoperative period, and the deficits may be in an anatomic distribution remote from the surgical site or regional anesthetic technique. Risk factors or potential triggers for PSIN include malignancy, diabetes mellitus, tobacco use, systemic infection, volatile anesthetic use, and recent blood transfusion. Suppression of the immune response with prolonged high-dose corticosteroids or intravenous immunoglobulin is the current treatment of choice. The goal of treatment is to sufficiently blunt the inflammatory response to allow for axonal regeneration. Fortunately, most patients improve with current treatment recommendations, with pain and sensory deficits improving before the resolution of motor deficits.
The degree to which inflammatory mechanisms play a role in postoperative neurologic dysfunction is unknown and poorly characterized, particularly within the anesthesia literature. As a result, anesthesia providers and surgeons rarely consider this potential etiology of nerve injury when evaluating patients with postoperative deficits. This is problematic, as the common approach of watchful waiting and conservative management will not be effective in patients with PSIN. Rather, PSIN is a clinical condition that must be suspected early in the disease process so that a definitive diagnosis can be obtained (via nerve biopsy) and aggressive immunotherapy initiated to attempt to improve neurologic outcome.
CENTRAL NERVOUS SYSTEM DISORDERS
Historically, neuraxial anesthesia techniques have not been offered to patients with preexisting neurologic disorders of the central nervous system (eg, multiple sclerosis, postpolio syndrome, amyotrophic lateral sclerosis) for fear of worsening neurologic outcome. In fact, many historians believe that the recommendation of Dripps and Vandam in 1956 to avoid regional anesthesia in patients with preexisting neurologic disorders has impacted clinical management for nearly half a century. Several theoretical mechanisms have been proposed based upon the double-crush phenomenon, including neurologic injury from needle or catheter-induced trauma, local anesthetic neurotoxicity, and neural ischemia due to local anesthetic additives. However, the avoidance of regional anesthesia within this patient population may also be due to physician and patient biases or potential medicolegal concerns. There are several confounding factors (age, body habitus, surgical trauma, tourniquet times and pressures, positioning, anesthetic technique) that make it difficult to determine the etiology of worsening postoperative neurologic deficits.
A recent review evaluated 139 patients with a history of one or more central nervous system disorders who subsequently underwent a neuraxial anesthetic technique. Preoperative neurologic disorders primarily included postpolio syndrome (PPS), multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS), and chronic spinal cord injury (SCI). In contrast to the findings of Vandam and Dripps several decades ago, the authors identified no new or worsening postoperative neurologic deficits (0.0%; 95% CI, 0.0%–0.3%) within their patient cohort. This was despite the fact that 74% of the patients reported active neurologic symptoms (paresthesias, dysesthesias, hyperreflexia) or sensorimotor deficits during the immediate preoperative period and subsequently received standard doses of local anesthetics. Two smaller reviews in parturients receiving smaller doses of local anesthetic for labor analgesia have reported similar results.
Clearly, further investigations with a larger number of patients are needed to make definitive recommendations. However, the current data suggest that the decision to perform neuraxial anesthesia in patients with preexisting central nervous system disorders be based on the risks and benefits for each individual patient. Some authors have postulated that the neurologic risk may be higher in patients who have progressive neurologic deficits compared to those with chronic, stable sensorimotor symptoms that have not changed over the course of several months or years.
Multiple Sclerosis
Multiple sclerosis is an inflammatory autoimmune disorder of the central nervous system with a lifetime risk of 1 in 400, making it the most common debilitating neurologic disease in young adults. It is a chronic, degenerative disease characterized by focal demyelination within the spinal cord and brain. This demyelination results in a fluctuating conduction block that causes a classic “waxing and waning” of symptoms that is characteristic of the disease. Signs and symptoms include sen-sory or motor deficits, diplopia or vision loss, bowel or bladder dysfunction, and ataxia. The precise etiology is unclear; however, a combination of genetic risk factors and environmental factors likely play a role. Twenty-five percent of MS patients are essentially asymptomatic, and their activities of daily living are unaffected. However, up to 15% of patients may become severely disabled with significant sensorimotor deficits within a short period of time. Several factors common to surgery can negatively impact the disease process, including hyperpyrexia, emotional stress, and infection. The mechanism of worsening neurologic function in patients with MS is unclear and may occur coincidentally within the postoperative period independent of anesthetic technique. Evidence regarding the risk of regional anesthesia in patients with MS is limited. Despite some evidence for demyelination of the peripheral nerves in MS, peripheral nerve block has traditionally been considered safe. However, a recent report of severe brachial plexopathy following an ultrasound-guided interscalene block has raised the concern that a segment of MS patients may have subclinical peripheral neuropathy. Several investigators have demonstrated evidence of axonal demyelinating peripheral lesions (sensory more than motor) in patients with MS. Misawa and colleagues demonstrated that peripheral demyelination may occur in 5% of MS patients, whereas Pogorzelski and colleagues have reported peripheral demyelination may occur in up to 47% of patients. Similarly, Sarova-Pinhas and colleagues have described nerve conduction abnormalities in up to 14.7% of peripheral nerves within MS patients compared to only 2.4% of nerves within the general population. Despite this evidence, the overall incidence and clinical relevance of this underlying peripheral neuropathy remains undefined in the setting of performing peripheral nerve block in patients with MS.
