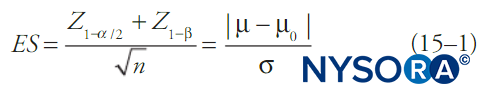Andrew Neice and Michael J. Barrington
INTRODUCTION
Regional anesthesia is best practiced in the context of a standardized anesthetic and surgical protocols; these plans are generally referred to as anesthetic pathways. For a patient having surgery that uses an anesthetic pathway, many of the decisions about the patient’s care are made not at the bedside in the immediate preoperative period, but instead long before the surgery by carefully considering the risks and benefits of various anesthesia and perioperative treatment options.When well designed, anesthetic pathways can improve patient care by ensuring that patients receive consistent, coordinated, evidenced-based care. They can also reduce costs by eliminating unnecessary interventions and reducing complications. Of course, pathways should not be indiscriminately adhered to as some patients will require modification to atone for specific medical conditions or patient preferences. Regardless, anesthetic pathways allow clinicians to focus on the unique characteristics of a patient instead of the common characteristics of an entire cohort, which were already examined during pathway development.
At their core, anesthetic pathways (or clinical pathways in any field) are a series of medical decisions. As leaders of the perioperative surgical home, anesthesiologists are best suited to lead their design. There are often many subtle issues that arise in the development of a pathway that may not be familiar to anyone other than a clinician who is frequently and personally involved in patient care. In addition, anesthesiologists’ working relations with surgeons, administrators, and the entire operating room team are paramount in development and success of patient pathways. Interspecialty coordination is vital to the successful development of anesthetic pathways. Anesthetic decisions will frequently affect patients’ ability to rehabilitate in the immediate postoperative period, so anesthetic and surgical pathways must be designed by a team effort.
Because there is evidence that use of regional anesthesia may affect the mortality and morbidity of common surgeries, and because pain control in the postoperative period is often challenging, regional anesthesia (either neuraxial or peripheral block) is often a key feature of anesthetic pathways. Therefore, development of pathways is of particular interest to practitioners whose clinical practice includes regional anesthesia.
Designing and implementing clinical pathways require skills often not taught during residency training. Physicians tailor their treatments with the unique characteristics of each patient in mind. This is related to physician training designed to elicit and synthesize data for a specific patient. Clinical pathways, in contrast, must be designed to optimize the average experience of a cohort of patients, often making trade-offs and compromises in the process. Knowledge of epidemiology and statistics are vital to effective clinical pathway design. Numerically estimating probable outcomes, and choosing those that rate most favorably, is central to the development of the pathway.
Benefits of an anesthesia pathway will of course depend on the peculiarities of the pathway itself, the institution, the surgical and anesthetic techniques, and the other health-care providers using the pathway (such as nursing care, physical therapy, etc.). A pathway designed for one institution may not be appropriate for another institution, which is why in this chapter we have chosen to emphasize the process of pathway development instead of presenting specific pathways. Furthermore, the goals of an anesthetic pathway may vary—some pathways may be designed to reduce morbidity or mortality, while others may be focused on reducing cost while maintaining a high level of patient care. Common goals of an anesthetic pathway include reducing in-hospital morbidity and mortality, reducing length of hospital stay, reducing costs, improving patient satisfaction, reducing readmissions, and improving long-term functional outcomes.
Despite this heterogeneity, there is evidence that, generally speaking, clinical pathways improve patient care. A recent meta-analysis of clinical pathways found that they were associated with lower rates of patient complications (eg, wound infections, bleeding, and pneumonia), as well as improved documentation. Most of the studies included in the meta-analysis also showed a reduction in the length-of-stay and hospital costs without increased risk of readmission rates and mortality. However, it is possible that some clinical pathways actually could improve these outcomes as well. The considerable heterogeneity of successful clinical pathways has not allowed investigators to identify features common to successful (or unsuccessful) clinical pathways. Nevertheless, basic principles of risk management may be deployed to aid in the design of clinical pathways, and developing a reusable, common framework for approaching clinical pathways seems likely to increase the probability of success.
In this chapter, we present a framework for developing clinical pathways and review some of the prerequisite knowledge required for this process. We also present case scenarios that illustrate the subtleties in applying existing medical literature to clinical pathways. Finally, we present components of a surgical pathway for total knee arthroplasty.
FRAMEWORK FOR ANESTHETIC PATHWAYS
An example of a single component of a clinical pathway is shown in Table 1. This is a component of a larger pathway for total joint arthroplasty, which is further discussed in the final section of this chapter. This component describes the standard oral premedications for the surgery and contains specific information about dose, common contraindications, and common additions to the pathway that may be needed for some patients. Although developing a clinical pathway often involves lengthy analyses with uncertainties, final pathway elements should be brief and specific. Besides recommended pre-medications, pathways may have many different elements; for example, see those shown in Table 2.
