Holly Evans, Karen C. Nielsen, M. Steve Melton, Roy A. Greengrass, and Susan M. Steele
INTRODUCTION
Continuous peripheral nerve blocks (CPNBs) provide a number of advantages in the perioperative period. These techniques provide the flexibility to prolong intraoperative anesthesia while avoiding the risks and side effects of general anesthesia. Following surgery, CPNBs offer extended postoperative analgesia. When compared to parenteral opioid analgesia, CPNBs are associated with superior analgesia, reduced opioid consumption, and decreased opioid-related side effects such as postoperative nausea and vomiting, sedation, and respiratory depression. Analgesia of similar quality to epidural anesthesia is the result; however, less hypotension, urinary retention, pruritus, and mobility restrictions occur with CPNBs.
There is also evidence supporting the beneficial effect of CPNBs on postoperative sleep patterns and cognitive function as well as early rehabilitation. Concurrent sympathectomy is ideal following microvascular, reimplantation, and free-flap surgery, as well as for treatment of accidental intra-arterial drug injection. Extended analgesia can also be provided for patients with chronic pain and those requiring palliation of terminal illness. Finally, preoperative use can reduce phantom limb sensation in patients undergoing amputation.
Despite these benefits, CPNBs have historically been relatively underused. This early lack of popularity was multifactorial; however, inadequate CPNB equipment likely contributed. The development of CPNB needles, catheters, and nerve localization technology has been essential for the safe use and advancement of these regional anesthesia techniques. This chapter summarizes the equipment required for continuous plexus anesthesia and will review the chronology of the development of modernday CPNB equipment.
PRE-BLOCK CONSIDERATIONS
Block Room
A perineural catheter can be placed in a block room just as the preceding patient’s procedure is finishing. This enhances operating room efficiency and flow. A block room allows the supplies and monitors discussed next to be stocked and stored in one location. The block room should be a clean, semi-sterile room in close proximity to the operating room suite.
Block Cart
Table 1 outlines block cart supplies necessary for the performance of CPNBs. Supplies are sterile where applicable.
TABLE 1 Block cart supplies necessary for continuous peripheral nerve blocks.
- Sterile gloves ± gown for anesthesiologist
- Disinfecting solution (2% chlorhexidine gluconate with 70% isopropyl alcohol)
- Clear drapes
- Needles for subcutaneous local anesthetic infiltration (ie, 25-gauge 1½ inch)
- Syringes for subcutaneous local anesthetic infiltration (ie, 3 mL)
- 2 × 2 in. gauze
- Selection of block needles and catheter sets of appropriate diameter and length
- Nerve stimulator and electrodes
- Ultrasound machine
- Selection of ultrasound probes of appropriate frequency, shape, and size
- Sterile ultrasound probe covers
- Sterile gel for ultrasound imaging
- Dextrose 5% in waterLocal anesthetics and adjuvants (see below)
- Occlusive dressing, Epi-Guard, tape, Mastisol, Dermabond®
- Connectors for proximal tip of catheter
- Oxygen source
- Oxygen masks
- Suction
Monitoring
Patients receiving a perineural catheter often receive sedation and large doses of local anesthetic. They should have standard American Society of Anesthesiologists (ASA) monitors applied. Furthermore, these patients should be monitored by an individual with Advanced Cardiac Life Support (ACLS) resuscitation knowledge and skills.
Resuscitative Medication and Equipment
A number of life-threatening complications can occur during placement of a CPNB. Table 2 lists the resuscitative medications and equipment that should be readily available.
TABLE 2. Necessary resuscitative medications and equipment.
- ACLS drugs (ie, epinephrine, vasopressin, atropine)
- Cardioverter/defibrillator
- Intralipid® 20% (1.5-mL/kg bolus over 1 minute and every 3–5 minutes up to 3 mL/kg, 0.25 mL/kg/min infusion, maximum total dose 8 mL/kg)
Premedication and Sedation
Table 3 lists various agents that can be used to provide sedation and analgesia for perineural catheter placement. Most advocate the use of light sedation, thus allowing communication with the patient regarding potential side effects.
TABLE 3. Agents for sedation and analgesia for perineural catheter placement.
- Benzodiazepines (ie, midazolam)
- Opiates (ie, fentanyl)
- N-Methyl–aspartate antagonists (ie, ketamine)
- Alpha-2 antagonists (ie, clonidine, dexmedetomidine)
- Anesthetics (ie, propofol, etomidate)
Local Anesthetic Solutions and Adjuvants Block Initiation
A variety of local anesthetics should be available on the block cart to initiate the nerve block. Short-acting agents such as lidocaine or mepivacaine allow for rapid onset yet early recovery of the sensorimotor block. This facilitates prompt assessment of neurological function following surgery and prior to starting a continuous perineural infusion. Block initiation with long-acting agents such as bupivacaine or ropivacaine extend the duration of dense anesthesia and analgesia. Ropivacaine is often selected instead of bupivacaine for its more favorable safety profile. Concentrated solutions provide effective intraoperative anesthesia and analgesia. Dilute local anesthetic solutions can provide selective sensory anesthesia while minimizing motor block after surgery.
When appropriate, epinephrine 1:200,000 or 1:400,000 is added to the local anesthetic solution. The epinephrine serves as a marker of intravascular injection and can limit the systemic absorption of perineurally placed local anesthetic.
Further adjuvants such as clonidine and dexamethasone have been used to enhance the duration and quality of analgesia.
Continuous Infusion
The nerve block is typically maintained with a continuous infusion of dilute, long-acting local anesthetic. Patient-controlled boluses are often added to the continuous infusion to enhance analgesia. The local anesthetic solution should be prepared in a clean environment, such as in a pharmacy using a laminar flow workbench. Minimizing bacterial contamination is critical to reducing the likelihood of infectious neurological complications.
NYSORA Tips
- Safe practice of regional anesthesia requires the immediate availability of monitors, resuscitation drugs, and equipment as well as a health care practitioner familiar with ACLS protocols.
NEEDLE-AND-CATHETER SYSTEMS
Historical Perspective
The earliest report of CPNB is attributed to Ansbro’s 1946 work (Figure 1). He attached a malleable blunt needle to injection tubing and a syringe. The needle was placed blindly in the supraclavicular area lateral to the pulsations of the subclavian artery and approximately 1 cm cephalad to the midpoint of the clavicle. A cork stopper from a can of ether was used to secure the needle in place. Intermittent injections of procaine were given to 27 patients to extend the duration of intraoperative anesthesia for up to 4 hours and 20 minutes.
Almost a quarter century later, continuous subclavian perivascular, interscalene, and axillary brachial plexus blocks were reported by DeKrey et al, Winnie, and Selander (Figure 2), respectively. In all reports, the authors used an intravenous-type needle-and-cannula set. The brachial plexus was identified with a paresthesia or fascial pop technique, the inner needle was removed, and the outer plastic cannula was advanced into a perineural location.
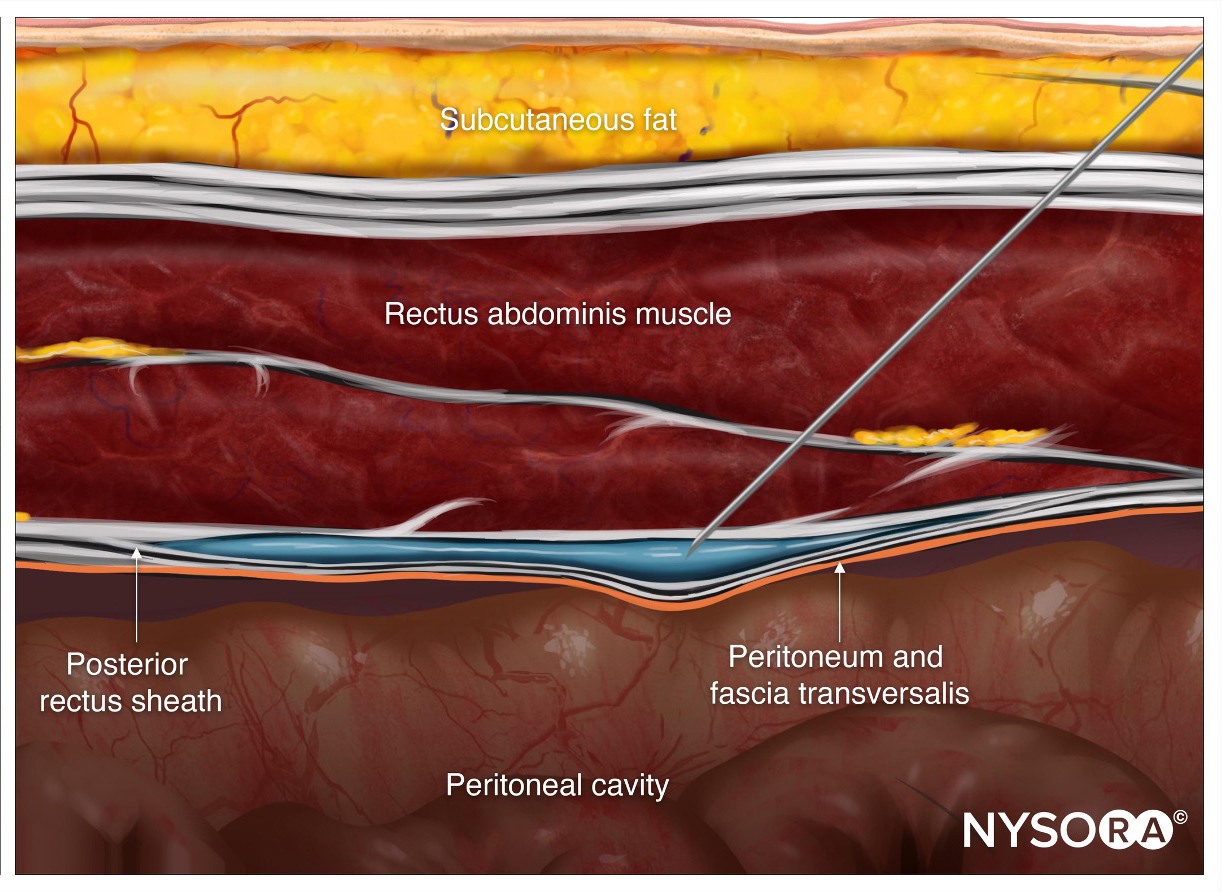
While these early accounts primarily involved “cannula-over-needle” devices, subsequent descriptions also included “catheter-through-needle” equipment. In the earliest report of a continuous lower extremity block, Brands and Callahan performed continuous lumbar plexus blocks that lasted 72 to 96 hours for patients who sustained femoral neck fractures. The authors used an 18-gauge, 15 cm long needle and the loss-of-resistance method to identify the lumbar plexus psoas compartment and subsequently threaded an epidural catheter through the needle.