In contrast to peripheral nerve block, the potential risk of new or progressive neurologic deficits in MS patients after spinal anesthesia was first described in 1937. Critchley and colleagues described three patients with [disseminated (multiple) sclerosis] who experienced a worsening of symptoms after spinal anesthesia. The authors concluded that [spinal anesthesia may be a precipitating agent in the evolution of disseminated (multiple) sclerosis.] Several subsequent studies demonstrated similar outcomes with the development of new or worsening neurologic deficits or a higher likelihood of symptom exacerbation after spinal anesthesia. In contrast, a more recent study demonstrated no new or worsening neurologic symptoms after spinal anesthesia in 35 MS patients undergoing a variety of surgical procedures.
The safety of epidural anesthesia and analgesia in MS patients has been focused almost exclusively within the obstetric population, which may not accurately represent the non-pregnant MS patient. Pregnancy is frequently associated with a decrease in disease relapse, whereas the postpartum period is often associated with an increased risk of relapse. The transition from cellular immunity to humoral immunity required for the mother’s immune system to tolerate the fetus is thought to be protective during pregnancy. However, as cellmediated immunity rebounds during the postpartum period, patients will often experience a transient worsening of neurologic symptoms that could be falsely attributed to recent regional anesthetic techniques.
Confavreux and colleagues have performed one of the few prospective studies evaluating risk factors associated with disease relapse during the postpartum period. They concluded that epidural analgesia during labor and delivery did not contribute to a higher risk of relapse compared to patients who did not receive neuraxial techniques. Similarly, Kuczkowski found no association between any form of obstetric regional analgesia and the worsening of MS symptoms among obstetric patients. Epidural anesthesia and analgesia have traditionally been recommended over spinal anesthesia in MS patients because the concentration of local anesthetic in the white matter of the spinal cord is one-fourth the level after epidural injection compared to intrathecal injection. It is believed that the lack of myelin may leave the spinal cord susceptible to the neurotoxic effects of local anesthetics. Although definitive studies on the pharmacological effects of local anesthetic concentrations and doses are lacking, many recommend limiting neuraxial local anesthetic doses and concentrations to the lowest level possible. There is some evidence that lidocaine can reversibly worsen symptoms of MS by blocking sodium channels in demyelinated areas enough to cause symptoms compared to healthy myelinated areas that remain unaffected. With regard to the obstetric patient, the risk of neuraxial anesthesia or analgesia needs to be weighed against the increased risk of general anesthesia. A recent survey from the United Kingdom demonstrated that 90% of obstetric anesthesiologists would perform spinal anesthesia for an emergency cesarean section in an MS patient after carefully weighing the potential risks and benefits.
In summary, there remains little conclusive evidence to support or refute the use of regional anesthesia in patients with MS. Peripheral nerve block has not been definitively shown to be harmful in the setting of MS, and therefore MS should not be considered an absolute contraindication to this regional technique. In contrast, given that demyelinated fibers may be more prone to the toxic effects of local anesthetics, epidural anesthesia and analgesia may be considered safer than spinal techniques. However, reducing the local anesthetic concentration and total dose to the lowest effective levels may be prudent for both peripheral and neuraxial block. All decisions regarding the use of regional anesthesia and analgesia in patients with MS need to be made after careful consideration of the potential risks and benefits. Regardless of the anesthetic technique chosen, patients should be informed of the risk of new or worsening neurologic symptoms during the postoperative period because of exposure to multiple exacerbating factors.
Postpolio Syndrome
Postpolio syndrome refers to new-onset neurologic symptoms that develop several years after an acute poliomyelitis infection. The onset of new or progressive symptoms may occur up to 30 years after the initial episode of poliomyelitis. PPS affects anterior horn cells within the anterior portion of the spinal cord and is therefore considered a lower motor neuron disorder. Initial symptoms include muscle weakness, fatigue, gait instability, joint pain, and muscle atrophy within muscle groups that were previously affected by the disease. Sensory deficits are generally not characteristic of the syndrome and are only observed if a secondary disorder is present (eg, compressive radiculopathy or disk herniation). The motor effects of PPS are thought to be related to an ongoing process of denervation and reinnervation that ultimately ends when denervation is no longer compensated for by reinnervation.