It should be noted that although the pathways developed by anesthesiologists are primarily concerned with the anesthetic management of the patient, they are designed to dovetail with the corresponding surgical pathway. For example, the choice of regional anesthesia may allow the patient to complete physical therapy on postoperative day 1.
TABLE 1. Example of a pathway item: oral premedication for total knee arthroplasty.
| Recommended Oral Premedications Prior to Surgery (Example Only) | |
|---|---|
| Acetaminophen 1g by mouth | Avoid in those with cirrhosis, elevated liver function tests |
| Celecoxib 400mg by mouth | Avoid in those with sulfa allergy, renal dysfunction |
| Gabapentin 600mg by mouth | Avoid in patients with renal dysfunction, outpatients, patients with advanced age or dementia |
TABLE 2. Examples of pathway elements.
- Enumeration of the goals of the pathway
- Criteria for patient selection, preparation for surgery, and education prior to surgery
- Preoperative optimization (eg, blood pressure goals, hematocrit goals, associated pharmacologic interventions)
- Recommended surgical anesthesia
- Recommended systemic multimodal anesthetic technique
- Recommended regional anesthesia/analgesia for the treatment of postoperative pain
- Recommended prophylactic therapy for postoperative nausea and vomiting
- Recommended intraoperative fluid or hematocrit goals or transfusion criteria
- Recommended intraoperative management of hemodynamics (eg, preferred blood pressures, preferred pressors, etc.)
- Recommended adjuvant drugs (eg, preferred antibiotics, anticoagulation, antifibrinolytics)
- Recommended adjuvant monitoring (eg, evoked potentials, bispectral index, cerebral oxygenation)
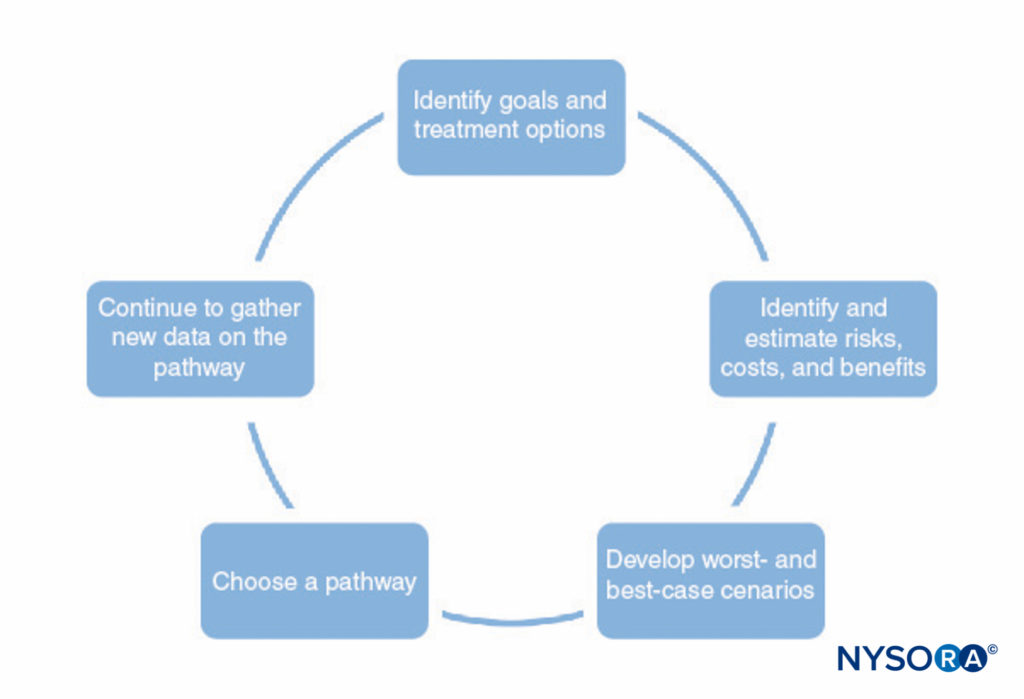
A process for developing clinical pathways is shown in Figure 1. This process consists of the following:
- Identifying the goals of the pathway and different treatment options to help achieve those goals. This should include all stages of anesthesia and surgery, including postoperative recovery.
- Identifying ways in which each treatment interacts with the others (eg, the type of regional anesthesia may affect the patient’s ability to perform physical therapy postoperatively, premedications may delay discharge due to sedation).
- Identifying possible risks, costs, and benefits of each treatment, including mortality, morbidity, patient satisfaction, institutional capabilities, financial cost, and other factors.
