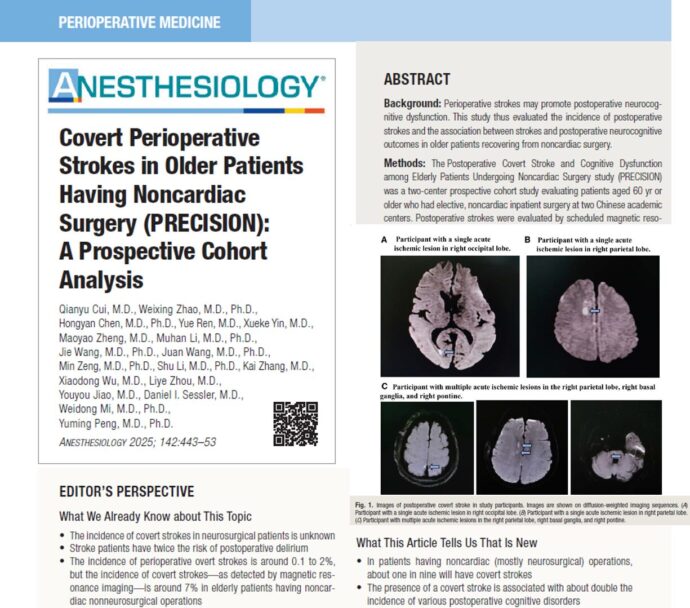
Perioperative continuation of buprenorphine in surgical patients with opioid use disorder
A new study published in Anesthesiology (2025) explores the impact of continuing versus interrupting buprenorphine in patients with opioid use disorder (OUD) undergoing surgery. The retrospective cohort study, led by Dr. James M. Hitt and colleagues, examined postoperative pain scores and opioid requirements in veterans receiving buprenorphine therapy before surgery.
The findings provide strong evidence supporting recent guidelines that recommend continuing buprenorphine perioperatively rather than interrupting it, addressing a critical debate in perioperative pain management for patients on medication-assisted treatment (MAT).
Why this study matters
- Opioid Use Disorder (OUD) affects ~3 million Americans, and opioid-related overdoses remain a leading cause of preventable deaths.
- Buprenorphine is increasingly used to treat OUD, reducing withdrawal symptoms, opioid cravings, and overdose risk.
- Perioperative pain management in OUD patients is complex, as buprenorphine’s high binding affinity and partial opioid receptor activity raise concerns about its impact on post-surgical analgesia.
- Current guidelines recommend continuing buprenorphine through surgery, but clinical evidence remains limited.
- This study provides high-quality real-world data from 1,881 surgical cases in 1,673 patients, addressing a critical knowledge gap.
Study overview
The research team analyzed Veterans Affairs (VA) data from 2010–2020, comparing patients who continued buprenorphine perioperatively to those who had their treatment interrupted before surgery.
Patient cohorts
- Continued buprenorphine group (63%) – Patients who remained on buprenorphine through the perioperative period.
- Interrupted buprenorphine group (37%) – Patients who stopped taking buprenorphine before surgery.
- Matched control group – Patients without a buprenorphine prescription.
Primary outcomes
- Postoperative pain scores (Numerical Rating Scale, NRS 0–10) within 72 hours of surgery.
- Postoperative opioid requirements (Morphine Milligram Equivalents, MME) within two weeks after surgery.
- Key findings
Pain scores: No significant clinical difference
-
- Average pain scores (0-10 scale) were:
- Control group: 4.1 ± 1.9
- Continued buprenorphine: 4.9 ± 2.0
- Interrupted buprenorphine: 5.5 ± 1.7
- Patients who interrupted buprenorphine reported higher pain scores than those who continued it (p < 0.001).
- Differences between continued buprenorphine and controls were clinically insignificant, supporting the safety of buprenorphine maintenance during surgery.