Postpolio syndrome is the most prevalent motor neuron disease in North America. Furthermore, because acute polio-myelitis continues to occur in developing countries, PPS will likely remain an anesthetic concern for years to come. It is not uncommon for patients with PPS to require orthopedic procedures; therefore, it is important to determine the safety of regional anesthetic techniques under these clinical circumstances. Although patients with PPS have fewer motor neurons than normal, it is difficult to know whether the remaining motor neurons are more susceptible to the toxic effects of local anesthetics. There have been no reports of worsening neurologic status following neuraxial anesthesia with normal doses of tetracaine and bupivacaine in patients with PPS. However, this finding does not imply that regional anesthetic techniques are without risk. As with all patients, the potential risk of regional anesthesia must be balanced against the disadvantages of general anesthesia, including a hypersensitivity to sedative or opioid medications, the risk of muscle relaxant use, and the risk of hypoventilation and aspiration. The largest series of patients with PPS (n = 79) undergoing neuraxial anesthesia or analgesia demonstrated no worsening of neurologic symptoms during the postoperative period. However, the paucity of clinical data on this topic prevents clear recommendations from being made regarding the safety of neuraxial anesthesia or peripheral nerve block in patients with PPS. Ultimately, the decision to use regional anesthesia should be made on an individual basis after a thorough discussion of the potential risks and benefits with each patient. Given the increased sensitivity to opioid and sedative medications within this patient subgroup, these medications should always be used with caution.
Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis is a progressive degenerative disease of upper and lower motor neurons. The cause is unknown, but theories include glutamate excitotoxicity, oxidative stress, mitochondrial dysfunction, paraneoplastic tumors, autoimmune disease, and viral infection. Initially, ALS presents as atrophy, weakness, and fasciculations in the intrinsic hand muscles. As it progresses, atrophy and weakness develop in all skeletal muscles including those of the tongue, pharynx, larynx, and respiratory muscles of the chest. Patients lose the ability to cough, increasing the risk of aspiration. Autonomic dysfunction may be evident and is manifested by orthostatic hypotension and an increased resting heart rate. Unfortunately, in the majority of patients, death from respiratory failure occurs within a few years of disease onset.
The existing evidence, albeit limited, has not supported the fear that neuraxial or peripheral block will exacerbate preexisting symptoms in ALS patients. However, given the potential for worsening respiratory failure following general anesthesia due to the use of muscle relaxants and opioid medications, the ability to avoid airway manipulation may be considered a benefit within this high-risk patient population. Regardless of anesthetic technique, the possibility of postoperative respiratory or neurologic deterioration is quite high in patients with ALS. Ultimately, the decision to use regional anesthesia should be made on an individual basis after a thorough discussion of the potential risks and benefits with each patient.
Spinal Stenosis and Lumbar Disk Disease
Spinal canal pathology has been proposed as a potential risk factor for neurologic complications following neuraxial block-ade. Several mechanisms of injury have been proposed, including an ischemic or compressive effect after the injection of large volumes of local anesthetic into a relatively confined space (ie, epidural anesthesia) and local anesthetic neurotoxicity (ie, spinal anesthesia). Although the precise mechanism(s) of injury remain unclear, there are several isolated case reports and large case series that are believed to support these hypotheses.
Spinal stenosis occurs as age-related changes within the inter-vertebral disks and facet joints resulting in a narrowing of the spinal canal or neural foramina. Changes include disk degeneration, facet joint hypertrophy, osteophyte formation, and an infolding of the ligamentum flavum. The precise mechanism by which spinal nerve root compression results in signs or symptoms of spinal stenosis is not completely understood. Classic symptoms include back and leg radicular pain that significantly worsens with extension and is alleviated with flexion. Preexisting spinal stenosis or compressive lumbar disk disease has been proposed as a potential risk factor for neurologic complications following a neuraxial (spinal or epidural) technique. Proposed mechanisms of injury include mechanical trauma, local anesthetic neurotoxicity, ischemia, or a multifactorial etiology. Pathophysiologically, patients with spinal stenosis have a reduction in the diameter of the spinal canal resulting in less anatomic space for fluid collections such as blood or local anesthetic. As a result, small quantities of fluid may result in significant increases in pressure around the neuraxis that would have no clinical effect in a widely patent spinal canal.
Two relatively large case series and several case reports have been published that suggest undiagnosed spinal stenosis may be a risk factor for neurologic complications following neuraxial block. The majority of cauda equina cases involved epidural analgesia, which may suggest an ischemic etiology (mechanical compression of the cord by the infusing local anesthetic) to the injury. Hebl and colleagues performed a retrospective review of 937 patients with preexisting spinal stenosis or lumbar disk disease with and without a history of prior spinal surgery and concluded that this cohort of patients were at an increased risk for the development or worsening of neurologic deficits when compared to the general population undergoing a neuraxial technique. In addition, patients with more than one neurologic diagnosis (eg, spinal stenosis, compressive radiculopathy, preexisting peripheral neuropathy) appeared to have an even higher risk of injury. Similarly, Moen and colleagues performed a large epidemiologic survey in Sweden that revealed similar trends. During a 10-year study period, 1,260,000 spinal anesthetics and 450,000 epidural blocks were evaluated. Overall, the authors identified 127 serious complications, including 85 (67%) patients with per-manent injuries. Although 14 patients had preexisting spinal stenosis, 13 (93%) of these were diagnosed in the postoperative period during the evaluation of the neurologic deficit. The authors concluded that the incidence of severe anesthesia-related complications may not be as low as previously reported and that preexisting spinal canal pathology may be a “neglected risk factor.” Finally, although patients with prior spine surgery may have an increased risk of paraplegia following transforaminal epidural steroid injections, no similar risk has been found in patients after neuraxial anesthesia or analgesia.