The identification of important neuroanatomical features occurred concurrently with the description of these early CPNBs. This knowledge was instrumental to the further understanding of plexus anatomy and to the development of continuous regional anesthesia techniques. Landmark papers were published by Winnie as well as Thompson and Rorie outlining the existence of a brachial plexus sheath and suggesting the potential for continuous plexus anesthesia. Tuominen et al provided early evidence for the safety of continuous plexus infusions of local anesthetic when they studied blood levels of bupivacaine during continuous axillary brachial plexus infusion.
Reflecting the popularity of the paresthesia, fascial pop, and loss-of-resistance techniques from the 1970s through the early 1990s, the majority of subsequent reports of CPNBs also involved the use of intravenous-type needles and cannula (cannula-over-needle devices) as well as epidural-type needles and catheters (catheter-through-needle equipment).
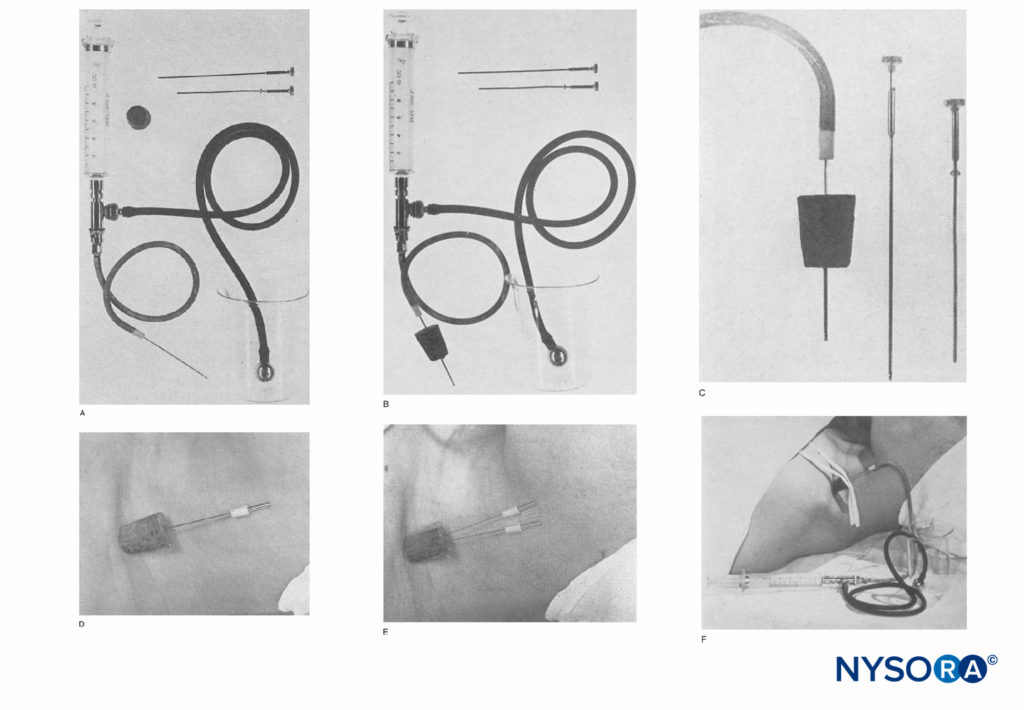
FIGURE 1. A: Apparatus consists of a 10-mL Luer lock syringe and the two-way valve as used in the Hingson-Edwards continuous caudal method. The tubing can be of any desired length (18 inches is sufficient). A malleable needle (Becton-Dickinson & Company) that has been filed to a blunt end to prevent perforation of blood vessels is used. A cork stopper from an ether can completes the apparatus. B: Apparatus with needle through the cork, usually 4 to 6 cm. C: Close view of blunted needle through the cork guard. The cork, when placed flush with the skin in the supraclavicular area, prevents the needle from going in deeper. D: Needle in place in the supraclavicular area. The cork prevents displacement inward and holds it upright.
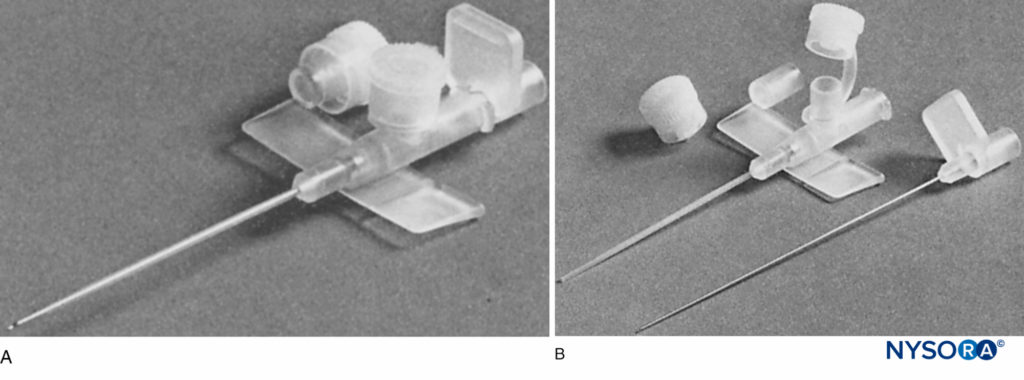
FIGURE 2. A, B: The Venflon cannula. (Reproduced with permission from Selander D: Catheter technique in axillary plexus block. Presentation of a new method. Acta Anaesthesiol Scand. 1977;21(4):324-329.)
Development of Insulated Systems
In the late 1970s, concern surfaced in the regional anesthesia literature over the potential for long-bevel needles and the paresthesia technique to cause neurologic complications. The introduction of nerve stimulator techniques led to a decline in the popularity of the paresthesia, fascial pop, and loss-or-resistance methods in favor of identification of neural structures by electrolocation. Uninsulated, short-bevel needles could be used with nerve stimulators; however, insulated needles were found to provide more focused current output and consequently more accurate localization of neural structures. As nerve stimulator techniques came into more widespread use in the 1990s, commercially insulated single-injection needles became available, but the design of CPNB needles and catheters initially failed to keep pace. For many years, regional anes-thesia practitioners assembled intravenous access, spinal, and epidural equipment to create their own insulated CPNB apparatus.
Numerous reports described connecting an intravenous needle and cannula to a current source to enable nerve stimulation. For example, Anker-Moller et al adapted a 14-gauge intravenous needle and cannula set (Viggo, Sweden) to provide a continuous femoral nerve block. The nerve stimulator was attached inside the hub of the metal needle. The femoral nerve was identified with nerve stimulator assistance, the inner needle was removed, and a 16-gauge epidural catheter (Portex, UK) was inserted through the intravenous cannula (Figure 3).
Ben-David et al inserted the metal needle of a 20-gauge intravenous catheter (Venflon, Viggo, Sweden) inside the proximal end of a 16-gauge central venous pressure needle (Secalon, Viggo, Sweden). They attached the negative electrode of the nerve stimulator to the exposed metal needle of the 20-gauge intravenous catheter and obtained appropriate stimulation during lumbar plexus block. The over-the-needle central venous cannula was then advanced beyond the tip of the needle and used for continuous lumbar plexus block (Figure 4).
Concepcion wrapped the stylet of a 26-gauge spinal needle around the metal introducer of a typical intravenous over-the-needle cannula. An alligator clip was then attached to the stylet to allow electrical stimulation using a nerve stimulator.
Prosser devised yet another method to provide nerve stimulation during CPNBs for pediatric patients. This author connected a jackplug electrode into the hub of an intravenous cannula (Abbocath-T Venisystems, Abbott, Ireland) to make an electrical contact between the nerve stimulator and the central metal needle of the intravenous set (Figure 5). The surrounding Teflon®-coated sheath insulated all but the tip of the cannula. This adaptation was successful with 20-, 22-, and 24-gauge intravenous catheters and consequently was ideal for the pediatric population.
Further advancing pediatric regional anesthesia, Tan et al used a radial artery catheterization set (#RA-04120; Arrow, Reading, PA) with a 20-gauge cannula over a 22-gauge, thin-walled, short-beveled needle for continuous axillary brachial plexus block. The set had a 0.018-inch integral spring wire that this group connected via an alligator clip to the negative pole of a nerve stimulator to enable electrolocation of the brachial plexus. Using a Seldinger technique, the guidewire was then advanced and used to direct the cannula into the brachial plexus sheath.