- Making numerical estimates of the risks, costs, and benefits identified in step 3. Some of this information (eg, information on cost) can be gathered and tabulated. However, most of the information cannot be known with any certainty. In many cases, the medical literature can provide estimates of the probabilities involved, as long as the physician understands the limitations of a particular study.
- Use the estimates developed in step 4 to develop probable outcomes and worst-case and best-case scenarios for each pathway.
- Choose the pathway most likely to benefit the patient based on step 5.
- Continually refine the estimates of the risks, costs, and benefits as new information becomes available and refine the clinical pathways.
If we could quantify the risks and benefits of each intervention as easily as we could quantify the financial costs of procedures and equipment, steps 4, 5, and 6 would be trivial. However, this is rarely the case. In many cases, there may be not adequate published data to guide the process. Sometimes, there may be some incongruity between available studies and the desired information—the only studies available may have been done on similar, but different, patient populations; have analyzed similar, but different, surgical procedures; or may describe interventions that you cannot exactly replicate at your institution. Nevertheless, the closest match to one’s institutional conditions can be used to estimate probabilities used in step 5.
Another risk to consider includes those complications that may not manifest until well after the treatment period. For instance, recently introduced drugs, procedures, or therapies by definition will have unknown long-term risks. Recognizing the limitations of the existing evidence for each intervention in the literature is crucial in protocol development. Although inferential statistics enhances a physician’s judgment beyond what can be achieved simply by relying on their historical personal observations, unfortunately any given study may have unforeseen biases that may make the study less than an ideal guidance for the protocol being developed. As such, when developing “worst-case” and “best-case” scenarios, one needs to take into account the possibility that a study has made a type I or (more commonly) a type II error.
REVIEW OF STATISTICAL CONCEPTS RELEVANT TO THE DEVELOPMENT OF ANESTHETIC PATHWAYS
A good analogy for the role of statistics in enhancing clinician judgment is comparing it to the role that a magnifying lens plays in enhancing vision. Drugs or interventions that have large, consistent effects (eg, the effect of insulin on glucose or epinephrine on heart rate) can be easily appreciated by the clinician without any sort of corroborating study, just as a magnifying lens is generally not necessary to see frank pus in a wound. Likewise, a study that is underpowered may be unable to detect subtle effects, just as a simple hand lens is not powerful enough to image individual bacteria. While few clinicians would attempt to diagnose a bacterial infection with only a magnifying glass, clinicians frequently make the error of assuming that a reported negative result is synonymous with no effect, without considering the limit of detection of the study. Rare but catastrophic complications are of particular concern as extremely large numbers of patients may be required to properly power a study.
A statistical test may report either that observed data are consistent with random chance (the null hypothesis, a “negative” result) or reject the null hypothesis and assert that there is an association between a treatment and an outcome (a “positive” result). In reality, there may or may not be an association between a treatment and an outcome. There are therefore four possibilities for any statistical test, two of which involve drawing the correct conclusion, and two of which are errors. These are traditionally shown using a 2 × 2 table as shown in Table 3.
TABLE 3. Possible outcomes of a statistical test.
| Hypothesis Is True | Hypothesis Is False | |
|---|---|---|
| Hypothesis is accepted | Correct decision | Type I error (alpha) |
| Hypothesis is rejected | Type II error (beta) | Correct decision |
The two types of possible errors are a type I error (alpha), which involves incorrectly concluding that an association exists when one does not, and a type II error (beta), which involves incorrectly concluding that no association exists when in fact one does. Obviously, the probabilities of making the two types of errors are related. Imagine a statistical test that always asserts there is an association between the treatment and outcome, regardless of the data. This technique will frequently make type I errors but will never make a type II error (because it never asserts a negative result).
By convention, statistical tests compute the probability that a set of data is due to random chance (the p value) and if this is below some fixed threshold (alpha), the notion that the data are due to random chance is rejected. Generally, a confidence interval is reported as well. When alpha is fixed, most commonly at 0.05, beta will be a function of the other features of the statistical test. One of the simpler formulas relating alpha, beta, change in mean, standard deviation of the data, and sample size is shown in Equation 1. Equation 1 describes power relationships for a test of a large sample of a continuous outcome variable in a single population with known mean and standard deviation:
where n is sample size, σ is sample standard deviation, μ is the sample mean, μ0 is the population mean, ES is effect size, and Z is the Z-score corresponding to the value in subscript. Different statistical tests (eg, t tests for continuous variables, t tests for proportions, χ2 tests, analysis-of-variance [ANOVA] tests, etc.) will have somewhat different formulas for beta, but in general beta (and hence the probability of a type II error) will decrease with larger sample sizes, less-restrictive choices of alpha, smaller standard deviations in the data, and larger effect sizes.