In summary, although it appears that patients with spinal stenosis or compressive lumbar disk disease may be at increased risk of neurologic complications following neuraxial block, the existing literature fails to provide a direct comparison of surgical patients with similar spinal pathology undergoing gen-eral anesthesia. Therefore, it is unclear whether the higher incidence of neurologic complications in this patient population is due to surgical factors, anesthetic technique, the natural progression of disease process, or a combination of these factors.
Spinal Cord Injury
Spinal cord injury affects over 10,000 Americans each year. Of these, approximately 50% of injuries occur at the cervical level. Most SCI cases are secondary to motor vehicle accidents, with a smaller percentage resulting from sports injuries, falls, or penetrating trauma. The ratio of complete to incomplete neurologic deficits in the United States appears to be decreasing over the past decade, reflecting a greater proportion of incomplete deficits. A potentially dangerous condition that may develop in the month(s) after the resolution of acute spinal shock is autonomic dysreflexia (AD). AD is a life-threatening syndrome resulting from cutaneous or visceral stimulation below the level of the spinal cord injury, leading to extreme vascular instability. The lifetime prevalence of AD has been estimated to range from 17% to 70%, with most episodes occurring in SCI cases if the level of injury is at or above T6.
General anesthesia with low-concentration volatile anesthetic does not offer protection against AD. Although higher concentrations of volatile anesthetic may be effective, anesthesia-related hemodynamic instability may not be well tolerated within this patient population. Therefore, neuraxial (spinal or epidural) regional anesthesia techniques can be valuable adjuncts in the management of patients with chronic SCI undergoing lower extremity, abdominal, obstetric, gynecologic, and urologic procedures. Numerous case reports and case series have demonstrated that neuraxial techniques are safe and effective in preventing episodes of AD in SCI patients, even those with high cord lesions. At this point, there is no clear evidence to suggest that use of regional techniques may potentially worsen preexisting neurologic deficits in patients with SCI. However, difficulty in determining appropriate anesthetic level, the potential for hemodynamic instability and respiratory difficulty, and challenging block placement are important considerations when evaluating patients with SCI for a neuraxial technique.
SUMMARY
Patients with preexisting neurologic disease present a unique challenge to the anesthesiologist who is contemplating a regional anesthetic technique. A thorough preoperative assessment is vital in order to establish the patient’s baseline neurologic status. Anesthesia providers should be aware of the risk factors for postoperative neurologic complications during their selection of suitable candidates for a central or peripheral block and adapt their technique to minimize these risks as much as possible. While most preexisting neurologic disorders are not absolute contraindications to regional anesthesia, the decision to proceed with a regional technique should be made on an individual, case-by-case basis as select patients may ben-efit from a regional anesthetic technique compared to other anesthetic or analgesic options.
REFERENCES
- Upton AR, McComas AJ: The double crush in nerve entrapment syndromes. Lancet 1973;2:359–362.
- Osterman AL: The double crush syndrome. Orthop Clin North Am 1988; 19:147–155.
- Neal JM, Bernards CM, Hadzic A, et al: ASRA practice advisory on neurologic complications in regional anesthesia and pain medicine. Reg Anesth Pain Med 2008;33:404–415.
- Jacob AK, Kopp, SL: Regional anesthesia in the patient with preexisting neurologic disorders. Advances in Anesthesia 2011;29:1–18.
- Skre H: Genetic and clinical aspects of Charcot-Marie-Tooth’s disease. Clin Genet 1974;6:98–118.
- Saporta AS, Sottile SL, Miller LJ, Feely SM, Siskind CE, Shy ME: Charcot-Marie-Tooth disease subtypes and genetic testing strategies. Ann Neurol 2011;69:22–33.
- Bui AH, Marco AP: Peripheral nerve block in a patient with Charcot-Marie-Tooth disease. Can J Anaesth 2008;55:718–719.
- Dhir S, Balasubramanian S, Ross D: Ultrasound-guided peripheral regional block in patients with Charcot-Marie-Tooth disease: a review of three cases. Can J Anaesth 2008;55:515–520.
- Fernandez Perez AB, Quesada Garcia C, Rodriguez Gonzalez O, Besada Estevez JC: [Obstetric epidural analgesia, a safe choice in a patient with Charcot-Marie-Tooth disease]. Rev Esp Anestesiol Reanim 2011;58: 255–256.
- Schmitt HJ, Muenster T, Schmidt J: Central neural block in Charcot-Marie-Tooth disease. Can J Anaesth 2004;51:1049–1050.
- Sugai K, Sugai Y: [Epidural anesthesia for a patient with Charcot-Marie-Tooth disease, bronchial asthma and hypothyroidism]. Masui 1989;38: 688–691.