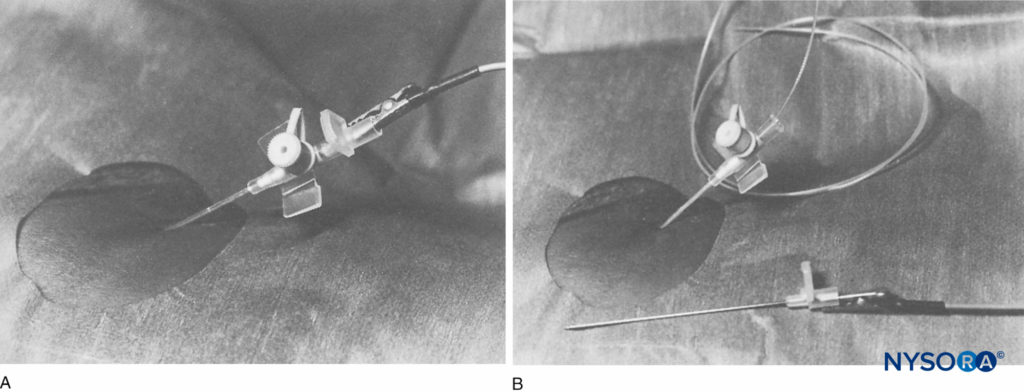
FIGURE 3. A: Placement of the infusion cannula. Nerve stimulator attached to the trocar (lateral view). B: The catheter is inserted through the infusion cannula (lateral view). (Reproduced with permission from Anker-Møller E1, Spangsberg N, Dahl JB, et al: Continuous block of the lumbar plexus after knee surgery: a comparison of the plasma concentrations and analgesic effect of bupivacaine 0.250% and 0.125%. Acta Anaesthesiol Scand. 1990 Aug;34(6):468-472.)
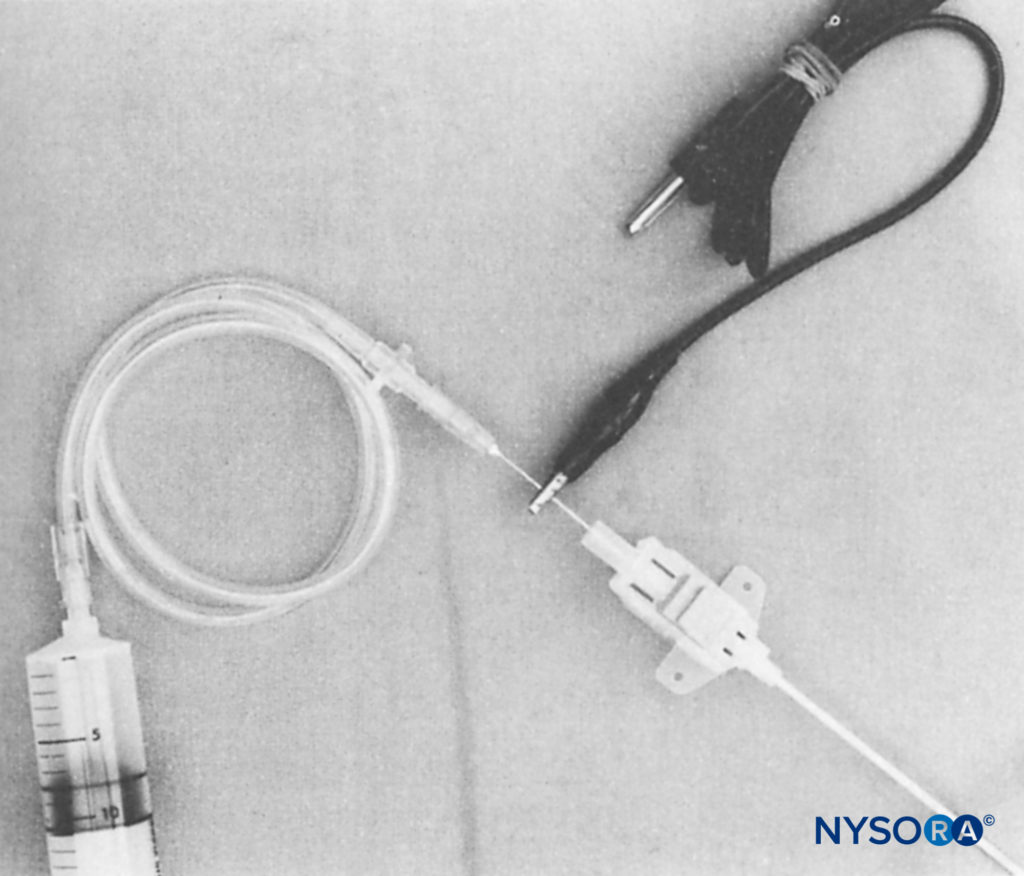
FIGURE 4. Assembly shows a 21-gauge needle inserted into the proximal end of the 16-gauge Secalon (Viggo, Sweden) central venous pressure catheter. Metal contact of the smaller needle inside the larger needle allows an electrical impulse to be conveyed to the Secalon needle tip. An alligator clip from the electrical stimulator is attached to the shaft of the 21-gauge needle. Intravenous extension tubing inserts into the hub of the 21-gauge needle. (Reproduced with permission from Ben-David B1, Lee E, Croitoru M: Psoas block for surgical repair of hip fracture: a case report and description of a catheter technique. Anesth Analg. 1990 Sep;71(3):298-301.)
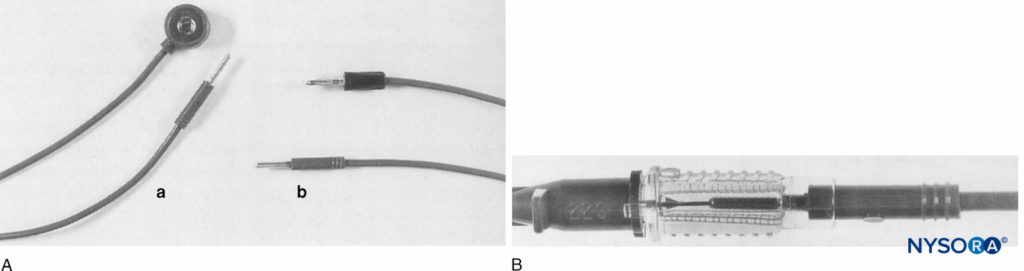
FIGURE 5. A: Peripheral nerve stimulator lead fitted with (a) standard press stud and jackplug connectors; (b) modified connectors, press stud replaced with second jackplug. B: Electrical contact between the Abbocath’s central metal cannula and original jackplug from the lead. (Reproduced with permission from Prosser DP: Adaptation of an intravenous cannula for paediatric regional anaesthesia. Anaesthesia. 1996 May;51(5):510.)
Spinal and epidural equipment has similarly been adapted for CPNBs performed with nerve stimulators. Several groups have placed an 18-gauge intravenous cannula over a 22-gauge spinal needle to provide insulation to the distal part of the needle. A current source was then attached to the bare proximal metal needle, and local anesthetic was injected on identification of neural structures. The cannula was then threaded off the spinal needle into the perineural space and used for continuous infusion of local anesthetic for up to 2 days.
Alternatively, spinal needles and microcatheters have been used. The spinal microcatheters, however, were of such small size that injection was difficult, and they were prone to kinking. This equipment was eventually withdrawn from the market due to neurotoxicity associated with continuous spinal anesthesia.
While these designs allowed nerve stimulation through an insulated needle, a number of drawbacks still existed. Many steps were required between identification of the nerve and threading the catheter. This increased the risk of catheter misplacement and the likelihood of committing a breach in sterility. Despite the insulation provided by an intravenous-type cannula over a metal needle, the uninsulated area of the distal needle tip was of significant size and could adversely affect the accuracy of nerve location. Unfortunately, in these self-assembled systems, the cannula rarely provided a snug fit over the needle. In addition, there was continued concern about the risk of neurologic complications from long-beveled intravenous-type needles. Finally, the shape of the needle tip did not facilitate catheter threading in directions other than parallel to the course of the needle.
Increased effort into the development of CPNB equipment and the introduction of the safer long-acting local anesthetic ropivacaine (Astra, Westborough, MA) in the 1990s further stimulated the expansion of CPNB techniques.
Commercially available cannula-over-needle systems were developed and marketed by various companies. B. Braun introduced a set that consisted of a cannula over a short-bevel needle with an accompanying catheter. The advantage was that the components were designed to fit snugly together (Figure 6). Both Pajunk (Geisengen, Germany) and B. Braun subsequently developed systems that involved a short-bevel needle with an integrated wire for nerve stimulation and with connection tubing for concurrent aspiration and injection (Figure 7). There was an accompanying catheter that could be threaded through the cannula. Some manufacturers offered the option of a Sprotte or Facet needle tip and a variety of sizes, some suitable for pediatric patients. Early Arrow equipment involved a bullet-tip needle for enhanced sensation of fascial penetration and a theoretical reduction in neurology injury (Figure 8).
NYSORA Tips
- Short-bevel block needles were used with the paresthesia technique to minimize nerve injury.
- Insulated needles are used with nerve stimulation to locate the nerve or plexus.
- The design of CPNB needles and catheters initially lagged behind the development of single-injection PNB equipment.
Modern Insulated Systems
The next advance in the development of insulated CPNB equipment involved catheter-through-needle systems (ie, Contiplex® Tuohy, B. Braun Medical, Bethlehem, PA, and Plexolong®, Pajunk). A Tuohy needle was insulated along its length with the exception of a pinpoint area at its most distal tip. A stimulating wire was attached to the needle. Early prototypes involved a detachable wire with an alligator clip on one end and a plug for a nerve stimulator on the other end (Figure 9). In subsequent models, the stimulating wire was permanently affixed to the metal needle (Figures 10 and 11). This wire also served as a marker for the open face of the needle bevel distally. A 20-gauge, multiorifice epidural catheter and connector were included. The curved distal tip of the Tuohy needle facilitated advancement of the catheter parallel to the nerve(s) in question. Various needle lengths were manufactured, allowing CPNB of nerves of varying depths. Sprotte and Facet needle tips were also manufactured to enable catheter threading at various needle angle approaches.
Some CPNB equipment (Contiplex Tuohy) incorporates an adapter with a Luer lock head and a hemostatic valve that can be fitted to the proximal end of the needle (Figure 10). The adapter has a central diaphragm that allows passage of a catheter through a port separate from where aspiration and injection occur. This eliminates the need for equipment disconnection and minimizes the likelihood of needle movement, catheter misplacement, and secondary block failure. This adapter also has a side arm connected to extension tubing that allows for continuous aspiration for blood and injection of solution by an assistant.