- Average pain scores (0-10 scale) were:
Opioid use: Interrupting buprenorphine increased supplemental opioid requirements
-
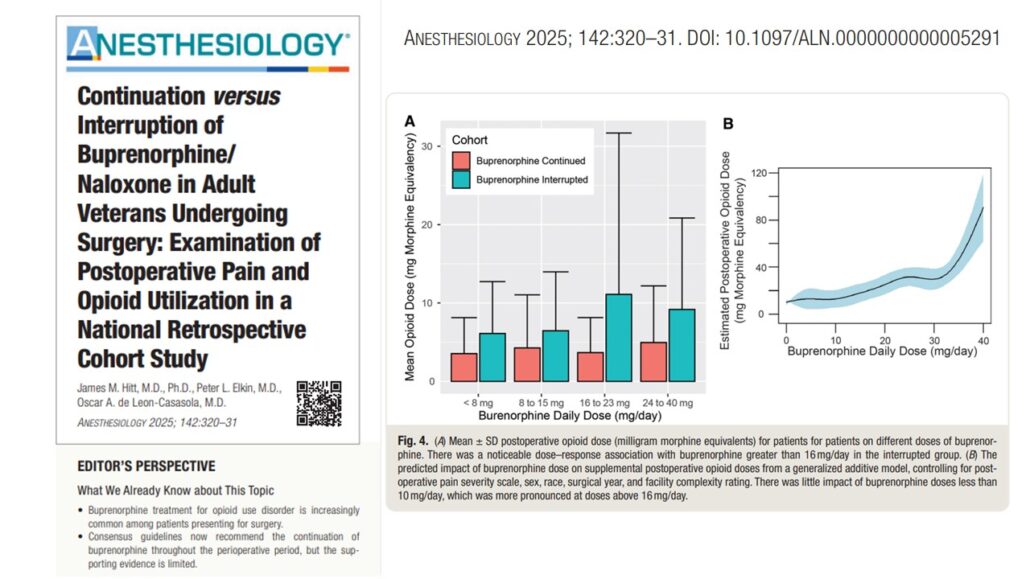
- Patients who interrupted buprenorphine required significantly more opioids than those who continued:
- Continued buprenorphine: 39.7 ± 1.9 mg MME/day
- Interrupted buprenorphine: 74.2 ± 4.5 mg MME/day (p < 0.001)
- No significant difference in supplemental opioid use between the continued buprenorphine group and the control group (p = 0.23).
- Higher preoperative buprenorphine doses (>16 mg/day) did not result in increased pain or opioid needs.
- Patients who interrupted buprenorphine required significantly more opioids than those who continued:
Perioperative buprenorphine management trends
-
- Buprenorphine continuation increased over time:
- 2012: 50% interruption rate
- 2020: Only 19% of patients interrupted buprenorphine before surgery
- This shift aligns with evolving clinical guidelines, recognizing the risks of withdrawal and increased opioid use when buprenorphine is stopped.
- Buprenorphine continuation increased over time:
Clinical implications: Why this matters for anesthesia & pain management
Supporting the shift toward perioperative buprenorphine continuation
-
- The study directly supports current guidelines, including:
- American Society of Regional Anesthesia (ASRA)
- Perioperative Pain and Addiction Interdisciplinary Network (PAIN)
- CDC Guidelines for Opioid Prescribing
- Stopping buprenorphine before surgery led to worse outcomes:
- Higher pain scores
- Increased opioid use postoperatively
- No significant benefit in pain control
- The study directly supports current guidelines, including:
Buprenorphine does not prevent effective postoperative pain management
-
- Concerns that buprenorphine may “block” full opioid agonists are not supported by data.
- Patients who remained on buprenorphine required no more opioids than opioid-naïve controls.
- Maintaining buprenorphine therapy may reduce the need for high-dose opioid analgesia, improving safety.
High-dose buprenorphine (≥16 mg/day) can be continued safely
-
- Higher-dose buprenorphine patients did not experience increased pain or opioid requirements.
- Guidelines recommending tapering before surgery may not be necessary for all patients.
Final thoughts: What’s next?
The perioperative management of buprenorphine is shifting as new research emerges. This study reinforces the safety and efficacy of continuing buprenorphine through surgery, aligning with modern clinical guidelines.
What this means for pain medicine & anesthesia:
-
- Continue buprenorphine perioperatively whenever possible.
- Patients who remain on buprenorphine do not have worse pain outcomes.
- Interrupting buprenorphine increases opioid use postoperatively.
- High-dose buprenorphine (>16 mg/day) does not necessarily need tapering.
For more information, refer to the full article in Anesthesiology.
Hitt JM, Elkin PL, de Leon-Casasola OA. Continuation versus Interruption of Buprenorphine/Naloxone in Adult Veterans Undergoing Surgery: Examination of Postoperative Pain and Opioid Utilization in a National Retrospective Cohort Study. Anesthesiology. 2025;142(2)
Stay informed with Pain Medicine Updates 2025, your go-to resource for the latest advancements in pain management. Curated by Dr. Guilherme Ferreira Dos Santos and NYSORA’s International Pain Medicine Educational Board, this book delivers concise, high-impact summaries of key research from 2023-2024, tailored for busy clinicians. Get your copy on Amazon or Google Books.