- Tanaka S, Tsuchida H, Namiki A: [Epidural anesthesia for a patient with Charcot-Marie-Tooth disease, mitral valve prolapse syndrome and IInd degree AV block]. Masui 1994;43:931–933.
- Lepski GR, Alderson JD: Epidural analgesia in labour for a patient with hereditary neuropathy with liability to pressure palsy. Int J Obstet Anesth 2001;10:198–201.
- Al-Nasser B: Toxic effects of epidural analgesia with ropivacaine 0.2% in a diabetic patient. J Clin Anesth 2004;16:220–223.
- Blumenthal S, Borgeat A, Maurer K, et al: Preexisting subclinical neuropathy as a risk factor for nerve injury after continuous ropivacaine administration through a femoral nerve catheter. Anesthesiology 2006; 105:1053–1056.
- Horlocker TT, O’Driscoll SW, Dinapoli RP: Recurring brachial plexus neuropathy in a diabetic patient after shoulder surgery and continuous interscalene block. Anesth Analg 2000;91:688–690.
- Waters JH, Watson TB, Ward MG: Conus medullaris injury following both tetracaine and lidocaine spinal anesthesia. J Clin Anesth 1996;8: 656–658.
- Kalichman MW, Calcutt NA: Local anesthetic-induced conduction block and nerve fiber injury in streptozotocin-diabetic rats. Anesthesiology 1992;77:941–947.
- Dyck PJ, Kratz KM, Karnes JL, et al: The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: the Rochester Diabetic Neuropathy Study. Neurology 1993;43:817–824.
- Ross MA: Neuropathies associated with diabetes. Med Clin North Am 1993;77:111–124.
- Centers for Disease Control and Prevention: National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2011.
- Lirk P, Birmingham B, Hogan Q: Regional anesthesia in patients with preexisting neuropathy. Int Anesthesiol Clin 2011;49:144–165.
- Hebl JR, Kopp SL, Schroeder DR, Horlocker TT: Neurologic complications after neuraxial anesthesia or analgesia in patients with preexisting peripheral sensorimotor neuropathy or diabetic polyneuropathy. Anesth Analg 2006;103:1294–1299.
- Krishnan AV, Kiernan MC: Altered nerve excitability properties in established diabetic neuropathy. Brain 2005;128:1178–1187.
- Sinnreich M, Taylor BV, Dyck PJ: Diabetic neuropathies. Classification, clinical features, and pathophysiological basis. Neurologist 2005;11: 63–79.
- Williams BA, Murinson BB, Grable BR, Orebaugh SL: Future considerations for pharmacologic adjuvants in single-injection peripheral nerve blocks for patients with diabetes mellitus. Reg Anesth Pain Med 2009;34:445–457.
- Kroin JS, Buvanendran A, Tuman KJ, Kerns JM: Safety of local anesthetics administered intrathecally in diabetic rats. Pain Med 2012; 13:802–807.
- Kroin JS, Buvanendran A, Williams DK, et al: Local anesthetic sciatic nerve block and nerve fiber damage in diabetic rats. Reg Anesth Pain Med 2010;35:343–350.
- Williams BA: Toward a potential paradigm shift for the clinical care of diabetic patients requiring perineural analgesia: strategies for using the diabetic rodent model. Reg Anesth Pain Med 2010;35:329–332.
- Lirk P, Flatz M, Haller I, et al. In Zucker diabetic fatty rats, subclinical diabetic neuropathy increases in vivo lidocaine block duration but not in vitro neurotoxicity. Reg Anesth Pain Med 2012;37:601–606.
- Williams BA, Murinson BB: Diabetes mellitus and subclinical neuropathy: a call for new paths in peripheral nerve block research. Anesthesiology 2008;109:361–362.
- Gebhard RE, Nielsen KC, Pietrobon R, Missair A, Williams BA: Diabetes mellitus, independent of body mass index, is associated with a “higher success” rate for supraclavicular brachial plexus blocks. Reg Anesth Pain Med 2009;34:404–407.
- Alvine FG, Schurrer ME: Postoperative ulnar-nerve palsy. Are there predisposing factors? J Bone Joint Surg Am 1987;69:255–259.
- Chaudhry V, Glass JD, Griffin JW: Wallerian degeneration in peripheral nerve disease. Neurol Clin 1992;10:613–627.
- Selander D, Edshage S, Wolff T: Paresthesiae or no paresthesiae? Nerve lesions after axillary blocks. Acta Anaesthesiol Scand 1979;23: 27–33.
- Bigeleisen PE: Nerve puncture and apparent intraneural injection during ultrasound-guided axillary block does not invariably result in neurologic injury. Anesthesiology 2006;105:779–783.
- Lok C, Kirk P: Problems performing a sciatic nerve block in an amputee. Anaesthesia 2003;58:289–290.