Statistical power is simply 1–β and is more frequently referenced than β. While alpha is most commonly fixed at 0.05, a statistical power of 0.8 is generally considered adequate for detection of associations, although in some cases this is increased to 0.9. Unfortunately, while the choice of alpha is almost always explicitly stated in a publication, it is often left to the clinician to infer the power of the study, particularly for secondary outcomes. Fortunately, formulas such as Equation 1 can be readily found in common textbooks or in statistical software packages. In addition, many academic statistics departments have made online tools available to calculate statistical power. Formulas such as Equation 1 can be used to calculate the smallest effect size that a particular study is likely to detect. It then falls to the clinician to decide whether this effect size is small enough that smaller effect sizes are clinically irrelevant. If smaller effect sizes are still clinically relevant, then the clinician developing a pathway needs to take into account the possibility that there is a small but real effect size, a type II error was made, and adjust the best- or worst- case scenarios accordingly.
EXAMPLES OF PATHWAY DEVELOPMENT
We now provide a few illustrative examples of the analysis discussed previously. First, we present a case scenario that illustrates the type of analysis described. Then, we review some examples of drugs that were introduced to routine surgical protocols that caused patient harm; this illustrates pitfalls in pathway development. Last, we present components of a pathway for total knee replacement.
Illustrative Case: Adjuvants to Spinal Anesthesia
Suppose a novel drug (drug X) has been developed that increases the duration of spinal anesthesia when added to bupivacaine. Your group performs total hip and knee arthroplasty under spinal anesthesia but occasionally has to convert to general anesthesia due to unexpectedly long surgical times. The total joint pathway currently uses bupivacaine-only spinals but is considering adding drug X to the total joint pathway to prolong the length of spinal anesthetic and reduce unexpected conversions to general anesthesia.An initial study compared spinal anesthesia with bupivacaine versus drug X plus bupivacaine, with 500 patients in the treatment group and 500 in the control group. The study found that spinal anesthesia with bupivacaine provided adequate surgical anesthesia only for 180 minutes, while bupivacaine with drug X provided adequate surgical anesthesia for 200 minutes (95% CI 195–205 minutes, p < .05). Secondary outcomes included incidence of vomiting on postoperative days 1–3 and urinary retention requiring Foley catheterization. In the control arm, 3% and in the treatment arm 4% of patients vomited postoperatively, but this result was not statistically significant. In the control arm, 2% and in the treatment arm 3% of patients had urinary retention; however, this was also not statistically significant. The drug costs $50 per dose.
Analysis of Costs and Benefits
Even for a relatively simple (and contrived) question such as this, a complete analysis can become complex. We therefore present an abbreviated version of the procedure:
- Identify goals and treatment options. We limit ourselves to the goal of lengthening spinal anesthesia to reduce conversions to general anesthesia. Alternatives to the inclusion of drug X should be explored. Drug X must be evaluated not in a vacuum but instead in competition with other techniques. For example, other drugs may be available that lengthen spinal anesthesia for a comparable time, or simply increasing spinal dosage may be feasible to lengthen surgical anesthesia. Alternatively, the subpopulation likely to have longer operative time may be identified, and these patients could automatically receive general anesthesia or drug X could be reserved for them only. The risks and benefits of each of these options must be individually considered and compared with the routine use of drug X.
- Identify ways each treatment interacts with the rest of the pathway. Although no restrictions were mentioned, we would need to confirm that the use of drug X does not require special postoperative monitoring (such as continuous pulse oximetry), an alteration in nursing care (such as changing fall precautions), or change in physical therapy (such as delaying mobilization) or require alterations to other parts of the anesthetic and surgical pathway. If any restrictions or interactions are identified, they must be accounted for in step 3.
- Identify risks, costs, and benefits. For this illustration, we restrict the analyses to the risks, benefits, and costs alluded to in the study. The primary benefit would be increased duration of surgical anesthesia and a reduced need to convert to general anesthesia, which of course carries a number of attendant risks and costs. The risks include increased risk of urinary retention and postoperative vomiting. Although the initial study did not link either of these outcomes to drug X, we shall see in step 4 that the worst-case scenario must include the risk that this study made a type II error. The costs are easiest to quantify: This will add $50 in drug costs to each surgery.
- Make numerical estimates of the risks, costs, and benefits identified in step 3. The benefits of this drug will depend on the specifics of your institution. For example, suppose in reviewing your records, you find that you performed 500 total joint arthroplasties in the past year, and 5 of them required unexpected conversion to general anesthesia. The total surgical times for the five cases were 195, 250, 200, 190, and 220 minutes. Using the published confidence interval, use of drug X may have eliminated need to convert to general anesthesia in 2 of the cases (if it lengthens time to 195 minutes) to 3 of the cases (if it lengthens time to 205 minutes).