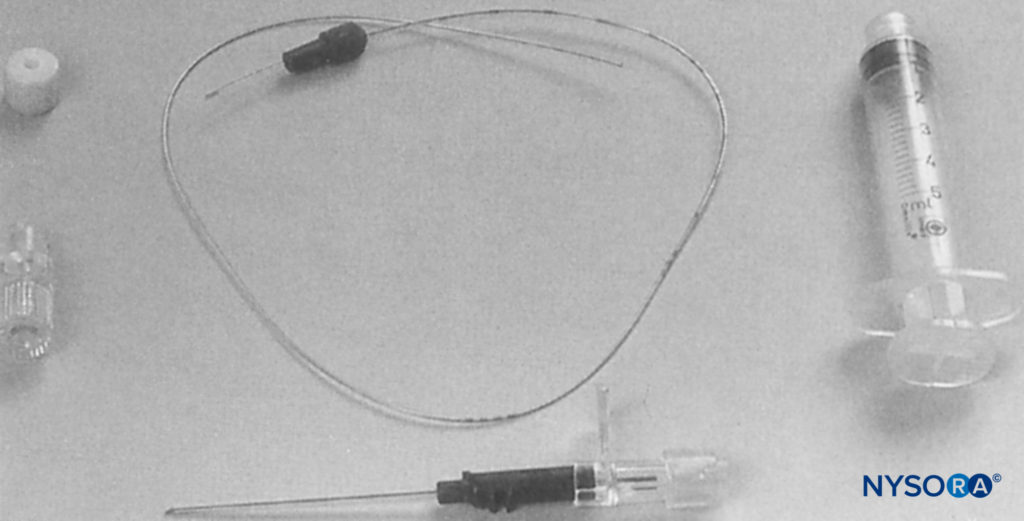
FIGURE 6. Brachial plexus infusion kit as used in this study (Contiplex®, B. Braun Australia Pty. Ltd.). The introducing cannula is 18 gauge, with a 0.85-mm diameter catheter, which is threaded into the axillary brachial plexus sheath. The needle within the cannula is a short-bevel type (30°). (Reproduced with permission from Mezzatesta JP, Scott DA, Schweitzer SA, et al: Continuous axillary brachial plexus block for postoperative pain relief. Intermittent bolus versus continuous infusion. Reg Anesth. 1997 Jul-Aug;22(4):357-362.)
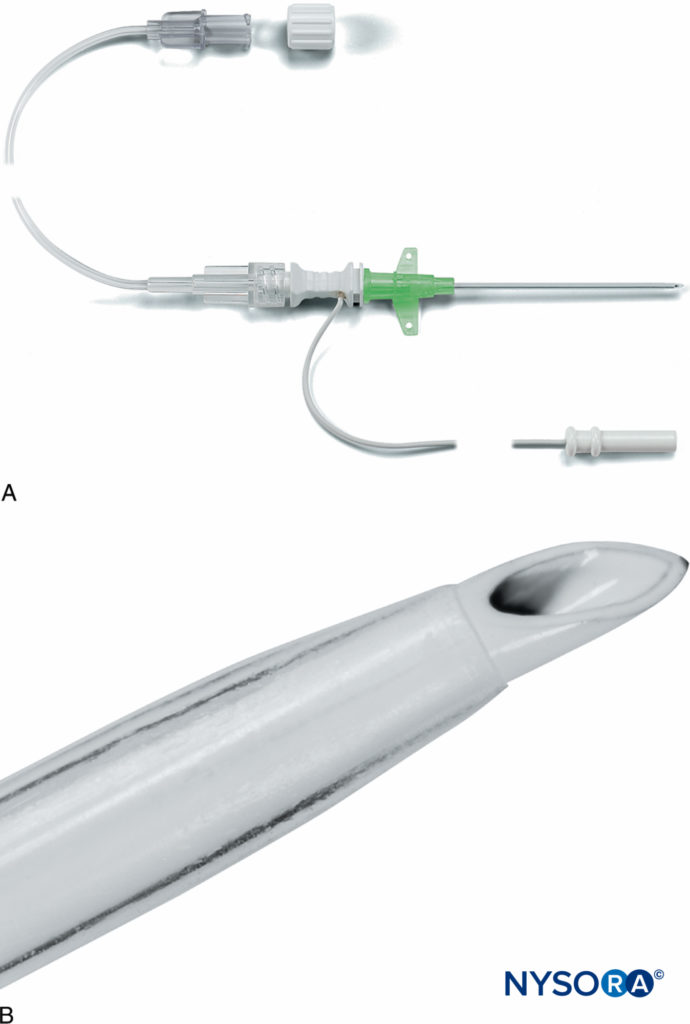
FIGURE 7. A: Pajunk MiniSet® consisting of a cannula-over-needle design and integrated stimulating cable and extension tubing. B: Pajunk MiniSet distal short-bevel needle tip and cannula. (Used with permission from Pajunk, Geisengen, Germany.)
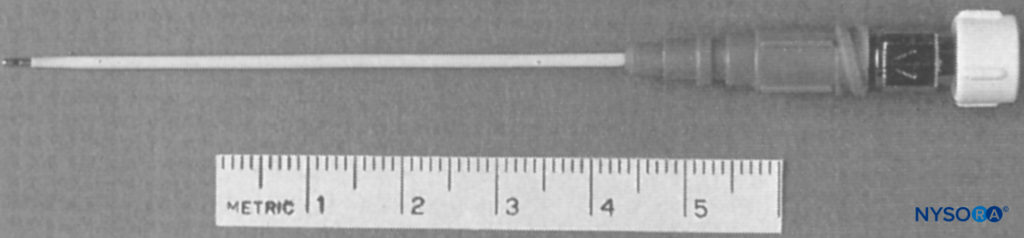
FIGURE 8. Arrow single-shot or continuous brachial plexus catheter. (Reproduced with permission from Longo SR, Williams DP: Bilateral fascia iliaca catheters for postoperative pain control after bilateral total knee arthroplasty: a case report and description of a catheter technique. Reg Anesth. 1997 Jul-Aug;22(4):372-377.)
Catheters initially manufactured for epidural use have been adapted for CPNBs. They are well suited for this application as they are nonirritating and flexible and generate minimal friction on passage through needles. Graduated markings provide an indicator of insertion depth and radiopacity provides an additional method to confirm placement location. Some advocate the use of styletted catheters, believing that they are easier to advance; however, these may result in greater tissue or blood vessel trauma. Multiorifice catheters have a closed distal tip and three distal openings (0.5, 1.0, and 1.5 cm from the tip). Single-orifice catheters have a single opening at the distal end of the catheter (Figure 12). Multiorifice catheters provide better spread of local anesthetic solution that is administered by bolus; consequently, they are associated with improved analgesia compared to single-orifice catheters.
NYSORA Tips
Continuous peripheral nerve block equipment typically includes the following:
- An insulated, large-bore needle (ie, Tuohy tip) with an integrated wire to allow nerve stimulation
- A flexible, atraumatic catheter that is threaded through the needle into a perineural location
- Clear, flexible tubing that can be attached to needle or catheter for injection and aspiration
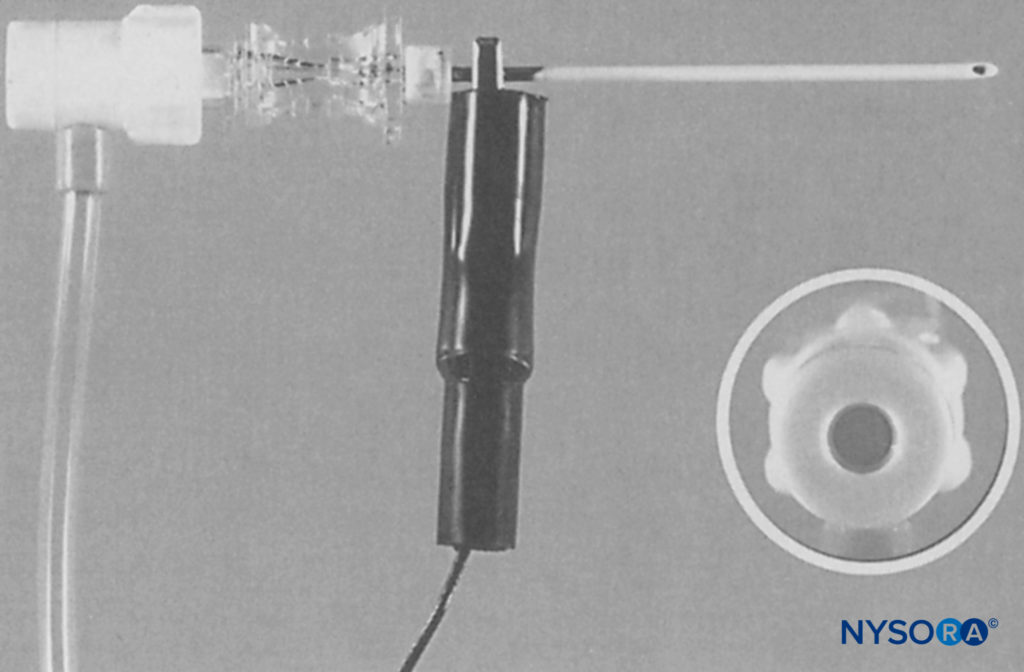
FIGURE 9. An 18-gauge, insulated Tuohy system (Braun, Contiplex®, B. Braun Medical, Bethlehem, PA). Inset shows a Luer lock head with central diaphragm at the proximal end of the needle. (Reproduced with permission from Klein SM, Grant SA, Greengrass RA, et al. Interscalene brachial plexus block with a continuous catheter insertion system and a disposable infusion pump. Anesthesia & Analgesia. Dec 2000;91(6):1473-1478.)