- Sites BD, Gallagher J, Sparks M: Ultrasound-guided popliteal block demonstrates an atypical motor response to nerve stimulation in 2 patients with diabetes mellitus. Reg Anesth Pain Med 2003;28:479–482.
- Liu SS, Ngeow JE, Yadeau JT: Ultrasound-guided regional anesthesia and analgesia: a qualitative systematic review. Reg Anesth Pain Med 2009; 34:47–59.
- Sites BD, Taenzer AH, Herrick MD, et al: Incidence of local anesthetic systemic toxicity and postoperative neurologic symptoms associated with 12,668 ultrasound-guided nerve blocks: an analysis from a prospective clinical registry. Reg Anesth Pain Med 2012;37:478–482.
- Rigaud M, Filip P, Lirk P, Fuchs A, Gemes G, Hogan Q: Guidance of block needle insertion by electrical nerve stimulation: a pilot study of the resulting distribution of injected solution in dogs. Anesthesiology 2008; 109:473–478.
- Lucchetta M, Pazzaglia C, Granata G, Briani C, Padua L: Ultrasound evaluation of peripheral neuropathy in POEMS syndrome. Muscle Nerve 2011;44:868–872.
- Riazi S, Bril V, Perkins BA, et al: Can ultrasound of the tibial nerve detect diabetic peripheral neuropathy? A cross-sectional study. Diabetes Care 2012;35:2575–2579.
- Eaton SE, Harris ND, Rajbhandari SM, et al: Spinal-cord involvement in diabetic peripheral neuropathy. Lancet 2001;358:35–36.
- Varsik P, Kucera P, Buranova D, Balaz M: Is the spinal cord lesion rare in diabetes mellitus? Somatosensory evoked potentials and central conduction time in diabetes mellitus. Med Sci Monit 2001;7:712–715.
- Selvarajah D, Wilkinson ID, Emery CJ, et al: Early involvement of the spinal cord in diabetic peripheral neuropathy. Diabetes Care 2006; 29:2664–2669.
- Drasner K: Local anesthetic neurotoxicity: clinical injury and strategies that may minimize risk. Reg Anesth Pain Med 2002;27:576–580.
- Koscielniak-Nielsen ZJ: Ultrasound-guided peripheral nerve blocks: what are the benefits? Acta Anaesthesiol Scand 2008;52:727–737.
- Pachman DR, Barton DL, Watson JC, Loprinzi CL: Chemotherapy-induced peripheral neuropathy: prevention and treatment. Clin Pharmacol Ther 2011;90:377–387.
- Peters CM, Jimenez-Andrade JM, Kuskowski MA, Ghilardi JR, Mantyh PW: An evolving cellular pathology occurs in dorsal root ganglia, peripheral nerve and spinal cord following intravenous administration of paclitaxel in the rat. Brain Res 2007;1168:46–59.
- Quasthoff S, Hartung HP: Chemotherapy-induced peripheral neuropathy. J Neurol 2002;249:9–17.
- Pignata S, De Placido S, Biamonte R, et al: Residual neurotoxicity in ovarian cancer patients in clinical remission after first-line chemotherapy with carboplatin and paclitaxel: the Multicenter Italian Trial in Ovarian cancer (MITO-4) retrospective study. BMC Cancer 2006;6:5.
- Kaley TJ, Deangelis LM: Therapy of chemotherapy-induced peripheral neuropathy. Br J Haematol 2009;145:3–14.
- Hebl JR, Horlocker TT, Pritchard DJ: Diffuse brachial plexopathy after interscalene block in a patient receiving cisplatin chemotherapy: the pharmacologic double crush syndrome. Anesth Analg 2001;92: 249–251.
- Caliandro P, La Torre G, Padua R, Giannini F, Padua L: Treatment for ulnar neuropathy at the elbow. Cochrane Database Syst Rev 2012;7:CD006839.
- Hebl JR, Horlocker TT, Sorenson EJ, Schroeder DR: Regional anesthesia does not increase the risk of postoperative neuropathy in patients undergoing ulnar nerve transposition. Anesth Analg 2001;93: 1606–1611, table of contents.
- Pithadia AB, Kakadia N: Guillain-Barré syndrome (GBS). Pharmacol Rep 2010;62:220–232.
- Bamberger PD, Thys DM: Guillain-Barré syndrome in a patient with pancreatic cancer after an epidural-general anesthetic. Anesthes Analg 2005;100:1197–1199.
- Gautier PE, Pierre PA, Van Obbergh LJ, Van Steenberge A: Guillain-Barre syndrome after obstetrical epidural analgesia. Reg Anesth 1989;14:251–252.
- Heyworth BE, Fabricant PD, Pizzurro MM, Beksac B, Salvati EA: Guillain-Barré syndrome mimicking nerve injury after total hip arthroplasty. HSS J 2011;7:286–289.
- Alici HA, Cesur M, Erdem AF, Gursac M: Repeated use of epidural anaesthesia for caesarean delivery in a patient with Guillain-Barré syndrome. Int J Obstet Anesth 2005;14:269–270.
