Vivian H. Y. Ip and Ban C. H. Tsui
INTRODUCTION
Regional anesthesia has become commonplace in many practices worldwide due to the increasing evidence of patient benefits, such as a reduction in pulmonary and thromboembolic complications, reduction in opioid consumption, as well as reduced pain and time to discharge and better quality of life in the immediate postoperative period. The increased popularity of regional anesthesia has resulted in advancement in techniques and equipment. The practice has evolved from using paresthesia for nerve localization to electrical nerve stimulation and, currently, to ultrasound. This chapter gives an overview of the equipment available in the practice of peripheral nerve blocks. It also outlines the equipment needed at various stages of the regional anesthesia procedure to ensure that it proceeds in an efficient and safe manner. The practice of regional anesthesia comprises equipment, protocols, and skills necessary to ensure that the block proceeds as smoothly and safely as possible—before, during, and after the block is administered.
REGIONAL block PREPARATION AND SETUP
Area and Monitoring
A quiet environment with all equipment necessary to perform regional anesthesia, together with resuscitation drugs and equipment within reach, is of paramount importance. An ideal location is an induction room where the patient can be monitored, premedicated and the regional block performed before transferring to the operating theater. A designated block area can be used to provide suitable, monitored procedure environment while optimizing operating room efficiency.
When performing the block, an assistant trained in regional anesthesia should be present to prepare and handle equipment and help with the injectate. The assistant should also be trained in performing resuscitation if it becomes necessary.
Regardless of where the block is performed, it is essential to have all equipment, drugs, and monitoring readily available. The best way to gather all the necessary equipment and drugs is the setup of a storage cart (Figure 1), which should be well labeled with the supplies readily identifiable.
NYSORA Tips
- An equipment cart should contain all of the drugs, needles, and catheters necessary for regional anesthesia as well as resuscitation medications and equipment.
Block Area General Equipment
Commonly used items should be stocked in the storage cart and refilled when necessary. The storage cart should contain the following:
- Sterile skin preparation solution, sponges/gauze, drape, marking pen, ruler for landmark identification, ultrasound gel, hypodermic needles for skin infiltration and drawing up 5% dextrose (5% dextrose in water, D5W).
- A selection of sedatives, for example, midazolam (0.5–3 mg IV), and short-acting opioids such as fentanyl (50–100 μg IV), and propofol (20–100 mg IV) for nerve blocks that are more uncomfortable and require deeper sedation (eg, ankle block).
- Local anesthetics and normal saline for drug dilution if necessary. Local anesthetics are ideally stored in a separate compartment from intravenous drugs to avoid drug error.
- Intravenous cannulas. All patients should have an intravenous cannula inserted in case of local anesthetic toxicity.
- Dressings for intravenous cannulas, clear dressings for covering the ultrasound transducer used in single-shot nerve blocks, transducer cover, gel, and dressings for nerve block catheter insertion.
Emergency Drugs and Resuscitation Equipment
The use of ultrasound allows the visualization of the injectate and therefore, it has significantly reduced, but not eliminated, the risk of severe local anesthetic systemic toxicity (LAST). However, resuscitation equipment and medications should always be immediately available in the block area.
Resuscitation Equipment
- Oxygen supply, nasal airway, and O2 masks
- Oral airways of different sizes, laryngeal masks, and endotracheal tubes
- Laryngoscopes (Macintosh and Miller blades)
- Bag-mask ventilation device
- Suction
- Selection of various size intravenous cannulas
- Defibrillator
Resuscitation Drugs and Suggested Doses Intravenously
- Atropine (300–600 μg).
- Epinephrine (10–100 μg).
- Suxamethonium (40–100 mg).
- Ephedrine (5–15 mg).
- Phenylephrine (100–200 μg).
- Glycopyrrolate (200–400 μg).
- Intralipid® 20% (1.5 mL/kg over 1–2 minutes as an initial bolus, which can be repeated two to three times for persistent asystole. After the bolus, an infusion can be started at 0.25 mL/kg/min for 30 to 60 minutes; increase the infusion rate for refractory hypotension). Ideally, Intralipid should be kept in a container with a protocol for use and equipment to draw up the drug.
NYSORA Tips
- During ultrasound-guided peripheral nerve block, visualization of injectate spread can minimize the risk of intravascular injection. The entire dose of local anesthetic should never be injected without seeing the spread of injectate on ultrasound, as this is suggestive of an intravenous injection
Documentation
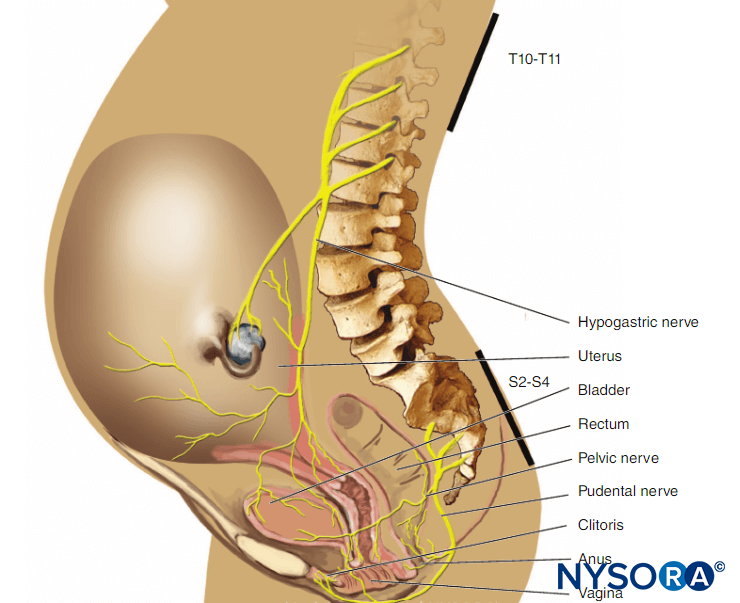
A pre-block checklist is paramount to ensure correct block performance at the appropriate site on the patient’s body and includes documenting preoperative conditions (eg, relevant neurological deficits and comorbidities) and discussing risks and benefits and obtaining proper consent. In most countries, standardized medical documentation protocols have been established for induction and maintenance of general anesthesia. This documentation includes information about arterial blood pressure, heart rate, oxygenation, and details of common procedures such as maintaining airway status and providing endotracheal intubation. Likewise, there are similar standard guidelines for documenting neuraxial anesthesia, including information about block-level; sterility provisions; equipment and technique used; the incidence of cerebrospinal fluid, blood, or paresthesia; and local anesthetic injection. In contrast, no such guidelines exist for documenting peripheral nerve blocks, even though they are used routinely in clinical practice and possess the same medicolegal implications as general and neuraxial anesthesia. One limitation of the lack of a documentation protocol for peripheral nerve blocks is the relative dearth of information available for those who wish to retrospectively review a regional procedure for quality assurance, research, or legal reasons. An example of block documentation is seen in Figure 2.
More information about monitoring and documenting of PNBs is addressed in “Monitoring, Documentation, and Consent for Regional Anesthesia Procedures“.
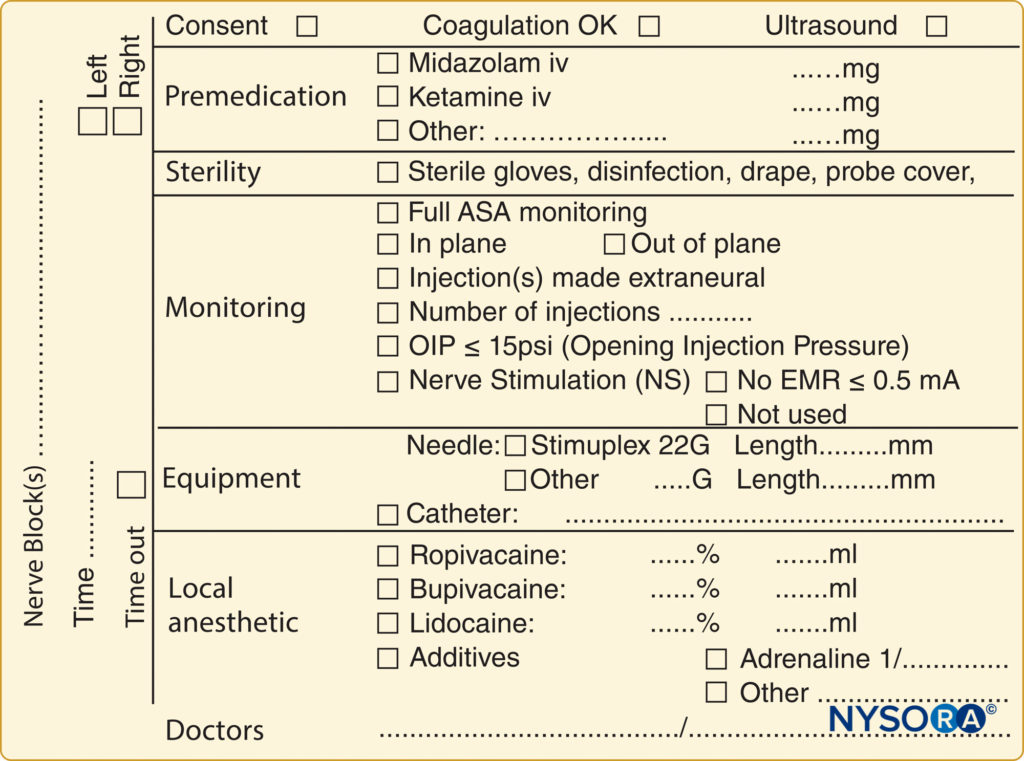
FIGURE 2. An example of block documentation as used at NYSORA-Europe CREER (Center for Research, Education and Enhanced Recovery) at ZOL (Ziekenhuis Oost-Limburg, Genk, Belgium).