The costs of this drug, if used on all patients, would be an extra $25,000 annually. If a high-risk subpopulation could be identified for use of the drug, this could potentially be reduced.The study found no link between urinary retention or postoperative vomiting with drug X, so in the best-case scenario, adding drug X does not introduce any new risks of complications. However, let us consider the power of this study. Presumably, the data on urinary retention and postoperative vomiting were analyzed using a t test for proportions.Using online power calculators or statistics packages, we can estimate the effect size that would be detected. Assuming a baseline rate of urinary retention of 2% (based on the control arm of the study), the drug would need to increase the rate to 5%–6% to achieve a power of approximately 0.8. Effect sizes lower than this will not be reliably detected. For postoperative vomiting, a power of 0.8 corresponds to the drug increasing the rate of vomiting to 7%. If rates of postoperative vomiting and urinary retention more than doubled, most clinicians would consider that a clinically relevant increase, but it would not be reliably detected by the study discussed. Therefore, we must consider the possibility that the study made a type II error and adjust our worst-case scenario appropriately.
If the study did make a type II error, what should we use for our estimates of effect size? The most reasonable estimate can be obtained by examining the study itself; as subjects are added, the rates converge toward their true values and eventually may cross a threshold of statistical significance. In the study discussed, postoperative vomiting was 3% in the control arm and 4% in the treatment arm, and urinary retention was 2% in the control arm and 3% in the treatment arm. We can use these increases to inform our estimates.
Assuming that our institution has similar rates of vomiting on postoperative days 1–3 (3%) and similar rates of urinary retention (2%), we would estimate that they would increase to 4% and 3%. With a surgical volume of 500 patients per year, this corresponds to 5 extra cases of urinary retention and 5 extra cases of postoperative vomiting per year. - Use the estimates to construct best- and worst-case scenarios. Best case: Eliminate three conversions to general anesthesia per year. Add $25,000 to health-care costs. Worst case: Eliminate two conversions to general anesthesia per year. Add $25,000 to health-care costs. Create five extra cases of urinary retention and five extra cases of postoperative vomiting. In this particular case, we note that the benefits of the drug may be improved if we can accurately identify a subpopulation likely to benefit. For example, if the drug is only given to the 10% of patients with the highest risk of long surgical times, both the cost and the morbidity associated with postoperative vomiting and urinary retention would decrease by a factor of 10. If the subpopulation is accurately identified, the number of general anesthesia conversions may be unaffected or minimally affected.
- Choose the pathway most likely to benefit the patient. Depending on the risks involved in conversions to general anesthesia, this drug may or may not be worth adding to the anesthetic pathway. Ultimately, clinical judgment is required to make a decision. However, by using the framework discussed, the clinician is significantly better informed than if the clinician simply made a decision based on intuition alone.
- Continually refine the estimates of risks, costs, and benefits. In this scenario, the institution’s annual surgical volume (500) is equal to the number of patients involved in each arm of the study. Given this, if the institution tracks its own complication rates before and after the change, it should be able to quickly ascertain the true risks, benefits, and costs of the intervention and make a more informed decision than the initial analysis.
Surprisingly, it is often the case that the number of patients enrolled in studies in the published literature is much smaller than the number of surgeries performed at even small institutions. For example, a recent meta-analysis on the effects of spinal opioids only included approximately 100–150 subjects and controls for analysis of postoperative vomiting and urinary retention for intrathecal fentanyl, despite the long and widespread use of fentanyl in spinal anesthesia. Because of this, analysis of internal data is often helpful in evaluating pathway success or failure. Use of internal data also sidesteps the problem of published studies using slightly different patient populations, drugs, or techniques than used at the home institution.
EXAMPLES OF PATHWAYS THAT CAUSED PATIENT HARM
The previous section was a hypothetical illustration of the development of one part of a pathway. In this section, we take a moment to discuss historical instances in which patient harm resulted from the introduction of a new drug as part of routine perioperative care. Although these drugs were introduced before the notion of surgical and anesthetic pathways became common, the experience with these drugs provides insight into some of the hazards of applying new treatments to large patient cohorts.
Enoxaparin and Venous Thromboembolism Prophylaxis
Enoxaparin was the first low molecular weight heparin approved by the United States Food and Drug Administration for general use. Shortly after the drug’s approval in May 1993, it entered widespread, routine use as prophylaxis for venous thromboembolism. Because many orthopedic surgeries, including total joint arthroplasty, pose a high risk for venous thromboembolism, and epidural or spinal anesthesia was frequently the preferred anesthetic for these cases, enoxaparin was often used in conjunction with neuraxial anesthesia.