- McGrady EM: Management of labour and delivery in a patient with Guillain-Barré syndrome. Anaesthesia 1987;42:899.
- Perel A, Reches A, Davidson JT: Anaesthesia in the Guillain-Barré syndrome. A case report and recommendations. Anaesthesia 1977;32: 257–260.
- Vassiliev DV, Nystrom EU, Leicht CH: Combined spinal and epidural anesthesia for labor and cesarean delivery in a patient with Guillain-Barre syndrome. Reg Anesth Pain Med 2001;26:174–176.
- Otsuka N, Igarashi M, Shimodate Y, Nakabayashi K, Asano M, Namiki A: [Anesthetic management of two patients with amyotrophic lateral sclerosis (ALS)]. Masui 2004;53:1279–1281.
- Steiner I, Argov Z, Cahan C, Abramsky O: Guillain-Barré syndrome after epidural anesthesia: direct nerve root damage may trigger disease. Neurology 1985;35:1473–1475.
- Staff NP, Engelstad J, Klein CJ, et al: Post-surgical inflammatory neuropathy. Brain 2010;133:2866–2880.
- Ahn KS, Kopp SL, Watson JC, Scott KP, Trousdale RT, Hebl JR: Postsurgical inflammatory neuropathy. Reg Anesth Pain Med 2011;36: 403–405.
- Bamford C, Sibley W, Laguna J: Anesthesia in multiple sclerosis. Can J Neurol Sci 1978;5:41–44.
- Dripps RD, Vandam LD: Exacerbation of pre-existing neurologic disease after spinal anesthesia. N Engl J Med 1956;255:843–849.
- Hebl JR, Horlocker TT, Schroeder DR: Neuraxial anesthesia and analgesia in patients with preexisting central nervous system disorders. Anesth Analg 2006;103:223–228, table of contents.
- Keschner M: The effect of injuries and illness on the course of multiple sclerosis. Res Publ Assoc Res Nerv Ment Dis 1950;28:533–547.
- Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T: Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N Engl J Med 1998;339:285–291.
- Crawford JS: Epidural analgesia for patients with chronic neurological disease. Anesth Analg 1983;62:621–622.
- Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG: Multiple sclerosis. N Engl J Med 2000;343:938–952.
- Compston A, Coles A: Multiple sclerosis. Lancet 2002;359: 1221–1231.
- Korn-Lubetzki I, Kahana E, Cooper G, Abramsky O: Activity of multiple sclerosis during pregnancy and puerperium. Ann Neurol 1984;16:229–231.
- Pollock M, Calder C, Allpress S: Peripheral nerve abnormality in multiple sclerosis. Ann Neurol 1977;2:41–48.
- Koff MD, Cohen JA, McIntyre JJ, Carr CF, Sites BD: Severe brachial plexopathy after an ultrasound-guided single-injection nerve block for total shoulder arthroplasty in a patient with multiple sclerosis. Anesthesiology 2008;108:325–328.
- Pogorzelski R, Baniukiewicz E, Drozdowski W: [Subclinical lesions of peripheral nervous system in multiple sclerosis patients]. Neurol Neurochir Pol 2004;38:257–264.
- Misawa S, Kuwabara S, Mori M, Hayakawa S, Sawai S, Hattori T: Peripheral nerve demyelination in multiple sclerosis. Clinical Neurophysiol 2008;119:1829–1833.
- Sarova-Pinhas I, Achiron A, Gilad R, Lampl Y: Peripheral neuropathy in multiple sclerosis: a clinical and electrophysiologic study. Acta Neurol Scand 1995;91:234–238.
- Critchley EP: Multiple sclerosis initially presenting as facial palsy. Aviat Space Environ Med 2004;75:1001–1004.
- Hammes E: Neurological complications associated with spinal anesthesia (eight cases). Minn Med 1943;36:339–345.
- Keschner M: The effect of injuries and illness on the course of multiple sclerosis. Res Publ Assoc Res Nerv Ment Dis 1950;28:533–547.
- Stenuit J, Marchand P: [Sequelae of spinal anesthesia]. Acta Neurol Psychiatr Belg 1968;68:626–635.
- Kuczkowski KM: Labor analgesia for the parturient with neurological disease: what does an obstetrician need to know? Arch Gynecol Obstet 2006;274:41–46.
- Warren TM, Datta S, Ostheimer GW: Lumbar epidural anesthesia in a patient with multiple sclerosis. Anesth Analg 1982;61:1022–1023.
- Sakurai M, Mannen T, Kanazawa I, Tanabe H: Lidocaine unmasks silent demyelinative lesions in multiple sclerosis. Neurology 1992;42: 2088–2093.
- Drake E, Drake M, Bird J, Russell R: Obstetric regional blocks for women with multiple sclerosis: a survey of UK experience. Int J Obstet Anesth 2006;15:115–123.
- Gonzalez H, Olsson T, Borg K: Management of postpolio syndrome. Lancet Neurol 2010;9:634–642.