EQUIPMENT FOR INDUCTION OF REGIONAL ANESTHESIA
Needles for Single-Shot Nerve Blocks
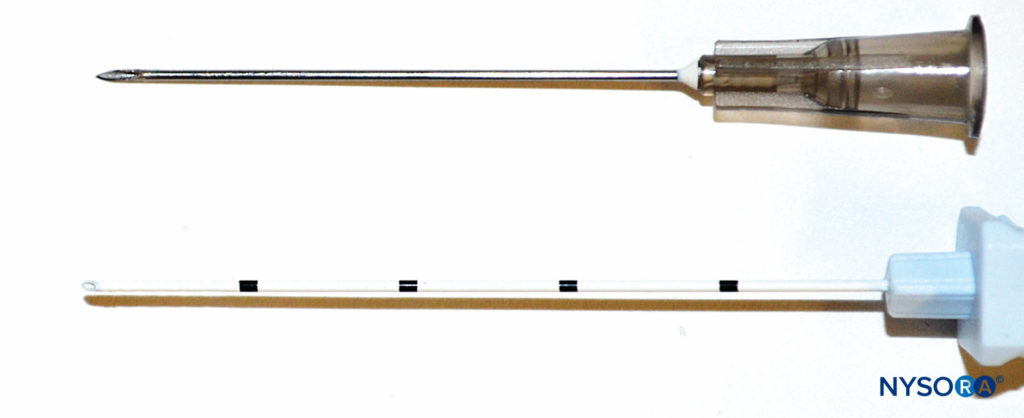
Currently, there are many different types of peripheral nerve block needles on the market. Insulated needles are commonly used with nerve stimulation. With the introduction of ultrasound, echogenic needles have been used widely for better visualization. Commercially available needles for single-shot nerve blocks usually come with pre-attached extension tubing to facilitate aspiration and injection of D5W or local anesthetics and feature a female attachment for connection to a nerve stimulator. One should note that the Luer lock for the attached tubing may occasionally be loose, which can lead to leakage of injected local anesthetic as well as air on aspiration.
Needle-Tip Design
Nerve injury can be caused by direct nerve penetration or forceful needle-nerve contact. The bevel of the needle can have an impact on the extent of damage on needle insertion close to a nerve. Short-beveled needles (Figure 3) may have the advantage of reducing nerve damage caused by cutting or penetration of the nerve, whereas long-beveled (14°) needles have been shown to be more likely to penetrate perineurium and cause fascicular injury than a short-beveled (45°) needle, especially when oriented transversely to the nerve fibers. On the other hand, short-beveled needles can cause greater injury in the case of nerve or fascicular penetration. Blunt, noncutting needles and Tuohy needles provide better feedback and enhanced feel for the “pop” that occurs when puncturing through the fascia. However, a needle that is too blunt may hinder fascial puncture, resulting in higher applied pressure and potentially “overshooting” after puncturing the fascia. Pencil-point and Tuohy needles can also cause greater post-traumatic inflammation, myelin damage, and intraneural hematoma.
Neuraxial blocks can be performed with needles of different tip styles. Despite their description as atraumatic, Whitacre or Sprotte needles (Figure 4) can be traumatic to the tissues on entry, with tearing and severe disruption of collagen fibers (see “Ultrastructural Anatomy of the Spinal Meninges and Related Structures“). This contrasts with the Quincke needle, a so-called cutting needle (Figure 4), also used for neuraxial blocks. Nevertheless, the overall consensus is that neuraxial blocks performed with an atraumatic needle are associated with less risk of post-dural puncture headache.
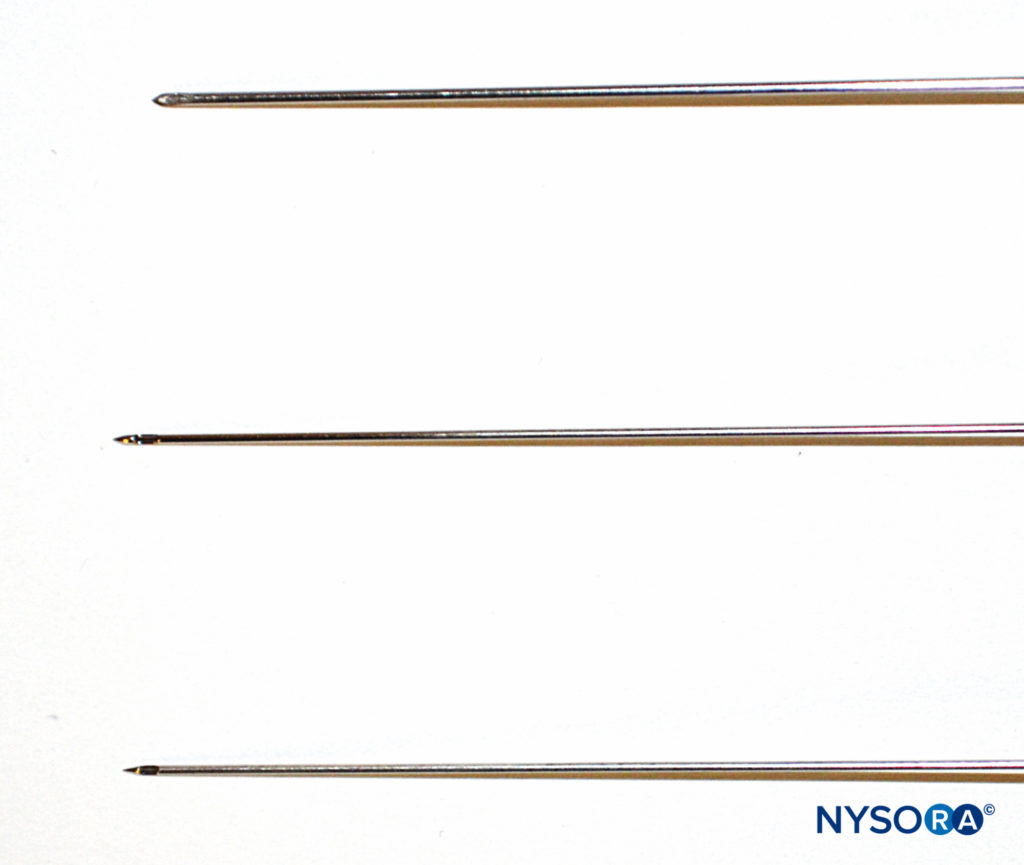
FIGURE 4. Different needles for spinal anesthesia: Whitacre (top), Sprotte (middle), and Quincke (bottom).
Needle Length
The choice of the needle length depends on the specific block. For instance, deeper blocks, such as sciatic nerve block, will require longer needles (eg, 100–120 mm). The use of ultrasound can help determine the distance of the trajectory toward the target nerve. A needle that is too short will not reach the target site, while a long needle may be difficult to maneuver and may be advanced too deeply. Needles should have depth markings (Figure 5) for monitoring of the depth of penetration into the tissue. The correct needle length (shortest possible) will allow for better handling and manipulation.

FIGURE 5. Nerve block needle showing centimeter markings that can be used as an example to monitor the depth of penetration. Cornerstone reflectors to aid in visualization under ultrasound can also be seen at the distal end.
Needle Gauge
In general, 22-gauge insulated needles are probably used most commonly for single-shot peripheral nerve blocks. With needle size, a balance must be sought between patient comfort and bending of the needle as it punctures through the skin. Because longer needles tend to bend more easily during advancement and are more difficult to steer during deep blocks, a larger-gauge needle may be required, as smaller-gauge needles lack the rigidity and bend more readily. Larger-gauge needles should be used with caution, as they are associated with increased severity of tissue injury and hematoma, while smaller-gauge needles carry the more serious risk of the tip being inserted intrafascicular. Also, resistance tends to be increased on injection with smaller-gauge needles, and it also takes longer for blood to be aspirated back should the tip be intravascular.
Echogenic Needles
Since the introduction of ultrasound-guided peripheral nerve blocks, there has been an effort to manufacture needles with improved visibility on ultrasound. Echogenic needles reflect ultrasound beams through a variety of mechanisms, including a special coating that traps micro–air bubbles, grooves near the needle tip, or echogenic “dots” made by “cornerstone” reflectors (see the distal end of needle in Figure 5). Needles with enhanced echogenicity may reduce visualization time during ultrasound-guided procedures. An echogenic needle, with or without ultrasound beam steering, is better visualized compared to a non-echogenic needle at 60°–70° angles of insertion. In contrast, the non-echogenic needle with beam steering was more visible at a 40° insertion angle compared with the echogenic needle.