Prior to the development of enoxaparin, unfractionated subcutaneous heparin had been widely used for venous thromboembolism prophylaxis. While the risk of epidural hematoma associated with enoxaparin administration was felt to be comparable to that of subcutaneous heparin administration initially, the pharmacological differences between low molecular weight and unfractionated heparin had been underestimated.
Shortly after introduction, the US Food and Drug Administration began to receive reports of epidural hematoma associated with enoxaparin administration. In December 1997, the administration issued a public health advisory stating that it had received over 30 reports of postneuraxial epidural hematoma associated with enoxaparin and required that enoxaparin carry a black box warning indicating a significant risk of patient harm. By April 1998, the number of reports had increased to more than 40. The United States Food and Drug Administration approached the American Society of Regional Anesthesia and Pain Medicine to develop new guidelines for use of enoxaparin with neuraxial anesthesia. These guidelines were published in November 1998 and recommended much more conservative use of enoxaparin.
Aprotinin and Reduction of Perioperative Transfusions
Aprotinin is a small peptide molecule that acts as an antifibrinolytic by inhibiting trypsin and related proteolytic enzymes. It was used most commonly in cardiac surgery, where it was shown to significantly reduce transfusion requirements, and its use was investigated in other types of surgeries, such as orthopedic procedures. Although one meta-analysis showed no increased risk of mortality, myocardial infarction, or renal failure, large observational studies contradicted these findings and demonstrated an increased risk of postoperative renal failure. Further observational studies focused on long-term follow-up and confirmed increased morbidity and mortality associated with aprotinin, particularly with regard not only renal failure, but also stroke, death, and nonfatal myocardial infarction. Sales of aprotinin were halted in 2008; aprotinin has been largely superseded by tranexamic acid and aminocaproic acid. There remains some controversy regarding whether the observed increases in renal failure were due to the effect of aprotinin or some other confounder.
Discussion
In both of these examples, significant patient harm resulted from the introduction of a new drug into an anesthetic and surgical pathway because there were insufficient data to indicate the potential for patient harm. Risks became apparent only after a larger number of patients were treated and significant patient complications had occurred.
In the first case, low molecular weight heparins and neuraxial anesthesia, the difficulty in predicting patient harm was due in large part to the extremely low frequency of the adverse event. If it is assumed that the baseline risk of spinal hematoma is 1:150,000, even a relatively large increase in the risk of hematoma requires large sample sizes to detect an increased risk. Sample sizes this large often cannot be realistically obtained prior to a drug’s introduction, and only postapproval surveillance or the development of high-quality clinical registries will detect such uncommon rare, but potentially catastrophic, adverse events.
In the second case, aprotinin and renal failure, a number of factors can be identified. Initial investigations were not focused on increased risk of renal failure and either they did not investigate this risk or the study was underpowered. In addition, some believed that the complications associated with aprotinin were transient in nature, and that there were no long-term risks associated with aprotinin. Obviously, this could not be investigated until years after aprotinin came into use.
These cases highlight the risks associated with new therapeutic agents or old therapeutic agents used in new situations. Preliminary studies may investigate the wrong risks or may be underpowered, or risks may be long term and may not become evident until well after the study period. Given this, when considering the risks and benefits to the patient, the clinician must also consider unknown or unquantified risk and include the agent only if the ratio of known, quantifiable risks and benefits is overwhelmingly positive. For drugs with a long history of use and well-defined risks, less caution is warranted.
COMPONENTS OF A FULL PATHWAY: TOTAL KNEE ARTHROPLASTY
In this section, we present components of a surgical pathway for total knee arthroplasty. This is shown in Table 4. However, instead of presenting final recommendations (as would be done in a completed pathway), we highlight the common issues that are faced when developing different aspects of the pathway, as well as drugs and techniques that are frequently employed. This is done to avoid implying that there is a final consensus around the “correct” pathway for total joint arthroplasty—even if such a consensus existed, it would rapidly become out of date as new studies, drugs, and techniques become available. Providers with different patient populations and different subspecialties may develop different pathways appropriate to their institution.