- Bordes J, Gaillard PE, Lacroix G, Palmier B: Spinal anaesthesia guided by computed tomography scan in a patient with severe post-polio sequelae. Br J Anaesth 2010;105:702–703.
- Higashizawa T, Sugiura J, Takasugi Y: [Spinal anesthesia in a patient with hemiparesis after poliomyelitis]. Masui 2003;52:1335–1337.
- Lambert DA, Giannouli E, Schmidt BJ: Postpolio syndrome and anesthesia. Anesthesiology 2005;103:638–644.
- Pratt AJ, Getzoff ED, Perry JJ: Amyotrophic lateral sclerosis: update and new developments. Degener Neurol Neuromuscul Dis 2012;2012:1–14.
- Chen LK, Chang Y, Liu CC, Hou WY: Epidural anesthesia combined with propofol sedation for abdominal hysterectomy in a patient with amyotrophic lateral sclerosis—a case report. Acta Anaesthesiol Sin 1998;36:103–106.
- Hara K, Sakura S, Saito Y, Maeda M, Kosaka Y: Epidural anesthesia and pulmonary function in a patient with amyotrophic lateral sclerosis. Anesth Analg 1996;83:878–879.
- Hobaika AB, Neves BS: Combined spinal-epidural block in a patient with amyotrophic lateral sclerosis: case report. Rev Bras Anestesiol 2009;59:206–209.
- Kitoh T, Kobayashi K, Ina H, et al: Effects of lumbar sympathetic ganglion block for a patient with amyotrophic lateral sclerosis (ALS). J Anesth 2006;20:109–112.
- Kochi T, Oka T, Mizuguchi T: Epidural anesthesia for patients with amyotrophic lateral sclerosis. Anesth Analg 1989;68:410–412.
- Katz JN, Harris MB: Clinical practice. Lumbar spinal stenosis. N Engl J Med 2008;358:818–825.
- Stambough JL, Stambough JB, Evans S: Acute cauda equina syndrome after total knee arthroplasty as a result of epidural anesthesia and spinal stenosis. J Arthroplasty 2000;15:375–379.
- Tetzlaff JE, Dilger JA, Wu C, Smith MP, Bell G: Influence of lumbar spine pathology on the incidence of paresthesia during spinal anesthesia. Reg Anesth Pain Med 1998;23:560–563.
- Moen V, Dahlgren N, Irestedt L: Severe neurological complications after central neuraxial blocks in Sweden 1990–1999. Anesthesiology 2004;101:950–959.
- Yuen EC, Layzer RB, Weitz SR, Olney RK: Neurologic complications of lumbar epidural anesthesia and analgesia. Neurology 1995;45: 1795–1801.
- de Seze MP, Sztark F, Janvier G, Joseph PA: Severe and long-lasting complications of the nerve root and spinal cord after central neuraxial block. Anesth Analg 2007;104:975–979.
- Hooten WM, Hogan MS, Sanemann TC, Maus TJ: Acute spinal pain during an attempted lumbar epidural blood patch in congenital lumbar spinal stenosis and epidural lipomatosis. Pain Physician 2008;11: 87–90.
- Usubiaga JE, Wikinski JA, Usubiaga LE: Epidural pressure and its relation to spread of anesthetic solutions in epidural space. Anesth Analg 1967;46:440–446.
- Hebl JR, Horlocker TT, Kopp SL, Schroeder DR: Neuraxial block in patients with preexisting spinal stenosis, lumbar disk disease, or prior spine surgery: efficacy and neurologic complications. Anesth Analg 2010; 111:1511–1519.
- Horlocker TT: Neuraxial block in patients with spinal stenosis: between a rock and a hard place. Anesth Analg 2010;110:13–15.
- Kubina P, Gupta A, Oscarsson A, Axelsson K, Bengtsson M: Two cases of cauda equina syndrome following spinal-epidural anesthesia. Reg Anesth 1997;22:447–450.
- Houten JK, Errico TJ: Paraplegia after lumbosacral nerve root block: report of three cases. Spine J 2002;2:70–75.
- Huntoon MA, Martin DP: Paralysis after transforaminal epidural injection and previous spinal surgery. Reg Anesth Pain Med 2004;29: 494–495.
- Devivo MJ: Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal Cord 2012;50:365–372.
- Hagen EM, Faerestrand S, Hoff JM, Rekand T, Gronning M: Cardiovascular and urological dysfunction in spinal cord injury. Acta Neurol Scand Suppl 2011:71–78.
- Crosby E, St-Jean B, Reid D, Elliott RD: Obstetrical anaesthesia and analgesia in chronic spinal cord-injured women. Can J Anaesth 1992;39: 487–494.
- Hambly PR, Martin B: Anaesthesia for chronic spinal cord lesions. Anaesth 1998;53:273–289.
- Agostoni M, Giorgi E, Beccaria P, Zangrillo A, Valentini G: Combined spinal-epidural anaesthesia for Caesarean section in a paraplegic woman: difficulty in obtaining the expected level of block. Eur J Anaesthesiol 2000;17:329–331.