Hidden risks of gadolinium-based contrast agents in interventional pain medicine
A recent study by Hallo-Carrasco et al. highlights the potential dangers of gadolinium-based contrast media (GCM) in interventional pain medicine. This research, published in Regional Anesthesia & Pain Medicine (2024), investigates adverse events linked to using GCM during spine procedures where inadvertent intrathecal administration is a risk. Here’s a comprehensive look at the findings, implications, and best practices surrounding GCM use in these procedures.
Overview of gadolinium-based contrast media (GCM)
Gadolinium-based contrast agents are an alternative for patients with documented allergies to iodinated contrast media (ICM). While traditionally safer for imaging, GCM carries specific risks:
- Toxicity risks: Gadolinium ions (Gd3+) are inherently toxic; chelating agents stabilize the compound and reduce these risks.
- Neurotoxicity: When inadvertently injected into the intrathecal (spinal) space, GCM can cause severe neurotoxic effects.
- Brain retention: There are concerns about gadolinium deposits in brain tissues following repeated exposure.
Key findings
Conducted via a retrospective review of medical records, the study investigated 508 patients who received GCM for spine-related procedures between 2019 and 2022. Here’s what was uncovered:
- Adverse event rate: 23 patients (3.3%) experienced adverse events potentially related to GCM. Common symptoms included severe pain, dizziness, headache, and, in one case, multifocal stroke.
- Patient demographics: A significant majority of patients were white females with a mean age of 67.55 years.
- Indications for GCM use: The predominant reason for using GCM was a documented iodine-related allergy. However, only 1% of these cases involved high-risk allergy reactions that could justify substituting ICM.
- Severity of adverse events: While most were manageable, some cases required hospitalization. The study documented one severe incident of stroke following GCM administration.
Adverse reactions and symptoms
Adverse effects following inadvertent intrathecal injection of GCM included:
- Severe pain: Reported within days of the procedure, sometimes requiring hospitalization.
- Headaches: Particularly postural headaches, which align with findings from other studies on intrathecal gadolinium.
- Neurological symptoms: Dizziness and vertigo led to falls in elderly patients.
- Multifocal stroke: One patient developed multiple small acute infarcts after a lumbar epidural steroid injection.
Understanding the mechanisms of gadolinium neurotoxicity
When gadolinium-based agents enter the spinal space, they can interact with neurons in harmful ways:
- Calcium channel interference: Gadolinium competes with calcium, disrupting cellular processes vital for neuron function.
- Oxidative stress: Gadolinium exposure triggers oxidative stress in neurons, which can lead to cell damage and death.
- Neuroinflammation: Prolonged or high-dose exposure may cause neuroinflammation, contributing to symptoms like pain, dizziness, and cognitive disturbances.
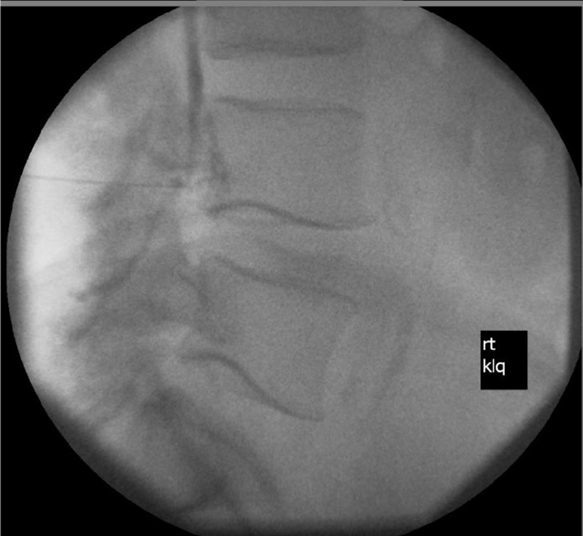
Fluoroscopy image consistent with possible or probable intrathecal injection of gadolinium-based contrast media. Hallo-Carrasco et al., RAPM 2024.
Guidelines for safer use of contrast media in interventional pain procedures
To minimize risks associated with GCM, follow these practices:
- Evaluate allergy labels carefully: Many ICM “allergies” are incorrectly documented. Only high-risk allergy labels should warrant consideration of GCM.
- Use multiple imaging views: To detect inadvertent intrathecal spread, employ anterior-posterior, contralateral oblique, and lateral views.
- Premedicate when necessary: For patients with mild ICM allergies, antihistamine and corticosteroid premedication may be safer than switching to GCM.
- Limit GCM volume and concentration: Use the lowest effective volume of GCM, typically 1 mL at a concentration of 0.5 mol/L, to reduce toxicity risks.
- Consider digital subtraction angiography: This technology can clarify gadolinium spread patterns, reducing the risk of inadvertent intrathecal injections.
Steps to take if adverse symptoms arise post-procedure
If a patient reports symptoms following a GCM procedure, consider the following:
- Evaluate symptom onset: Note the time from procedure to symptom onset, particularly for severe symptoms like dizziness, headache, or pain.
- Administer pain management as needed: Use non-invasive methods such as heating pads or medication.
- Consult radiology for imaging: Conduct an MRI to detect any gadolinium deposits or neurotoxic effects in severe cases.
- Document thoroughly: Clear documentation of all symptoms and follow-up treatments is crucial for future case management and research.
Conclusion and future directions
This study highlights the underappreciated risks of gadolinium-based contrast agents in spine procedures and underscores the importance of accurate allergy documentation. Healthcare professionals are encouraged to exercise caution and follow established guidelines to minimize unnecessary GCM use in pain management procedures.
For more detailed information, refer to the full article in RAPM.
Hallo-Carrasco A, Eldrige J, Provenzano DA, et al. Hidden risk of gadolinium-based contrast agents during interventional pain medicine procedures: a retrospective chart review. Regional Anesthesia & Pain Medicine 2024;49:751-756.
To learn more about fluoroscopy-guided interventional pain procedures, download NYSORA’s Pain Rx App!