TABLE 4
| Anesthetic Pathway for Total Joint Arthroplasty |
|---|
| Goals of the pathway The goals of the pathway should be clearly stated and commonly include reduction in mortality, morbidity, and costs; increases in patient satisfaction; and improved pain control. Goals should involve real clinical endpoints when possible and be agnostic to means, for example, reduce postoperative pain scores is a more appropriate goal than reduce postoperative opioid consumption. |
| Patient selection Modifiable risk factors, such as smoking, poorly controlled diabetes, obesity, and recreational drug use, may affect rates of surgical complications. The pathway may address when surgery should be delayed to address these factors. In addition, nonmodifiable comorbidities may create an unacceptable surgical risk; criteria for patient selection may be included in a pathway. Obviously, this must be coordinated with orthopedics. |
| Preoperative education and preadmission planning Frequently, patients are seen routinely in an anesthesia preoperative clinic. This part of the pathway provides an opportunity to identify patient characteristics that conflict with the default elements in the anesthetic pathway and address them preoperatively. For example, a patient with a penicillin allergy may receive skin-prick testing to determine whether the patient may be administered cephalosporins. In addition, if the pathway includes continuous peripheral nerve catheters, this may provide an opportunity for patient education. |
| Preprocedure checklist This element of the pathway frequently includes patient identification, site marking, confirmation of allergies and comorbidities, confirmation of availability of blood products, confirmation of anticoagulation status, and final checks of laboratory values. |
| Oral premedication/multimodal analgesia Optimization of pain control must be balanced with undesirable side effects such as excessive sedation. The following lists common agents used, along with their benefits and drawbacks. |
| Agent | Benefits | Drawbacks |
|---|---|---|
| Acetaminophen | Reduce postoperative pain scores, opioid sparing | Hepatotoxicity |
| Gabapentin/pregabalin | Reduce postoperative pain scores, opioid sparing, may reduce incidence of chronic postsurgical pain and have benefit in patients with existing chronic pain | Increased sedation, particularly in elderly; increased respiratory depression with doses > 300 mg |
| Cyclooxygenase 2 inhibitors | Reduce postoperative pain scores, opioid sparing | Renal impairment; evidence base weakened by retracted publications |
| Oral opioids (eg, oxycodone SR) | Reduce postoperative pain scores | Increased risk of respiratory depression with oxycodone dose > 10 mg |
| Anesthetic Pathway for Total Joint Arthroplasty |
|---|
| Use of regional anesthesia for postoperative pain control A wide spectrum of regional anesthetic techniques associated with total knee arthroplasty exists, along with significant variation in cost and efficacy. The choice of technique will affect other elements of the anesthetic and surgical pathway. Furthermore, approaches are rapidly evolving as new techniques, equipment, and agents become available. The following lists common sites used for regional anesthesia, along with benefits and drawbacks. |
| Technique | Benefits | Drawbacks |
|---|---|---|
| Epidural block | Considered the gold standard for postoperative analgesia for a range of surgeries | The side-effect profile may interfere with modern care pathway and small risk of catastrophic outcome (eg, epidural hematoma). |
| Femoral nerve block | Relieves majority of postoperative pain, without drawbacks of epidural,; associated with improved outcomes at 6 weeks in one trial | Muscle weakness may interfere with rehabilitation. Small risk (2–4 per 10,000) of long-term nerve injury, but overall choice of anesthetic does change risk of nerve injury. |
| Sciatic nerve block | Reduced posterior knee pain | Risk of neuropathy similar to femoral nerve block. Results of studies vary, ranging from improving analgesia and early mobilization to adding little or no analgesia to existing femoral block. Sciatic block use is controversial and unlikely to improve long-term outcomes. |
| Selective tibial nerve block | Reduced likelihood of foot drop | Injection closer to popliteal crease; risk of peroneal nerve injury with lateral-to-medial approach or vascular injury. |
| Adductor canal | Similar pain relief to femoral nerve block with reduced muscle weakness; effective in treating existing severe pain | Closer to surgical site; evolving technique. |
| Local infiltration analgesia | Easy and quick to perform, no muscle weakness | Evolving evidence for efficacy in this context. However, experts point to poor quality of some of the existing studies. Success of technique likely operator-dependent. Associated with transient peroneal nerve palsy. |
| Once a site or sites is chosen for regional anesthesia, the provider may utilize different techniques to obtain postoperative analgesia, summarized next. | ||
| Single shot | Quick to perform; low cost; effective. | Shortest duration (may be benefit if rapid recovery of muscle strength is required for physical therapy). |
| Nerve catheter | Improved analgesia compared to single-injection technique. Longest duration of analgesia; titratable; control over degree of motor block by changing flow rate. | More difficult and time consuming to perform; more expensive; requires postoperative surveillance. |
| Extended-release formulations of local anesthetic (eg, liposomal bupivacaine) | As quick to perform as single-shot block, with longer block duration. | Compared with bupivacaine, currently little evidence of efficacy. Costly. Limits ability to redo block. Safety and side-effect profile currently emerging. |