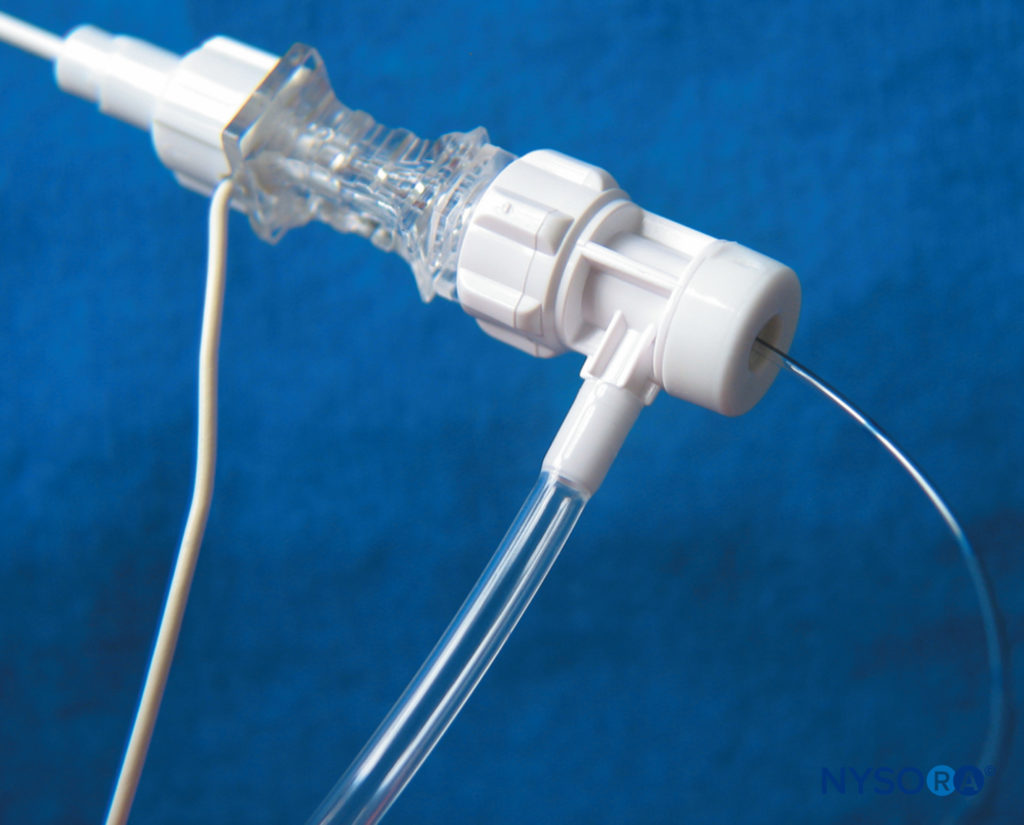
FIGURE 10. The Contiplex® Tuohy (B. Braun, Melsungen AG, Germany) consists of an insulated Tuohy needle with a pinpoint uninsulated tip, an integrated stimulating wire, extension tubing, and a connector with a hemostatic valve allowing the insertion of a catheter. This design enables simultaneous nerve stimulation, aspiration, and injection and allows for an immobile needle during catheter insertion. (Used with permission from Holly Evans, MD.)
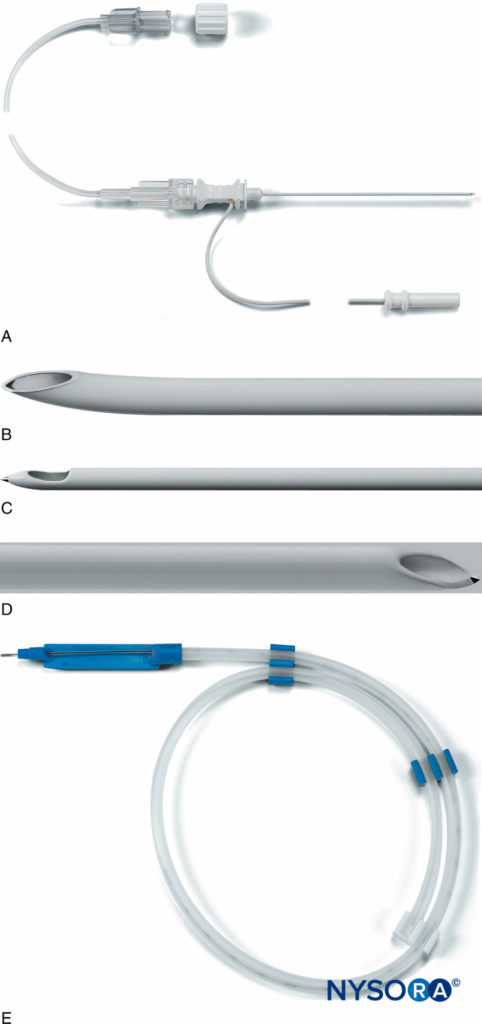
FIGURE 11. A: The Plexolong® system (Pajunk, Geisengen, Germany) incorporates an insulated needle, integrated stimulating wire, and extension tubing. B: The Plexolong Tuohy tip. C: The Plexolong Sprotte tip. D: The Plexolong Facet (or short-bevel) tip. E: The Plexolong catheter with thread-assist device. (Used with permission from Pajunk, Geisengen, Germany.)
Stimulating Catheters
Stimulating catheters provide the ability to conduct current to their distal end. They were developed as a tool to assess the proximity of the catheter’s distal tip to the neural structures and in an attempt to reduce the likelihood of secondary block failure. In one of the first published reports, Sutherland used a 1.0-mm outer-diameter ureteral catheter (Portex-Boots, Kent, UK) with a metal stylet. This author adapted the catheter by removing 50 mm from its distal tip and by rethreading the metal stylet so that it just protruded from the distal end (Figure 12). The proximal part of the stylet was folded over the proximal end of the catheter to maintain the correct length and to facilitate an electrical connection to the nerve stimulator. This equipment was used successfully for continuous sciatic nerve block following foot surgery.
Boezaart et al subsequently described their adaptation of a wire-reinforced epidural catheter for use as a stimulating catheter. They used a polyurethane (Tecothane®) thermoplastic catheter with an inner steel spring reinforcement and a nonferromagnetic stainless steel stylet (Arrow Theracath®) and removed part of the outer catheter to provide a 5-mm unsheathed metal tip. These authors described removing the inner stylet from an uninsulated 17-gauge Tuohy needle and advancing the epidural catheter through the needle such that the metal tip of the catheter did not make contact with the metal needle. This effectively allowed electrolocation during needle and catheter placement for continuous interscalene brachial plexus block. A later modification involved the addition of an insulated needle with an inner metal stylet to the set (StimuCath®, Arrow International). Further revisions resulted in the addition of an alligator clip to allow direct connection to a nerve stimulator (Figure 13) and, more recently, the addition of an integrated stimulating wire attached to the needle.
While this stimulating catheter was a significant advancement, several limitations existed. The needle bevel was sharper relative to other manufacturers’ equipment and was associated with concern about neural injury. The opacity of the blue 20-gauge and white 19-gauge catheters made identification of aspirated blood challenging. The relatively large uninsulated segment at the distal tip of the needle could decrease the accuracy of nerve identification. And, there was a risk that the area of exposed wire at the distal tip of the catheter could uncoil in vivo if cut or traumatized.
In an attempt to address some of these shortcomings, Kick et al developed another stimulating catheter (Stimulong® Catheter Set, Pajunk) and reported its reliability in a series of 10 supraclavicular brachial plexus blocks (Figures 14A and 14B). It consisted of a 20-gauge, single-hole, 400-mm polyamide catheter with a 405-mm indwelling removable con-ducting stylet. The metal wire stylet was insulated with Teflon coating along its length with the exception of the distal 0.3 mm. The proximal end had a plug to allow connection to a nerve stimulator. Subsequent modification involved incorporation of an integrated wire within the catheter (Stimulong Plus®, Pajunk) (Figure 15).

FIGURE 12. A closed-tip multiorifice catheter (top) and an open-tip single-orifice catheter (bottom). (Used with permission from Holly Evans, MD.)
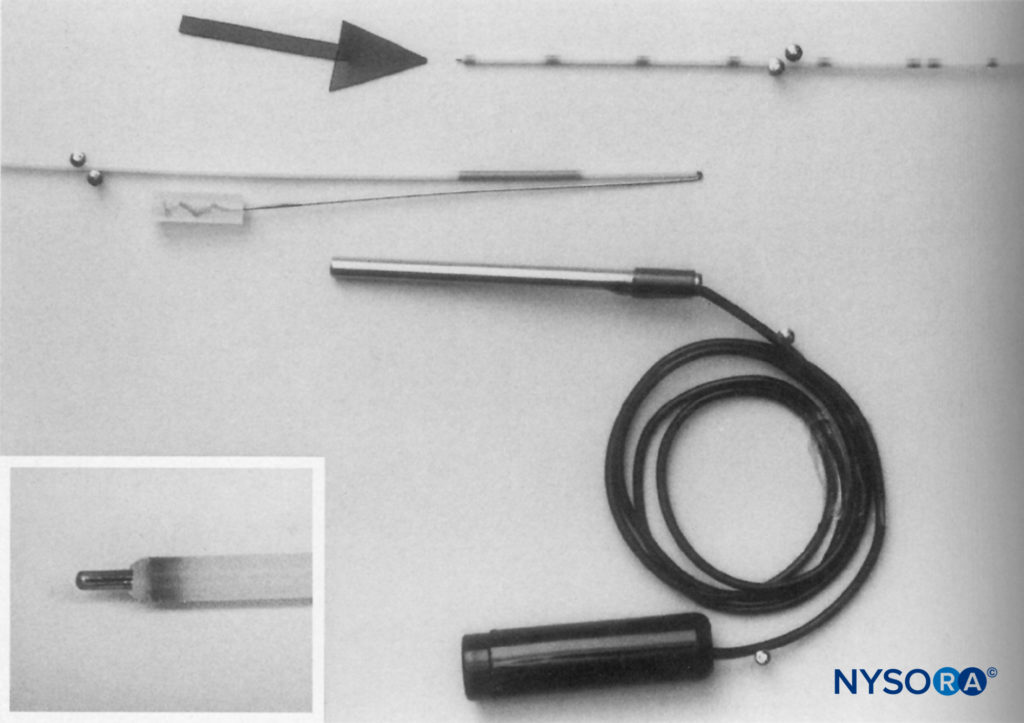
FIGURE 13. Shortened catheter with stylet just protruding from the distal end (inset) and folded over to maintain the position at the proximal end. Electrical connection is made by sliding a firmly fitting metal tube over the proximal end of the catheter. (Reproduced with permission from Sutherland ID: Continuous sciatic nerve infusion: expanded case report describing a new approach. Reg Anesth Pain Med. 1998 Sep-Oct;23(5):496-501.)