Continuous Catheter Assemblies
Continuous infusion of local anesthetic has proved effective in providing long-term postoperative analgesia in a variety of settings. Peripheral nerve block catheters also enable titration of medication in small-dose aliquots. Equipment for continuous peripheral nerve blocks is discussed in detail in “Equipment for Continuous Peripheral Nerve Blocks“. Catheter-over-needle assemblies have been gaining popularity for delivering continuous regional anesthesia and analgesia and are discussed briefly in this chapter.
Historically, it has been well recognized that perineural catheters are associated with leakage and migration. However, the catheter-over-needle system design may reduce these obstacles and has renewed interest in continuous regional techniques. The difference between this assembly and traditional catheter-through-needle assemblies is also the position of the needle in relation to the catheter, whether within or surrounding the catheter (Figure 6). With catheter-through-needle assemblies, a gap is left between the skin and the catheter on the removal of the needle. In contrast, withdrawal of the needle in the catheter-over-needle assembly does not affect the snug fit of the catheter in the skin because the needle is housed inside the catheter.
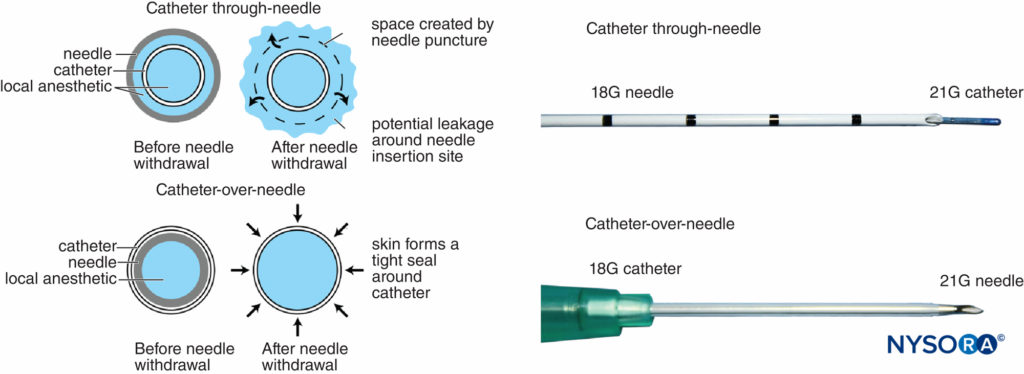
FIGURE 6. Left, schematic showing the difference between traditional catheter-through-needle (top) and catheter-over-needle (bottom) designs with respect to risk of leakage from the skin puncture site. In the former, the needle hole diameter is larger than the catheter diameter, leaving space for local anesthetic to leak when injected. In the latter, the puncture hole is smaller than the catheter diameter, allowing the catheter to be held tightly by the surrounding skin. Right, differing distal ends of catheter-though-needle (top) and catheter-over-needle (bottom) assemblies.
There are a few variations on this design that are marketed by different brands. For example, Contiplex (B-Braun Medical, Melsungen, Germany) design features the catheter-over-needle as a single unit (Figure 7).
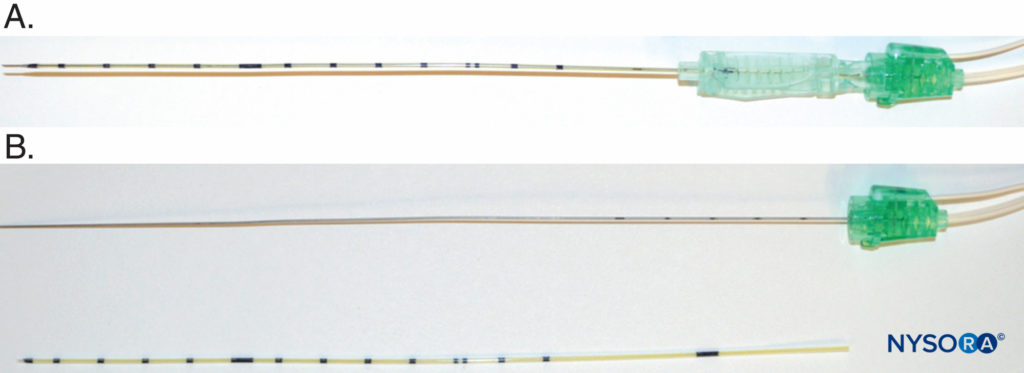
FIGURE 7. A: The Contiplex (B-Braun Medical, Melsungen, Germany) catheter-over-needle assembly, which features a long needle covered by the catheter and a clip that can be moved along the needle length for easy manipulation of the unit. B: B. Braun catheter-over-needle components: needle with extensions for nerve stimulation and injection (top) and catheter with centimeter marks (bottom).
Another variation is the recently introduced E-Cath (Pajunk MEDIZINTECHNOLOGIE GmbH, Geisingen, Germany kit with a “catheter-within-catheter” design, which features two components, the outer catheter sheath, and the flexible inner catheter, that create a nonkinkable unit (Figure 8). The initial device resembles an intravenous cannula, with a needle within the outer catheter, which is inserted proximal to the target nerve under ultrasound guidance. The distal end of the needle protrudes for its electrical conductive property. Once in place, the needle is withdrawn from the unit, leaving the outer catheter in situ, and an inner catheter is inserted into the outer catheter to replace the needle and is Luer locked in place for injection (Figure 8). The inner catheter literally replaces the needle, and the inner catheter tip is essentially at the exact location where the needle tip was before needle withdrawal.
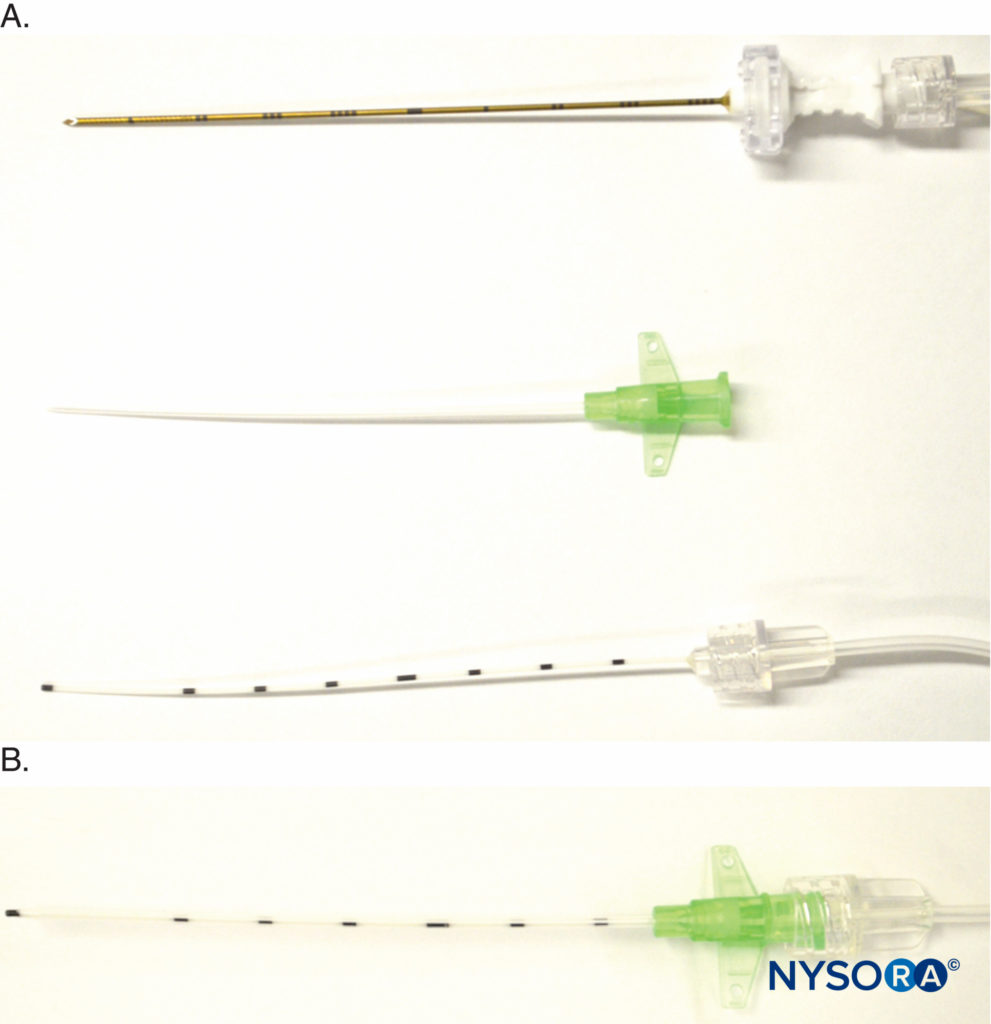
FIGURE 8. Detail of E-Cath (Pajunk MEDIZINTECHNOLOGIE GmbH, Geisingen, Germany catheter-over-needle components. A: Top, needle with extension tubing for nerve stimulator and a fluid giving set; middle, the catheter is placed over the needle to create a single unit that is inserted near the target nerve; bottom, the inner catheter is inserted into the outer catheter following needle withdrawal once the catheter-over-needle unit is at the desired position. B: Luer lock holding together the inner and outer catheters.
Several advantages of the catheter-over-needle design include potential:
- Simple to use with an insertion technique compared to that of a single-shot nerve block
- Less leakage from the catheter insertion site, for example, during shoulder surgery when the patient is in a sitting position with potential for surgical field contamination
- Less risk of dressing adhesive disruption
- Fewer steps and less risk of dislodgement
- Easy visualization of the catheter, especially the catheter tip
Nerve-Locating DevicesPeripheral Nerve Stimulators
Peripheral nerve stimulators were the primary nerve-seeking device in the decades preceding widespread use of ultrasound guidance. The combined use of ultrasound and nerve stimulation creates a more objective method of achieving accurate and safe blocks while allowing monitoring and visualization of the block needle and targets in real-time. With the introduction of ultrasound, the role of nerve stimulators has changed from nerve seeking to monitoring for needle-nerve contact or intraneural needle tip placement. In addition, nerve stimulation can be used as a confirmation technique and guide in placing epidural catheters using the electrical epidural stimulation (Tsui) test. Key properties of commercially available nerve stimulators are briefly highlighted next (Figure 9).
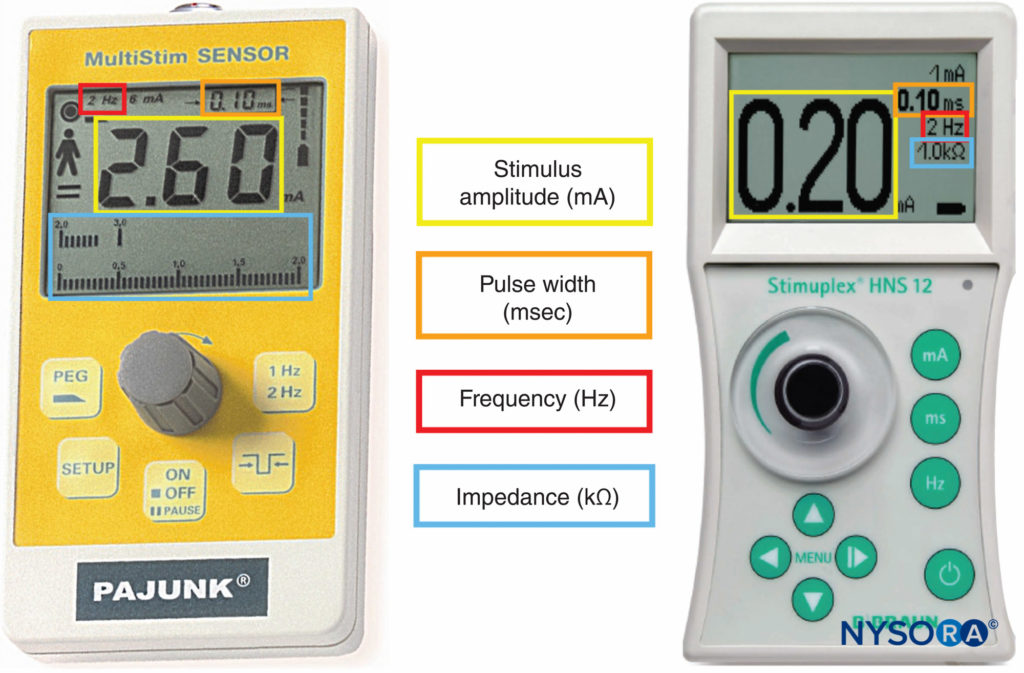
FIGURE 9. Peripheral nerve stimulators allowing measurement of stimulus amplitude, pulse width, frequency, and electrical impedance.
Constant Current Output and Display
Most modern models now deliver constant current, and current output can be set in frequency, pulse width, and current milliamperes (mA). The primary advantage of a constant current output nerve stimulator is its ability to deliver a stable current output in the presence of varied resistances.
Display
A clear digital display with accuracy to two decimal places is an important feature of the electrical nerve stimulator. This display must indicate the actual current delivered to the patient and not simply the target current setting. Some nerve stimulators are equipped with low- (up to 6 mA) and high-output (up to 80 mA) ranges. The lower range is used primarily to alert for potential intraneural needle placement, while the higher range is mainly used for the epidural stimulation test (1–10 mA).
Variable Pulse Width
Short pulse widths (ie, 0.04 ms) are a better indicator of the distance between the needle and the nerve, based on changes in current. In contrast, with long pulse widths (ie, 1 ms), there is little difference in the current required to stimulate the nerve, regardless of whether the stimulating needle is in direct contact with the nerve or 1 cm away. At a pulse width of 0.04 ms, there is a large difference in the stimulating current required when comparing direct contact with the nerve versus a distance of 1cm away.
Pulse width also has a role in the successful use of the electrical epidural stimulation test. The proper pulse width must be used for different applications of the test, be it a peripheral or neuraxial block. Table 1 summarizes the appropriate pulse width for different applications.
TABLE 1. Pulse widths for different applications during peripheral nerve block and neuraxial block.
| Pulse Width | Application | Typical Threshold Range |
|---|---|---|
| 0.1 ms | Motor peripheral nerve | Avoid < 0.3 mA |
| 0.2 ms | Epidural spaceIntrathecal space | 1–15 mA < 1 mA |
| 1 ms | Epidural space | 6 mA |
Clearly Marked Polarity of the Electrodes
The polarity of the needle will affect its ability to stimulate the nerve at a given current and should be clearly marked. The cathode (black) is selected as the stimulating electrode because it is three to four times more effective than the anode at depolarizing the nerve membrane.
Variable Pulse Frequency
Most new stimulators have an option to change the frequency at which the electrical pulse is delivered. Although some commercially available peripheral nerve stimulators can allow adjustment of frequency up to 5 Hz, the optimal frequency of the electrical pulse is between 0.5 and 4 Hz. Most users select a frequency of 2 Hz. When using a lower frequency, such as 1 Hz (one stimulus per second), the needle must be advanced slowly to avoid missing the nerve between stimulations.
Disconnect and Malfunction Indicators
The disconnection and malfunction of nerve stimulators should be easily detectable, and an indication of battery power is essential. Most nerve stimulators use a change in tone or light to warn when the circuit is not complete and if the pulse current cannot be delivered. The value of the tone/light change on disconnection of the circuit was demonstrated recently with a novel use for a peripheral nerve stimulator in guiding an insulated needle with an uninsulated tip into the tracheal lumen for airway topicalization. The change in tone/light indicated whether the tip was in contact with tissue (closed circuit) or suspended in the air-filled trachea (open circuit) (Figure 10).
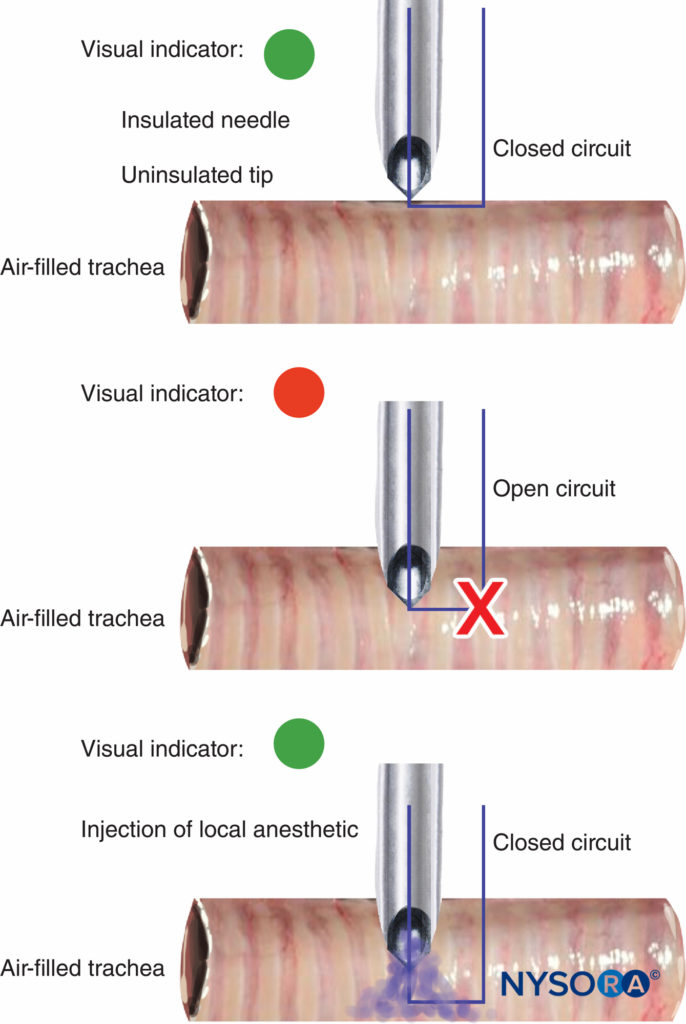
FIGURE 10. Change in peripheral nerve stimulator light and tone signal on the entry of uninsulated needle tip into tracheal lumen during airway topicalization (top and middle). With the injection of local anesthetic, electrical current is closed, causing another light/tone signal change (bottom).