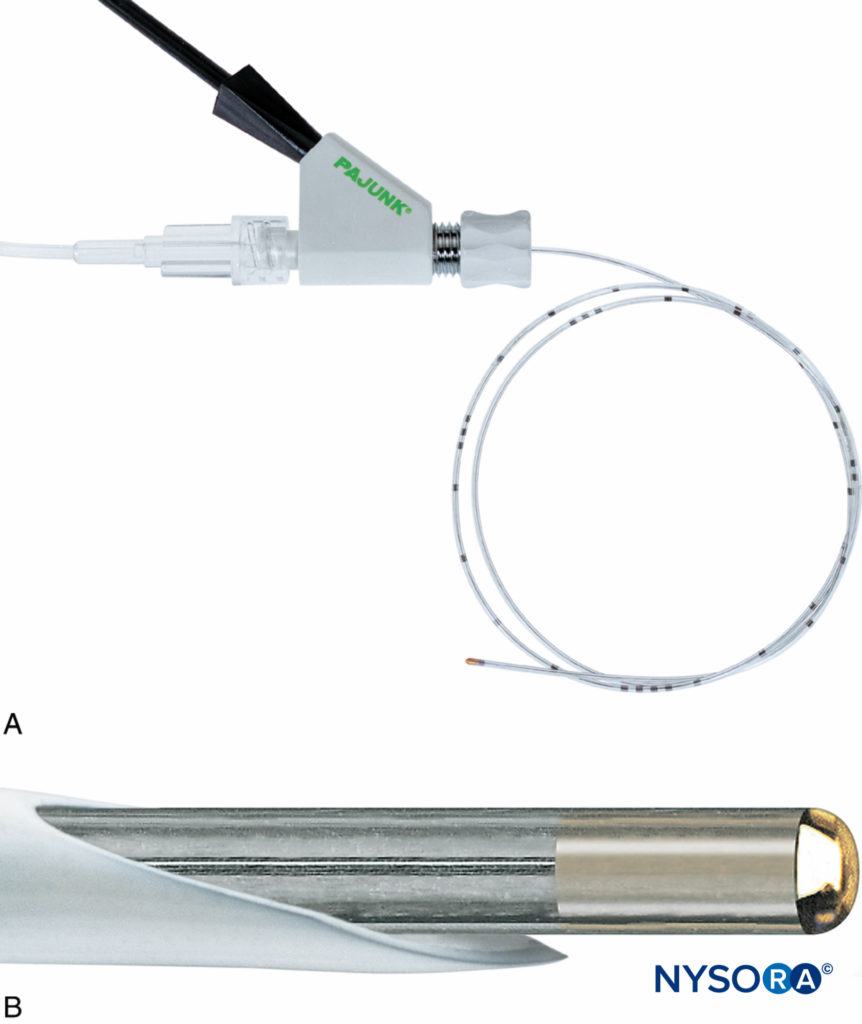
FIGURE 14. A: The stimulating catheter with integrated wire, Stimulong Plus® (Pajunk, Geisengen, Germany), is shown with its screw connector. The connector accepts an electric cable and extension tubing. B: The tip of the Stimulong Plus stimulating catheter is shown with its conductive golden tip. (Used with permission from Pajunk, Geisengen, Germany.)
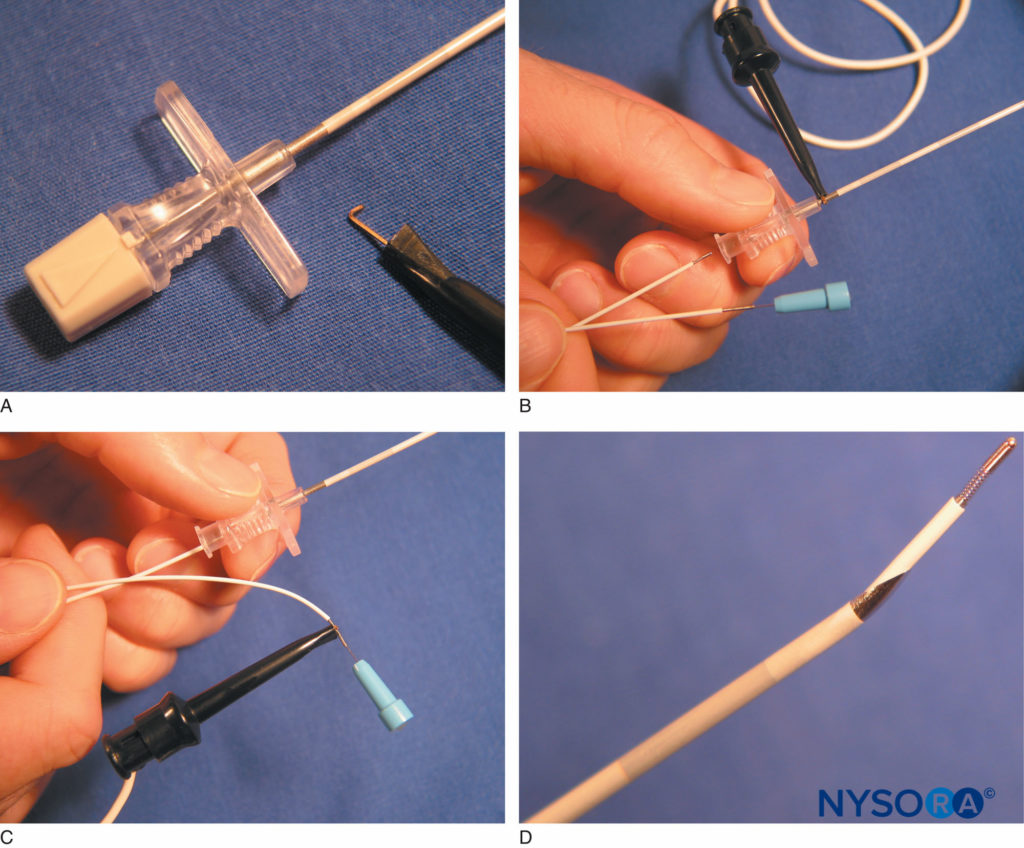
FIGURE 15. A: An alligator clip connects to an uninsulated segment on the proximal needle shaft of the StimuCath® system (Arrow International, Reading, PA). B: The StimuCath system consists of an insulated Tuohy tip needle, a stimulating catheter, and an alligator clip connector. C: The alligator clip is removed from the needle and attached to the catheter’s proximal end. This provides current output to the catheter’s distal tip. D: The Tuohy needle tip with a 5-mm long uninsulated segment is seen with the distal tip of the StimuCath stimulating catheter. (Used with permission from Holly Evans, MD.)
Stimulating catheters have been compared to nonstimulating catheters for CPNB. A stimulating catheter is associated with lower local anesthetic consumption and reduced requirement for additional opioid analgesia,; however, it may be more technically challenging to place under ultrasound guidance.
NYSORA Tips
- A stimulating catheter can confirm perineural location and may reduce the requirement for supplemental analgesia.
Echogenic Systems
The use of ultrasound to assist in the placement of CPNBs is now routine. Consequently, many manufacturers have incorporated echogenic features into their CPNB equipment. B. Braun incorporates laser reflectors into the needle of their Contiplex Ultra series. Pajunk catheters are wire reinforced and contain radiocontrast strips at their distal end.
NYSORA Tips
- Echogenic needles and catheters improve visualization of equipment inserted under ultrasound guidance.
NERVE LOCALIZATION SYSTEMS
Nerve Stimulator
Nerve stimulators generate an electrical current that passes through to the tip of an insulated needle or stimulating catheter to stimulate a nerve/plexus. A motor twitch appropriate for the nerve in question is sought. The limits of this technique must be appreciated. Studies of nerve blocks performed with both nerve stimulation and ultrasound guidance have shown that a needle tip can contact a nerve without resulting in a motor twitch. Furthermore, a needle tip can be within a nerve even when there is no motor twitch at relatively low current.
Ultrasound
Neural and perineural anatomy can be viewed with ultrasound imaging. Anatomical variants can be seen. The precise location and depth to neural structures can be estimated. Surrounding structures and vasculature can be identified and avoided. An image of the nerve block needle, catheter, and local anesthetic injection can be seen in relation to the nerve/plexus in real time. Though associated with the need for additional operator training and greater equipment expense, potential advantages of ultrasound include enhanced block success and reduced complications. Further details are provided in the relevant chapters.
Global Positioning System (Ultrasonix)
One of the newest nerve localization techniques involves the use of a global positioning system (ie, SonixGPS, Ultrasonix, Richmond, BC, Canada). Both the needle and the ultrasound probe contain sensors. As a result, the ultrasound screen displays the predicted needle trajectory and the location where the needle will intersect with the ultrasound beam.
Other: Fluoroscopy, Paresthesia, Fascial Click
There are additional nerve localization techniques. Fluoroscopy uses continuous x-ray imaging to localize a nearby bony structure. When appropriate, injection of contrast dye can be used to outline the location of perineural structures.
The paresthesia technique involves advancing the nerve block needle to contact a nerve/plexus. While sensory paresthesia may result, it is not guaranteed. Consequently, the paresthesia technique may be inaccurate and potentially harmful.