Electrical Impedance
Some modern nerve stimulators display the total impedance between the needle tip and ground electrode. The significance of this property in monitoring intraneural needle-tip placement is discussed in the section on monitoring devices in this chapter.
Other Accessories
A probe may be used for performing percutaneous electrode guidance during surface nerve mapping (Figure 11). A small remote hand control or foot pedal allows a single operator to adjust the current output of a nerve stimulator without an assistant, although this is seldom used clinically (Figure 12).

FIGURE 12. Remote hand-controlled (left) and foot-controlled (right) devices for adjusting nerve stimulator current output.
Ultrasound
The introduction of ultrasound technology has revolutionized the field of regional anesthesia, allowing nerve structures, needles, and other subcutaneous objects to be visualized in real-time. Ultrasound undoubtedly can improve the safety and ease of performing nerve blocks; however, this is largely operator dependent.
There are a number of commercially available portable ultrasound machines that are suitable for regional anesthesia (Figure 13). These machines can be transported easily, and image quality and resolution are equivalent or similar to those of stationary ultrasound machines. The transducer (or probe) is the most important element of the ultrasound machine; transducers of various footprints and beam planes are available, allowing the user to scan most surfaces on individuals of various body habitus. The quality of ultrasound machines is constantly improving, with better ergonomic options and ease of use, a higher resolution with improved transducers, improved portability, and reduction in cost.
Monitoring Devices Monitoring for the Patient
It is important to apply routine monitoring for patients undergoing regional anesthesia with or without sedation. Toxicity from local anesthetic overdose and intravascular injection and oversedation are potential complications of regional anesthesia. Therefore, one should be vigilant with regard to patient monitoring. One should also be aware that toxicity from local anesthetic can occur within the first half-hour after injection of medication due to the peak in plasma concentration (typically 20–30 minutes). Local anesthetic systemic toxicity is discussed in detail elsewhere.
General monitoring for the patient includes examining the following:
- Electrocardiogram.
- Noninvasive blood pressure.
- Pulse oximetry.
- For ventilation: The adequacy of ventilation for regional anesthesia without sedation can be performed by qualitative clinical monitoring. However, for patients who require sedation, capnography should be used unless it is precluded by the patient, procedure, or equipment.
Monitoring for Intraneural Injection Paresthesia
Prior to the introduction of nerve stimulation technology, paresthesia was the only means of nerve localization. However, there has been emerging evidence suggesting painful paresthesia can lead to persistent neurological symptoms and neuropathy. Thus, most clinicians are not only abandoning seeking paresthesia but also, with awake or lightly sedated patients, are using paresthesia as a signal to warn of needle-nerve proximity.
Electrical Nerve Stimulation
The role of the nerve stimulator has shifted since the introduction of ultrasound. In most cases, nerve stimulation is no longer used as a primary tool for nerve localization but is instead used for monitoring to minimize intraneural injection. Nerve stimulators allow the user to monitor two electrophysiological properties during nerve block performance: the nerve stimulation threshold and electrical impedance.
- Nerve stimulation threshold: A nerve stimulation threshold of less than 0.2 mA may suggest intraneural needle-tip location or needle-nerve contact. When used alone, nerve stimulation lacks sensitivity and should, therefore, be used in conjunction with other monitoring as described in this section. Observation of a motor response signifies needle-nerve proximity with a low threshold current; however, a lack of motor response at a 0.2 mA or less threshold with a pulse width of 0.1 ms does not always rule out intraneural needle placement.
- Electrical impedance: Many modern nerve stimulators are capable of measuring impedance. In an electrical circuit, direct current (DC) is the flow of electric charge in only one direction, whereas alternating current (AC) describes the flow of electric charge that reverses direction periodically. In nerve stimulation, pulsating DC is used. Because pulsating DC shares characteristics of both AC and DC waveforms, the electrical resistance of the nerve stimulation circuit is often referred to as impedance. Impedance is highly sensitive to tissue composition and varies depending on the water content of the tissue. Because there is variation in water and lipid content between intraneural and extraneural spaces, with the former having a considerably greater amount of nonconduction lipid and lower water content, a significant difference in intraneural and extraneural impedance has been demonstrated. A recent study in adult patients also showed that an increase in impedance greater than 4.3% may indicate intraneural needle placement. Furthermore, discrimination between nerve tissue and other tissue types has been improved by combining several impedance variables at multiple measurement frequencies to increase precision. Electrical impedance also changes on intravascular and perineural injection of D5W. A change in impedance may, therefore, warn against injection in a location that could potentially cause nerve damage or other sequelae.
NYSORA Tips
- A sudden change in impedance may indicate that the needle is entering different tissues.
Ultrasound Imaging
With the rising popularity of ultrasound guidance for peripheral nerve blocks, there has been a common misunderstanding that ultrasound can help avoid intraneural injection. To improve the safety margin, the needle tip should be visualized at all times during advancement of the needle during an in-plane approach; however, this can be challenging, even in experienced hands. In addition, there is a significant learning curve associated with using ultrasound in regional anesthesia, both with in-plane and out-of-plane approaches. When an out-of-plane approach is used, the needle shaft could be mistaken for the tip, which would be further downstream of the ultrasound beam. During injection, nerve swelling as a result of intraneural injection can also be difficult to note in real-time. Moreover, by the time nerve swelling is noticed, it may be too late to prevent nerve injury as it takes only a minuscule volume of local anesthetic to rupture the perineurium when the needle is placed intrafascicular. Finally, current ultrasound resolution is not high enough to recognize intrafascicular injection, the most severe event in terms of nerve damage. As such, nerve injury with peripheral nerve blocks despite the use of ultrasound continues to be reported. The rate of residual paresthesia or numbness after ultrasound-guided peripheral nerve block is estimated to be 0.18% up to 16%. Therefore, ultrasound should not be used as the sole guidance device but, rather, should be used in conjunction with other monitoring modalities to minimize the risk of intraneural injection.
Injection Pressure Monitoring
Monitoring injection pressure can help distinguish needle-tip location in the perineural tissue versus the needle-nerve contact or intrafascicular needle placement (ie, perineural vs. intraneural-intrafascicular).Results of several studies suggest that high-pressure injection into the intraneural space, even with small volumes, can be a major contributor to mechanical injury of neurological tissue during peripheral nerve blocks. The rationale and basis for nerve damage from high-pressure injection are likely a combination of mechanical injury from breaching the perineurium, leading to interference with endoneural microcirculation, and chemical injury from neurotoxicity of local anesthetics.
Using canine models, it was shown that high injection pressure (>20 psi) can result in persistent neurological damage indicative of intrafascicular injection. However, not all intraneural injection results in high injection pressure and subsequent neurological deficit. This could be due to an intraneural extrafascicular injection or the beveled needle tip not being completely within the nerve. In these cases, the injectate may push the nerve out of the way, avoiding a high-pressure injection. Nevertheless, intraneural injection is generally not recommended. Forceful needle-nerve contact and displacement have also been shown to cause inflammatory changes to nerves. Since a recent study demonstrated that high opening injection pressure (≥15 psi)—the pressure that must be overcome before injection can commence—may be indicative of intraneural needle placement, it is important to monitor injection pressure carefully during local anesthetic injection. Furthermore, high injection pressure can also cause undesired neuraxial spread during certain regional blocks close to the neuraxis, for example, lumbar plexus block or brachial plexus block.
NYSORA Tips
- It has been suggested that opening injection pressure should be kept below 15 psi to improve safety. Opening pressure is not dependent on needle size, needle type, injection speed, and syringe size.
Methods of monitoring injection pressure include the following: syringe feel, inline pressure manometer, and compressed air injection technique (CAIT).
- Syringe feel. Traditionally in clinical practice, a subjective “syringe feel” technique has been used to assess resistance to injection of local anesthetic and is performed by the anesthesiologist or assistant while the anesthesiologist maintains the correct needle position. Needless to say, this subjective approach is not reliable and is operator dependent. Different needle lengths, diameter, and syringe types also affect the feel.
- Inline pressure manometer. Commercially available objective disposable devices to measure injection pressure, such as BSmart™ (B-Braun Medical, Melsungen, Germany) (Figure 14), continuously displays the pressure during injection, allowing clinicians to quantify the injection pressure information, which can be documented. The manometer is color-coded such that when the pressure is 20 psi or greater, the indicator will be red to warn the operator. The inline pressure monitor is placed proximal to the needle and in line with the non-distensible tubing. The other end of the pressure monitor is attached directly to the syringe. The principle is the same as in-line pressure sensors in devices such as syringe pumps for continuous pressure monitoring. The main principle behind the use of an injection pressure monitor is that a certain amount of injection pressure (opening pressure) must be reached before the injection (the flow of the anesthetic) can commence. The critical opening pressure necessary to inject local anesthetic when the needle is in contact with the nerve or is intrafascicular has been estimated in several studies to be more than 15 psi. Therefore, if the injection is halted before the opening pressure is reached and the flow of anesthetic is initiated, injection in vulnerable needle-nerve interaction can be avoided.
- Compressed air injection technique. This is the clinical application of Boyle’s law (Pressure × Volume = Constant). At a constant temperature, a set volume of gas (air) varies inversely with the pressure; for instance, if the volume of the gas is reduced by 50%, the pressure will be increased from 1 atmosphere to 2 atmospheres. This technique, designed by Tsui, involves aspirating a set amount of air above a volume of injectate within a syringe. During injection, the volume of air is compressed and maintained at a certain percentage (Figure 15).
At 50% air compression, the injection pressure was 760 mm Hg or less, well below the threshold of less than 25 psi (1293 mm Hg). CAIT is a simple and practical way to standardize the local anesthetic injection pressures in real-time, ensuring the injection pressure is constantly below the threshold and minimizing the risk of clinically significant nerve injury. This method also inevitably reduces the speed of injection, which in turns decreases the risk of intrafascicular injection or siphoning the local anesthetic to unwanted tissue planes. The pressures generated by CAIT also remain consistently stable throughout the injection period, unlike the syringe feel the technique, which produces high peak pressures. This is likely due to the “cushioning” effect from the volume of air, which dampens the initial high pressure.

FIGURE 14. Commercially available disposable in-line pressure-monitoring device for monitoring injection pressure during peripheral nerve block (BSmart, BBraun Medical, Melsungen, GE).
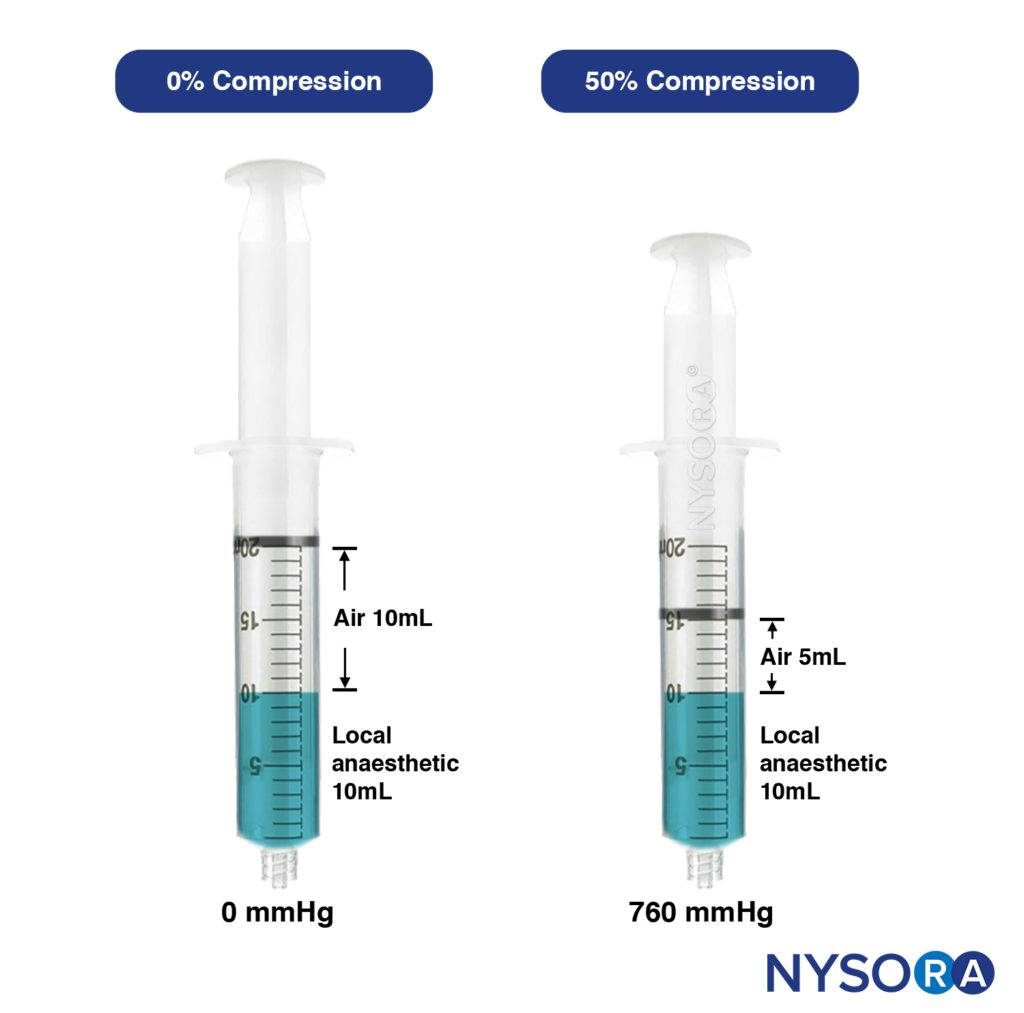
FIGURE 15. Left syringe: Uncompressed, containing 10mL of air and 10mL of local anaesthetic. Right syringe: Air compressed by 50% results in exerted pressure of 760mmHg (approx. 15 psi).
NYSORA Tips
- In the era of ultrasound-guided peripheral nerve blocks, it is important not to dismiss the value of nerve stimulator and injection pressure monitoring to minimize the potential for nerve injury.
- It is important to use a combination of monitors to minimize the risk of intraneural, intrafascicular injection and needle-nerve trauma.
EQUIPMENT FOR POSTblock MANAGEMENT
Block Assessment Tools
Various tools and techniques are available to monitor the progress of a regional block (Figure 16). Ideally, the monitoring tool or device should be as objective as possible, but due to physiological differences among individuals receiving a block, this is rarely the case. To date, there is no consensus on which is the most effective method. Nevertheless, most block-monitoring tools generally offer an accept-able interpretation of when surgical anesthesia is achieved. Similarly, tools and scales used to assess sensory and motor block vary greatly and offer subjective feedback on the degree to which a nerve block is achieving its desired goal. Commonly, pain scales or scores are used to indicate a patient’s comfort level; as with block-monitoring tools, these scales and scores provide a more objective and reproducible means to assess the severity of pain.

FIGURE 16. Selection of nerve block–monitoring equipment, including devices to measure current perception threshold (upper left), monofilaments and alcohol swabs for sensory perception (lower left), infrared scanning device (lower right), and force transducers with wireless data collection capability (upper right).
Sensory (Dermatome) Testing
Regional block assessment tools are based on the assumption that the patient will not be able to perceive a stimulus on the area being blocked. These stimuli are usually temperature based (ice, alcohol swab), but a graded filament can also be used to measure diminishing and returning cutaneous sensation. In the case of trunk/neuraxial blocks, these methods can help determine and follow block spread by observing which derma-tomes are responsive to the stimulus.
Temperature/Infrared Recording
Recently, infrared thermal imaging has also been tested as a means to monitor block progression. This test is based on the knowledge that skin temperature in the digits increases following brachial plexus block. The studies showed that infrared thermography of the digits had high positive predictive value for block success following brachial plexus anesthesia.
Current Perception Threshold
Current perception threshold (CPT) is a means of applying electrical current via a percutaneous electrode connected to a specialized current generator (eg, Neurometer) to test sensory level. This method has been used to quantify degrees of neuropathy in conditions such as diabetes mellitus. Recently, the reproducibility of this method has been tested in volunteers with acceptable results using a common peripheral nerve stimulator. In this study, a peripheral nerve stimulator (see the previous discussion) was used to apply an electrical stimulus to the blocked area; if the current required to elicit a sensory response was greater over time than the baseline (pre block or unblocked region) current, this was an indicator of block progression. Indeed, a subsequent study demonstrated that CPT can be an objective, reliable tool to monitor block onset in clinical scenarios.
Pain Assessment
Numerous validated pain rating scales exist, with the most popular being variations on the 0–10 scale, where 0 indicates “no pain at all,” and 10 indicates “worst pain ever.” The numeric rating scale (NRS) and visual analog scale (VAS) are two examples of this type. Other pain rating scales, such as the Defense and Veterans Pain Rating Scale (DVPRS), feature notes on how the pain affects everyday living that can be used to more precisely define the severity of the pain. The DVPRS also features facial cartoons that can be used to obtain feedback on pain severity from individuals with limited communication ability. For elderly patients, the Pain Assessment Checklist for Seniors With Limited Ability to Communicate (PACSLAC) can be used to assess pain in individuals with dementia or cog-nitive impairment and who have trouble communicating. For children, a variety of pain scales are available that can be used for different age groups and communication abilities.
Motor Block Assessment
The most common motor assessment tool is the Bromage score, a 4-point scale ranging from 0 (full movement) to 3 (complete block/no movement). The original Bromage score was applied for cases of lower extremity block but can also be adapted to the assessment of upper extremity block. Another more objective method that can assess the onset of and recovery from a nerve block is strength testing. This can be performed with a portable force transducer: The patient is asked to exert force against the transducer with the limb or body part that has been blocked (eg, elbow extension to assess radial nerve function).