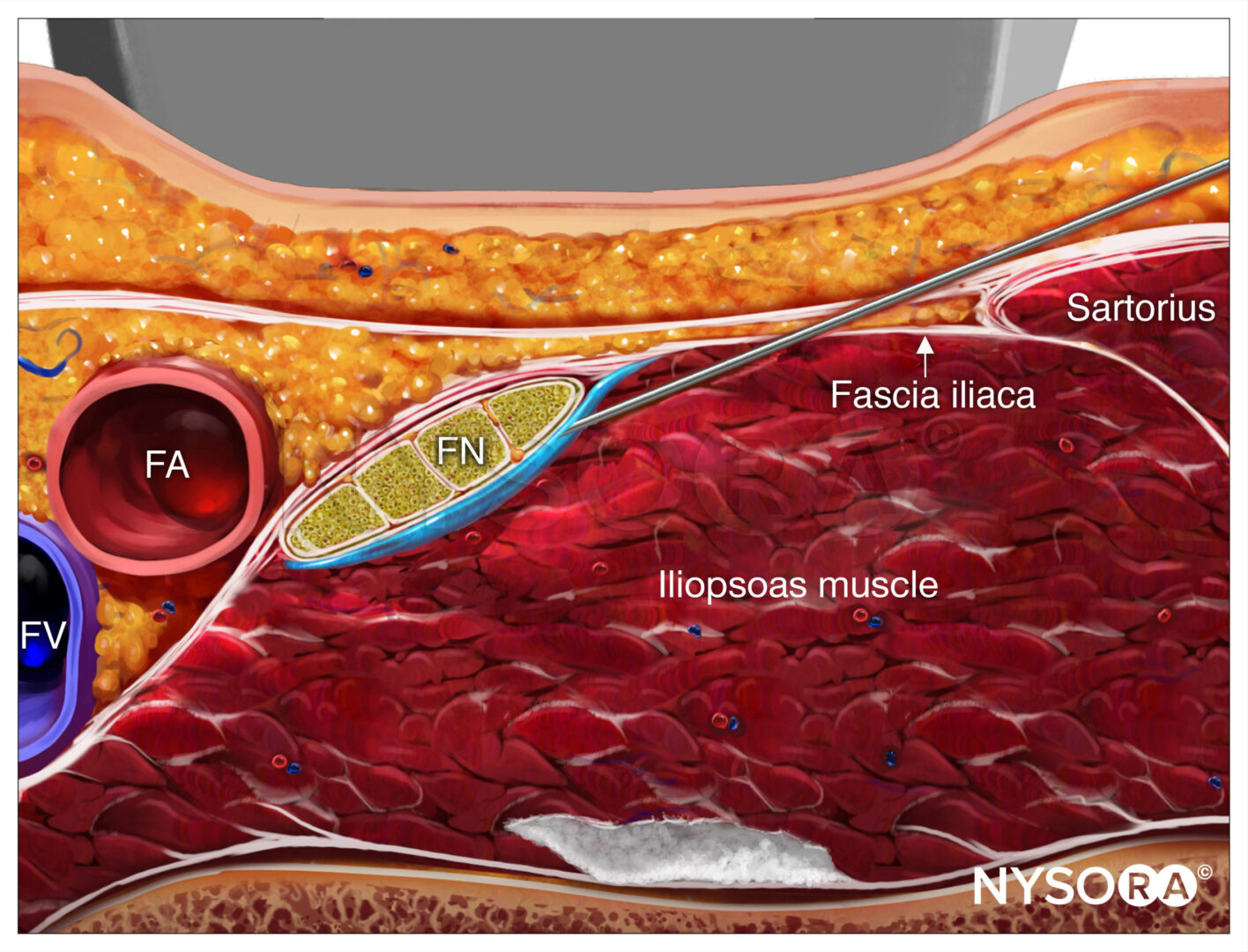
The fascial click technique involves a tactile “pop” sensation as a nerve block needle penetrates fascial layers. While this technique may be used for field blocks (i.e., fascia iliaca), its accuracy for nerve localization is limited.
NYSORA Tips
- Nerve stimulation can assist with identification of neural structures; however, limitations exist.
- Ultrasound guidance allows identification of important neural and perineural structures and allows confirmation of appropriate perineural injection of solute.
POSTBLOCK CONSIDERATIONS
Catheter Fixation Systems
One of the most common etiologies of secondary CPNB failure involves catheter displacement. Various techniques can be used to reduce the chance of catheter dislodgement. Subcutaneous tunneling of the catheter can be used in suitable locations. A liquid adhesive can be applied where the catheter exits the skin and under the clear occlusive dressing. There are adhesive dressings that have been specifically designed to fix a catheter to the skin (i.e., Epi-Guard, Copenhagen MedLab, Glostrup, Denmark).
Infusion Pumps
The infusion pump selected should be reliable and accurate. It should contain an appropriate volume of local anesthetic solution for the anticipated duration of infusion. The pump should be adjustable and have the ability to provide a continuous infusion as well as patient-controlled boluses at specifically timed intervals. These features enable the CPNB to be tailored to changing patient requirements.
Outpatient perineural infusion pumps must be reliable, accurate, and compact in size. Electronic or elastomeric pumps are available for outpatient use. Some are disposable, whereas others are reusable and must be returned by the patient to the hospital.
Patient Follow-up
Patients with perineural catheters must be followed daily. The catheter site is inspected for cleanliness, integrity, and possible dislodgement. The catheter site is evaluated for signs of infection, such as redness, warmth, or purulent discharge. Adequacy of analgesia is assessed, and modifications to the pain management plan are made. The patient is educated about the care of the numb extremity and fall prevention strategies if applicable. Symptoms of local anesthetic toxicity are sought. Pump function is reviewed.
Outpatient Perineural Catheters
Outpatients are contacted daily by telephone, at which time the follow-up outlined previously is performed. The procedure for catheter removal is discussed and performed by the patient and the patient’s caregiver at home. Disposable pumps are placed in the trash, while nondisposable pumps are returned in person or mailed to the hospital.
NYSORA Tips
- The safety and efficacy of CPNBs relies on properly securing the catheter to prevent dislodgement, appropriate follow-up by a knowledgeable health care worker, and adequate patient education regarding the care of the numb extremity, potential complications, and local anesthetic pump function.
CONCLUSION
This chapter highlights the equipment required for safe and effective perineural infusion of local anesthetic solutions. The development of specialized needle-and-catheter systems for this purpose was initially slow; however, currently available systems are reliable and applicable to most clinical situations.When considering deterrents to the more widespread adoption of CPNBs, lack of operator training has frequently been cited in the past. Currently, there are numerous learning opportunities available, including hands-on courses, preceptorships, virtual reality, and simulation training. There has also been improvement in patient and surgeon education regarding the potential benefits of CPNBs. Nevertheless, some concern persists about potential side effects, including nerve injury and falls. While ongoing research has enhanced our understanding of the mechanism of nerve injury related to regional anesthesia, further information is required. Other factors limiting the clinical application of CPNBs may be institutional and should be addressed at a local level. These include time pressure in busy operating rooms, lack of qualified assistants for block performance, lack of funds for equipment, or lack of support for postoperative care.
REFERENCES
- Serpell MG, Millar FA, Thomson MF: Comparison of lumbar plexus block versus conventional opioid analgesia after total knee replacement. Anaesthesia 1991;46(4):275–277.
- Edwards ND, Wright EM: Continuous low-dose 3-in-1 nerve block for postoperative pain relief after total knee replacement. Anesth Analg 1992;75(2):265–267.
- Matheny JM, Hanks GA, Rung GW, Blanda JB, Kalenak A: A comparison of patient-controlled analgesia and continuous lumbar plexus block after anterior cruciate ligament reconstruction. Arthroscopy 1993;9(1):87–90.
- Borgeat A, Schappi B, Biasca N, Gerber C: Patient-controlled analgesia after major shoulder surgery: Patient-controlled interscalene analgesia versus patient-controlled analgesia. Anesthesiology 1997;87(6):1343–1347.
- Singelyn FJ, Aye F, Gouverneur JM: Continuous popliteal sciatic nerve block: An original technique to provide postoperative analgesia after foot surgery. Anesth Analg 1997;84(2):383–386.
- Borgeat A, Tewes E, Biasca N, Gerber C: Patient-controlled interscalene analgesia with ropivacaine after major shoulder surgery: PCIA vs PCA. Br J Anaesth 1998;81(4):603–605.
- Singelyn FJ, Deyaert M, Joris D, Pendeville E, Gouverneur JM. Effects of intravenous patient-controlled analgesia with morphine, continuous epidural analgesia, and continuous three-in-one block on postoperative pain and knee rehabilitation after unilateral total knee arthroplasty. Anesth Analg 1998;87(1):88–92.
- Capdevila X, Barthelet Y, Biboulet P, Ryckwaert Y, Rubenovitch J, d’Athis F. Effects of perioperative analgesic technique on the surgical outcome and duration of rehabilitation after major knee surgery. Anesthesiology 1999;91(1):8–15.
- Lehtipalo S, Koskinen LO, Johansson G, Kolmodin J, Biber B. Continuous interscalene brachial plexus block for postoperative analgesia following shoulder surgery. Acta Anaesthesiol Scand 1999;43(3):258–264.
- Chelly JE, Greger J, Gebhard R, et al. Continuous femoral blocks improve recovery and outcome of patients undergoing total knee arthroplasty. J Arthroplasty 2001;16(4):436–445.
- White PF, Issioui T, Skrivanek GD, Early JS, Wakefield C: The use of a continuous popliteal sciatic nerve block after surgery involving the foot and ankle: Does it improve the quality of recovery? Anesth Analg 2003;97(5):1303–1309.
- Matthews PJ, Govenden V: Comparison of continuous paravertebral and extradural infusions of bupivacaine for pain relief after thoracotomy. Br J Anaesth 1989;62(2):204–205.
- Schultz P, Anker-Moller E, Dahl JB, Christensen EF, Spangsberg N, Fauno P: Postoperative pain treatment after open knee surgery: Continuous lumbar plexus block with bupivacaine versus epidural morphine. Reg Anesth 1991;16(1):34–37.