Some modern force transducers come equipped with a universal serial bus (USB) stick that, along with a portable computer, allows force data to be collected wirelessly in real-time (Figure 16).
Maintenance of Regional Anesthesia
Regional anesthesia practice has relied on infusion pumps to provide continuous delivery of local anesthetic through a perineural catheter. This method remains the most popular method of continuous regional anesthesia, but new developments in technology and practice have allowed for flexibility in postoperative analgesia. Conventional methods for continuous nerve blocks are discussed in more detail in “Continuous Peripheral Nerve Blocks: Local Anesthetic Solutions and Infusion Strategies” and “Equipment for Continuous Peripheral Nerve Blocks“; new developments are covered here briefly.
Intermittent Bolus
In addition to the traditional continuous infusion regimen, it has become increasingly popular to use intermittent bolus for continuous peripheral nerve block management. With its ability to target nerve structures with precision, catheter-over-needle technology (see the previous discussion) greatly reduces the risk of catheter migration or dislodgement. An advantage of intermittent boluses is that the risk of local anesthetic toxicity is also reduced because constant delivery of local anesthetic is avoided and the total dose is generally reduced. An intermittent bolus regimen can be achieved by either a patient-controlled or preprogrammed approach.
NYSORA Tips
- One should consider and select pumps that allow delivery of intermittent boluses as well as continuous infusion.
- Because infusion pumps will be maintained and transported by the patient if they are mobile, the pump should be portable and easy to use.
Future Advances
Recently, the exciting prospect of controlling local anesthetic infusion by remote control was described. In this system, pumps were set to adjust to patient need based on responses to questions about their pain control, and, in the event that settings needed to be changed, the practitioners were able to access the pump information via a secure server remotely, avoiding the need for a nurse or physician to be physically present.
CONCLUSION
With improvements in technology and equipment, regional anesthesia has progressed from an “art” practiced by few to a “science” that, with adequate training and experience, can be practiced by many. Regardless of who is performing the block or where it is being performed, there are several key guidelines that should be followed to ensure safe and effective regional anesthesia. It is essential that there be a designated area for performing nerve blocks and that all drugs and equipment are readily available. Careful documentation of the block procedure should be every institution’s standard. Adequate patient monitoring is essential and should include standard ASA monitoring as well as objective ultrasound–nerve stimulator and injection pressure monitoring to help prevent nerve injury. The use of proper equipment, including appropriate needle length and gauge, will result in easier and more accurate needling. If a continuous block is desired, new catheter-over-needle assemblies can help mitigate the problems of traditional catheter-through-needle designs, and recent developments in long-term local anesthetic delivery methods, including intermittent bolus and remote control, represent valuable options.
In summary, current regional anesthesia practice depends on numerous tools, methods, and monitoring equipment. Although time is needed to gain adequate competency with some of these methods and tools, they are critical for ensuring that the regional block is performed in the safest and most effective manner possible during every stage of the procedure.
REFERENCES
- van Lier F, van der Geest PJ, Hoeks SE, et al: Epidural analgesia is associated with improved health outcomes of surgical patients with chronic obstructive pulmonary disease. Anesthesiology 2011;115:315–3121.
- Davies J, Fernando R: Effect of ropivacaine on platelet function. Anaesthesia 2001;56:709–710.
- Richman JM, Liu SS, Courpas G, et al: Does continuous peripheral nerve block provide superior pain control to opioids? A meta-analysis. Anesth Analg 2006;102:248–257.
- Salviz EA, Xu D, Frulla A, et al: Continuous interscalene block in patients having outpatient rotator cuff repair surgery: A prospective randomized trial. Anesth Analg 2013;117:1485–1492.
- Sites BD, Taenzer AH, Herrick MD, et al: Incidence of local anesthetic systemic toxicity and postoperative neurologic symptoms associated with 12,668 ultrasound-guided nerve blocks: An analysis from a prospective clinical registry. Reg Anesth Pain Med 2012;37(5):478–482.
- Cave G, Harvey M: Intravenous lipid emulsion as antidote beyond local anesthetic toxicity: A systematic review. Acad Emerg Med 2009;16:815–824.
- Weinberg G, Ripper R, Feinstein DL, Hoffman W: Lipid emulsion infusion rescues dogs from bupivacaine-induced cardiac toxicity. Reg Anesth Pain Med 2003;28:198–202.
- Weinberg, G: Treatment regimes. 2015. http://www.lipidrescue.org/.
- Abbal B, Choquet O, Gourari A, et al: Enhanced visual acuity with echogenic needles in ultrasound-guided axillary brachial plexus block: A randomized, comparative, observer-blinded study. Minerva Anestesiol 2015;81:369–378.
- Selander D, Dhuner KG, Lundborg G: Peripheral nerve injury due to injection needles used for regional anesthesia. An experimental study of the acute effects of needle point trauma. Acta Anaesthesiol Scand 1977;21:182–188.
- Selander D: Peripheral nerve injury caused by injection needles. Br J Anaesth 1993;71:323–325.
- Selander DE: Labat lecture 2006. Regional anesthesia: Aspects, thoughts, and some honest ethics; about needle bevels and nerve lesions, and back pain after spinal anesthesia. Reg Anesth Pain Med 2007;32:341–350.
- Mackinnon SE, Hudson AR, Llamas F, et al: Peripheral nerve injury by chymopapain injection. J Neurosurg 1984;61:1–8.
- Steinfeldt T, Graf J, Schneider J, et al: Histological consequences of needle-nerve contact following nerve stimulation in a pig model. Anesthesiol Res Pract 2011;2011:591851.
- Steinfeldt T, Werner T, Nimphius W, et al: Histological analysis after peripheral nerve puncture with pencil-point or Tuohy needletip. Anesth Analg 2011;112:465–470.
- Reina MA, de Leon-Casasola OA, Lopez A, et al: An in vitro study of dural lesions produced by 25-gauge Quincke and Whitacre needles evaluated by scanning electron microscopy. Reg Anesth Pain Med 2000;25:393–402.
- Castrillo A, Tabernero C, Garcia-Olmos LM, et al: Postdural puncture headache: impact of needle type, a randomized trial. Spine J 2015;15:1571–1576.
- Hammond ER, Wang Z, Bhulani N, et al: Needle type and the risk of post-lumbar puncture headache in the outpatient neurology clinic. J Neurol Sci 2011;306:24–28.
- Uppal V, Sondekoppam RV, Ganapathy S: Effect of beam steering on the visibility of echogenic and non-echogenic needles: A laboratory study. Can J Anaesth 2014;61:909–915.
- Ip VH, Tsui BC: The catheter-over-needle assembly facilitates delivery of a second local anesthetic bolus to prolong supraclavicular brachial plexus block without time-consuming catheterization steps: a randomized controlled study. Can J Anaesth 2013;60:692–699.
- Tsui BC, Tsui J: Less leakage and dislodgement with a catheter-over-needle versus a catheter-through-needle approach for peripheral nerve block: An ex vivo study. Can J Anaesth 2012;59:655–661.
- Ip VH, Rockley MC, Tsui BC: The catheter-over-needle assembly offers greater stability and less leakage compared with the traditional counterpart in continuous interscalene nerve blocks: A randomized patient-blinded study. Can J Anaesth 2013;60:1272–1273.
- Ip V, Bouliane M, Tsui B: Potential contamination of the surgical site caused by leakage from an interscalene catheter with the patient in a seated position: A case report. Can J Anaesth 2012;59:1125–1129.
- Tsui BC, Ip VH: Catheter-over-needle method reduces risk of perineural catheter dislocation. Br J Anaesth 2014;112:759–760.
- Ip V, Tsui B. The safety of an interscalene catheter-over-needle technique. Anaesthesia 2013;68:774–775.
- Tsui BC, Gupta S, Finucane B: Confirmation of epidural catheter placement using nerve stimulation. Can J Anaesth 1998;45:640–644.
- Green JS, Tsui BC: Use of a nerve stimulator to assist cricothyroid membrane puncture during difficult airway topicalization. Can J Anaesth 2015;62:1126–1127.
- Becker DE, Reed KL: Essentials of local anesthetic pharmacology. Anesth Prog 2006;53:98–108.
- Bigeleisen PE, Moayeri N, Groen GJ: Extraneural versus intraneural stimulation thresholds during ultrasound-guided supraclavicular block. Anesthesiology 2009;110:1235–1243.
- Robards C, Hadzic A, Somasundaram L, et al: Intraneural injection with low-current stimulation during popliteal sciatic nerve block. Anesth Analg 2009;109:673-677.
- Chan VW, Brull R, McCartney CJ, et al: An ultrasonographic and histological study of intraneural injection and electrical stimulation in pigs. Anesth Analg 2007;104:1281–1284, tables.
- Byrne K, Tsui BC: Practical concepts in nerve stimulation: Impedance and other recent advances. Int Anesthesiol Clin 2011;49:81–90.
- Tsui BC, Pillay JJ, Chu KT, Dillane D: Electrical impedance to distinguish intraneural from extraneural needle placement in porcine nerves during direct exposure and ultrasound guidance. Anesthesiology 2008;109:479–483.
- Bardou P, Merle JC, Woillard JB, et al: Electrical impedance to detect accidental nerve puncture during ultrasound-guided peripheral nerve blocks. Can J Anaesth 2013;60:253–258.
- Kalvoy H, Sauter AR: Detection of intraneural needle-placement with multiple frequency bioimpedance monitoring: a novel method. J Clin Monit Comput 2106;30(2):185–192.
- Chin J, Tsui BC: No change in impedance upon intravascular injection of D5W. Can J Anaesth 2010;57:559–564.
- Cohen JM, Gray AT: Functional deficits after intraneural injection during interscalene block. Reg Anesth Pain Med 2010;35:397–399.
- Reiss W, Kurapati S, Shariat A, Hadzic A: Nerve injury complicating ultrasound/electrostimulation-guided supraclavicular brachial plexus block. Reg Anesth Pain Med 2010;35:400–401.
- Hara K, Sakura S, Yokokawa N, Tadenuma S: Incidence and effects of unintentional intraneural injection during ultrasound-guided subgluteal sciatic nerve block. Reg Anesth Pain Med 2012;37:289–293.
- Sites BD, Taenzer AH, Herrick MD, et al: Incidence of local anesthetic systemic toxicity and postoperative neurologic symptoms associated with 12,668 ultrasound-guided nerve blocks: An analysis from a prospective clinical registry. Reg Anesth Pain Med 2012;37:478–482.
- Liu SS, YaDeau JT, Shaw PM, et al: Incidence of unintentional intraneural injection and postoperative neurological complications with ultrasound-guided interscalene and supraclavicular nerve blocks. Anaesthesia 2011;66:168–174.
- Widmer B, Lustig S, Scholes CJ, et al: Incidence and severity of complications due to femoral nerve blocks performed for knee surgery. Knee 2013;20:181–185.
- Bilbao Ares A, Sabate A, Porteiro L, et al: [Neurological complications associated with ultrasound-guided interscalene and supraclavicular block in elective surgery of the shoulder and arm. Prospective observational study in a university hospital]. Rev Esp Anestesiol Reanim 2013;60:384–391.
- Brull R, Hadzic A, Reina MA, Barrington MJ: Pathophysiology and etiology of nerve injury following peripheral nerve block. Reg Anesth Pain Med 2015;40(5):479–490.
- Neal JM, Barrington MJ, Brull R, et al: The second ASRA practice advisory on neurologic complications associated with regional anesthesia and pain medicine: Executive summary 2015. Reg Anesth Pain Med 2015;40(5):401–430.
- 46. Gadsden J, Latmore M, Levine DM, Robinson A: High opening injection pressure is associated with needle-nerve and needle-fascia contact during femoral nerve block. Reg Anesth Pain Med 2016;41(1):50–55.
- Gadsden JC, Choi JJ, Lin E, Robinson A: Opening injection pressure consistently detects needle-nerve contact during ultrasound-guided interscalene brachial plexus block. Anesthesiology 2014;120(5):1246–1253.
- Hadzic A, Dilberovic F, Shah S, et al: Combination of intraneural injection and high injection pressure leads to fascicular injury and neurologic deficits in dogs. Reg Anesth Pain Med 2004;29:417–423.
- Kapur E, Vuckovic I, Dilberovic F, et al: Neurologic and histologic outcome after intraneural injections of lidocaine in canine sciatic nerves. Acta Anaesthesiol Scand 2007;51:101–107.
- Gentili F, Hudson A, Kline DG, Hunter D: Peripheral nerve injection injury: An experimental study. Neurosurgery 1979;4:244–253.
- Gentili F, Hudson AR, Hunter D, Kline DG: Nerve injection injury with local anesthetic agents: A light and electron microscopic, fluorescent microscopic, and horseradish peroxidase study. Neurosurgery 1980;6:263–272.
- Myers RR, Kalichman MW, Reisner LS, Powell HC. Neurotoxicity of local anesthetics: Altered perineurial permeability, edema, and nerve fiber injury. Anesthesiology 1986;64:29–35.
- Selander D, Brattsand R, Lundborg G, et al: Local anesthetics: importance of mode of application, concentration and adrenaline for the appearance of nerve lesions. An experimental study of axonal degeneration and barrier damage after intrafascicular injection or topical application of bupivacaine (Marcain). Acta Anaesthesiol Scand 1979;23:127–136.
- Bigeleisen PE: Nerve puncture and apparent intraneural injection during ultrasound-guided axillary block does not invariably result in neurologic injury. Anesthesiology 2006;105:779–783.
- Steinfeldt T, Wiesmann T, Nimphius W, et al: Perineural hematoma may result in nerve inflammation and myelin damage. Reg Anesth Pain Med 2014;39(6):513–519.
- Steinfeldt T, Poeschl S, Nimphius W, et al: Forced needle advancement during needle-nerve contact in a porcine model: histological outcome. Anesth Analg 2011;113(2):417–420.
- Gadsden JC, Lindenmuth DM, Hadzic A, et al: Lumbar plexus block using high-pressure injection leads to contralateral and epidural spread. Anesthesiology 2008;109:683–688.
- Orebaugh SL, Mukalel JJ, Krediet AC, et al: Brachial plexus root injection in a human cadaver model: injectate distribution and effects on the neuraxis. Reg Anesth Pain Med 2012;37:525–529.
- Claudio R, Hadzic A, Shih H, et al: Injection pressures by anesthesiologists during simulated peripheral nerve block. Reg Anesth Pain Med 2004;29(3):201–205.
- Theron PS1, Mackay Z, Gonzalez JG, Donaldson N, Blanco R. An animal model of “syringe feel” during peripheral nerve block. Reg Anesth Pain Med 2009;34(4):330–332.
- Tsui BC, Li LX, Pillay JJ: Compressed air injection technique to standardize block injection pressures. Can J Anaesth 2006;53: 1098–1102.
- Asghar S, Bjerregaard LS, Lundstrom LH, et al: Distal infrared thermography and skin temperature after ultrasound-guided interscalene brachial plexus block: a prospective observational study. Eur J Anaesthesiol 2014;31:626–634.
- Asghar S, Lundstrom LH, Bjerregaard LS, Lange KH: Ultrasound-guided lateral infraclavicular block evaluated by infrared thermography and distal skin temperature. Acta Anaesthesiol Scand 2014;58:867–874.
- Masson EA, Veves A, Fernando D, Boulton AJ: Current perception thresholds: A new, quick, and reproducible method for the assessment of peripheral neuropathy in diabetes mellitus. Diabetologia 1989;32:724–728.
- Matsutomo R, Takebayashi K, Aso Y: Assessment of peripheral neuropathy using measurement of the current perception threshold with the neurometer in patients with type 2 diabetes mellitus. J Int Med Res 2005; 33:442–453.
- Nather A, Keng LW, Aziz Z, et al: Assessment of sensory neuropathy in patients with diabetic foot problems. Diabet Foot Ankle 2011;2:6367. doi:10.3402/dfa.v2i0.6367
- Tsui BC, Shakespeare TJ, Leung DH, et al: Reproducibility of current perception threshold with the Neurometer((R)) vs the Stimpod NMS450 peripheral nerve stimulator in healthy volunteers: an observational study. Can J Anaesth 2013;60:753–760.
- Gaudreault F, Drolet P, Fallaha M, Varin F: The reliability of the current perception threshold in volunteers and its applicability in a clinical setting. Anesth Analg 2015;120:678–683.
- Huskisson EC: Visual analogue scales. In Melczak R (ed): Pain Measurement and Assessment. Raven Press, 1983, pp 33–37.
- Buckenmaier CC III, Galloway KT, Polomano RC, et al: Preliminary validation of the Defense and Veterans Pain Rating Scale (DVPRS) in a military population. Pain Med 2013;14:110–123.
- Fuchs-Lacelle S, Hadjistavropoulos T: Development and preliminary validation of the pain assessment checklist for seniors with limited ability to communicate (PACSLAC). Pain Manag Nurs 2004;5:37–49.
- Bromage PR. Epidural Analgesia. Saunders, 1978.
- Byeon GJ, Shin SW, Yoon JU, et al: Infusion methods for continuous interscalene brachial plexus block for postoperative pain control after arthroscopic rotator cuff repair. Korean J Pain 2015;28:210–216.
- Patkar CS, Vora K, Patel H, et al: A comparison of continuous infusion and intermittent bolus administration of 0.1% ropivacaine with 0.0002% fentanyl for epidural labor analgesia. J Anaesthesiol Clin Pharmacol 2015;31:234–238.
- Spencer AO, Tsui BC: Intermittent bolus via infraclavicular nerve catheter using a catheter-over-needle technique in a pediatric patient. Can J Anaesth 2014;61:684–685.
- Macaire P, Nadhari M, Greiss H, et al: Internet remote control of pump settings for postoperative continuous peripheral nerve blocks: A feasibility study in 59 patients. Ann Fr Anesth Reanim 2014;33:e1–e7.