- Turker G, Uckunkaya N, Yavascaoglu B, Yilmazlar A, Ozcelik S: Comparison of the catheter-technique psoas compartment block and the epidural block for analgesia in partial hip replacement surgery. Acta Anaesthesiol Scand 2003;47(1):30–36.
- Nielsen KC, Greengrass RA, Pietrobon R, Klein SM, Steele SM: Continuous interscalene brachial plexus block provides good analgesia at home after major shoulder surgery-report of four cases. Can J Anaesth 2003;50(1):57–61.
- Zaric D, Boysen K, Christiansen J, Haastrup U, Kofoed H, Rawal N:. Continuous popliteal sciatic nerve block for outpatient foot surgery—A randomized, controlled trial. Acta Anaesthesiol Scand 2004;48(3):337–341.
- Buettner J, Klose R, Hoppe U, Wresch P: Serum levels of mepivacaine-HCl during continuous axillary brachial plexus block. Reg Anesth 1989;14(3):124–127.
- van den Berg B, Berger A, van den Berg E, Zenz M, Brehmeier G, Tizian C: Continuous plexus anesthesia to improve circulation in peripheral microvascular interventions. Handchir Mikrochir Plast Chir 1983;15(2):101–104.
- Camprubi Sociats I, Garcia Huete L, Sabate Pes A, Bartolome Sarvise C, Cochs Cristia J. Use of axillary perivascular blockage of the brachial plexus with a catheter as treatment in accidental intra-arterial injection of drugs. Rev Esp Anestesiol Reanim 1989;36(3):167–170.
- Haynsworth RF, Heavner JE, Racz GB: Continuous brachial plexus block using an axillary catheter for treatment of accidental intra-arterial injections. Reg Anaesth 1985;10:187.
- Berger JL, Nimier M, Desmonts JM: Continuous axillary plexus block in the treatment of accidental intraarterial injection of cocaine. N Engl J Med 1988;318(14):930.
- Aguilar JL, Domingo V, Samper D, Roca G, Vidal F: Long-term brachial plexus anesthesia using a subcutaneous implantable injection system. Case report. Reg Anesth 1995;20(3):242–245.
- Fischer HB, Peters TM, Fleming IM, Else TA: Peripheral nerve catheterization in the management of terminal cancer pain. Reg Anesth 1996;21(5):482–485.
- Smith BE, Fischer HB, Scott PV. Continuous sciatic nerve block. Anaesthesia 1984;39(2):155–157.
- Ansbro FP: A method of continuous brachial plexus block. Am J Surg 1946;71:716–722.
- DeKrey JA, Schroeder CF, Buechel DR: Continuous brachial plexus block. Anesthesiology 1969;30(3):332.
- Winnie AP: Interscalene brachial plexus block. Anesth Analg 1970;49(3):455–466.
- Selander D: Catheter technique in axillary plexus block. Presentation of a new method. Acta Anaesthesiol Scand 1977;21(4):324–329.
- Brands E, Callanan VI: Continuous lumbar plexus block–analgesia for femoral neck fractures. Anaesth Intensive Care 1978;6(3):256–258.
- Thompson GE, Rorie DK: Functional anatomy of the brachial plexus sheaths. Anesthesiology 1983;59(2):117–122.
- Tuominen M, Rosenberg PH, Kalso E: Blood levels of bupivacaine after single dose, supplementary dose and during continuous infusion in axillary plexus block. Acta Anaesthesiol Scand 1983;27(4):303–306.
- Manriquez RG, Pallares V: Continuous brachial plexus block for prolonged sympathectomy and control of pain. Anesth Analg 1978;57(1):128–130.
- Economacos G, Skountzos V: Nerve blocking of the sciatic and femoral nerves. Continual block with vein catheter on 44 patients. Acta Anaesthesiol Belg 1980;31 Suppl:223–228.
- Vatashsky E, Aronson HB: Continuous interscalene brachial plexus block for surgical operations on the hand. Anesthesiology 1980;53(4):356.
- Sada T, Kobayashi T, Murakami S: Continuous axillary brachial plexus block. Can Anaesth Soc J 1983;30(2):201–205.
- Neimkin RJ, May JW Jr, Roberts J, Sunder N: Continuous axillary block through an indwelling Teflon catheter. J Hand Surg Am 1984;9(6):830–833.
- Conacher ID, Kokri M. Postoperative paravertebral blocks for thoracic surgery. A radiological appraisal. Br J Anaesth 1987;59(2):155–161.
- Lonnqvist PA: Continuous paravertebral block in children. Initial experience. Anaesthesia 1992;47(7):607–609.
- Vaghadia H, Kapnoudhis P, Jenkins LC, Taylor D: Continuous lumbosacral block using a Tuohy needle and catheter technique. Can J Anaesth 1992;39(1):75–78.
- Chan V, Ferrante FM: Continuous thoracic paravertebral block. In Ferrante FM, VadeBoncoeur TR (eds): Postoperative Pain Management. Churchill Livingstone, 1993:403–414.
- Selander D, Dhuner KG, Lundborg G: Peripheral nerve injury due to injection needles used for regional anesthesia. An experimental study of the acute effects of needle point trauma. Acta Anaesthesiol Scand 1977;21(3):182–188.
- Selander D, Edshage S, Wolff T: Paresthesiae or no paresthesiae? Nerve lesions after axillary blocks. Acta Anaesthesiol Scand 1979;23(1):27–33.
- Montgomery SJ, Raj PP, Nettles D, Jenkins MT: The use of the nerve stimulator with standard unsheathed needles in nerve block. Anesth Analg 1973;52(5):827–831.
- Ford DJ, Pither C, Raj PP: Comparison of insulated and uninsulated needles for locating peripheral nerves with a peripheral nerve stimulator. Anesth Analg 1984;63(10):925–928.
- Anker-Moller E, Spangsberg N, Dahl JB, Christensen EF, Schultz P, Carlsson P: Continuous block of the lumbar plexus after knee surgery: A comparison of the plasma concentrations and analgesic effect of bupivacaine 0.25% and 0.125%. Acta Anaesthesiol Scand 1990;34(6):468–472.
- Ben-David B, Lee E, Croitoru M: Psoas block for surgical repair of hip fracture: A case report and description of a catheter technique. Anesth Analg 1990;71(3):298–301.
- Conception M: Continuous brachial plexus techniques. In Ferrante FM, VadeBoncoeur TR (eds): Postoperative Pain Management. Churchill Livingstone, 1993:359–402.
- Prosser DP: Adaptation of an intravenous cannula for paediatric regional anaesthesia. Anaesthesia 1996;51(5):510.
- Tan TS, Watcha MF, Safavi F, McCulloch D, Payne CT, Tuefel A: Cannulation of the axillary brachial sheath in children. Anesth Analg 1995;80(3):640–641.
- Rosenblatt R, Pepitone-Rockwell F, McKillop MJ: Continuous axillary analgesia for traumatic hand injury. Anesthesiology 1979;51(6):565–566.
- Steele SM, Klein SM, D’Ercole FJ, Greengrass RA, Gleason D: A new continuous catheter delivery system. Anesth Analg 1998;87(1):228.
- Fredrickson MJ, Ball CM, Dalgleish AJ: Catheter orifice configuration influences the effectiveness of continuous peripheral nerve block. Reg Anesth Pain Med 2011;36(5):470–475.
- Sutherland ID: Continuous sciatic nerve infusion: Expanded case report describing a new approach. Reg Anesth Pain Med 1998;23(5):496–501.
- Boezaart AP, de Beer JF, du Toit C, van Rooyen K: A new technique of continuous interscalene nerve block. Can J Anaesth 1999;46(3):275–281.
- Boezaart AP, De Beer JF, Nell ML: Early experience with continuous cervical paravertebral block using a stimulating catheter. Reg Anesth Pain Med 2003;28(5):406–413.
- Borene SC, Rosenquist RW, Koorn R, Haider N, Boezaart AP: An indication for continuous cervical paravertebral block (posterior approach to the interscalene space). Anesth Analg 2003;97(3):898–900.
- Kick O, Blanche E, Pham-Dang C, Pinaud M, Estebe JP: A new stimulating stylet for immediate control of catheter tip position in continuous peripheral nerve blocks. Anesth Analg 1999;89(2):533–534.
- Pham-Dang C, Kick O, Collet T, Gouin F, Pinaud M: Continuous peripheral nerve blocks with stimulating catheters. Reg Anesth Pain Med 2003;28(2):83–88.
- Casati A, Fanelli G, Koscielniak-Nielsen Z, et al: Using stimulating catheters for continuous sciatic nerve block shortens onset time of surgical block and minimizes postoperative consumption of pain medication after halux valgus repair as compared with conventional nonstimulating catheters. Anesth Analg 2005;101(4):1192–1197.
- de Tran QH, Munoz L, Russo G, Finlayson RJ: Ultrasonography and stimulating perineural catheters for nerve blocks: a review of the evidence. Can J Anaesth 2008;55(7):447–457.
- Morin AM, Kranke P, Wulf H, Stienstra R, Eberhart LH: The effect of stimulating versus nonstimulating catheter techniques for continuous regional anesthesia: a semiquantitative systematic review. Reg Anesth Pain Med 2010;35(2):194–199.
- Mariano ER, Loland VJ, Sandhu NS, et al: Comparative efficacy of ultrasound-guided and stimulating popliteal-sciatic perineural catheters for postoperative analgesia. Can J Anaesth 2010;57(10):919–926.
- Gandhi K, Lindenmuth DM, Hadzic A, et al: The effect of stimulating versus conventional perineural catheters on postoperative analgesia following ultrasound-guided femoral nerve localization. J Clin Anesth 2011;23(8):626–631.
- Robards C, Hadzic A, Somasundaram L, et al: Intraneural injection with low-current stimulation during popliteal sciatic nerve block. Anesth Analg 2009;109(2):673–677.