| Surgical anesthesia Options for surgical anesthesia are summarized next. |
||
| Anesthesia | Benefits | Drawbacks |
|---|---|---|
| Spinal | Associated with improved outcomes, decreased requirements for critical care services. | Can be technically difficult on certain patients. Occasional catastrophic outcomes (eg, epidural hematoma). Duration of spinal anesthesia may be inadequate for surgery. Patients may be reluctant to be “awake” for surgery |
| Epidural | Similar benefits as spinal, but can be used for postoperative analgesia and longer-duration surgeries. | Can be technically difficult on certain patients. Occasional catastrophic outcomes (eg epidural hematoma). Patients may be reluctant to be “awake” for surgery. |
| General | Complete amnesia. | Associated with poor outcomes compared to spinal. Occasional catastrophic outcome (eg, difficult airway); increased risk of respiratory depression. |
| Neuraxial anesthesia has been associated with improved outcomes; however, this modality is not always the preferred modality. If neuraxial anesthesia is employed, decisions regarding the inclusion or exclusion of long- or short-acting opioids must be made; this decision can be complex as it affects many subsequent pathway elements (postoperative monitoring, rehabilitation, etc.). Even patients who receive neuraxial anesthesia usually require sedation, and some pathways may specify the desired agents or levels of sedation, in part to avoid excessively sedating the patients. In addition, some patients are likely unsuited for neuraxial anesthesia, for example, due to expected surgical time for joint revisions; this and other criteria (such as difficult spinal) for proceeding to general anesthesia can be described in this section. | ||
| Intraoperative drugs Intraoperative drugs may include first- and second-line antibiotics, preferred antiemetics for spinal anesthesia versus general anesthesia, and preferred sedatives if spinal or epidural anesthesia is chosen. Anticoagulation is usually started in the postoperative period by the surgery team but may be commented on here. Of note, intraoperative dexamethasone appears to improve postoperative pain scores as well as providing an effective antiemetic, without increasing risk of infection or other perioperative complications. |
| Anesthetic Pathway for Total Joint Arthroplasty |
|---|
| Intraoperative transfusion goals and blood conservation options Blood transfusion has a number of risks,53 and one of the goals of the pathway may be to minimize blood loss and hence transfusion requirements. A wide variety of techniques can minimize blood loss, some of which are summarized next. |
| Technique | Benefits | Drawbacks |
|---|---|---|
| Intraoperative hypotension | Reduced blood loss. | Increased vigilance and monitoring required. Risk of end-organ ischemia. Underresuscitation may contribute to postoperative orthostatic intolerance, impairing early mobilization. |
| Tourniquet use | Reduced blood loss and protocols exist for appropriate use. | Risk of ischemic injury or axonal neuropathy or effect on quadriceps function. |
| Appropriate thermoregulation | Reduced blood loss via maintenance of coagulation cascade, improved recovery. | |
| Cell scavenging | Reduced allogenic blood product requirements. | Added cost and complexity. |
| Reinfusion drains | Reduced allogenic blood product requirements. | Added cost and complexity. |
| Tranexamic acid | Reduced blood loss due to antifibrinolysis. | Association with seizures. No known increased risk of thrombotic events but has only recently come into use in this surgical population. |
| Postoperative pain control Pathways often address pain control for patients with chronic pain or opioid use, as well as those without. Generally, this section will comment on both the expected infusion rates for any peripheral nerve catheters, as well as adjuvants such as patient-controlled opioid administration, ketamine, or other agents. |
||
| Considerations regarding orthopedic surgical pathway Pathways sometimes comment on ways in which they interact with the surgeon's pathway to make it clear why particular recommendations are made. |
||
The individual elements of the pathway include such diverse topics as preoperative planning, patient education, intraoperative anesthetic management, postoperative pain management, and fluid and hemodynamic goals. A completed pathway, in addition to containing firm, detailed recommendations on management, would contain an addendum that outlines the evidence that was used to make pathway decisions. As we have seen, however, even after identifying the appropriate literature, there is often a significant amount of analysis and judgment that must be used in applying the literature to the pathway.
A final pathway is only useful if it is distributed widely to all relevant providers. In addition to electronic distribution, pathways can be displayed in the form of a poster. This allows the anesthesia provider to have the steps in the pathway easily available at different phases of the patient’s care. Common places to display pathway information include preoperative areas, regional anesthesia bays, operating rooms, and anesthesia work-rooms. In addition, with the advent of electronic health record systems, many institutions have the capability to create order sets, which automatically create orders associated with the pathway.
CONCLUSION
Perioperative anesthesia and analgesia pathways provide a unique way of improving patient care and lowering cost by deploying existing resources and technology in an evidence-based way. Pathway development should, therefore, be considered a vital component of the anesthesiologist’s practice.
REFERENCES
- Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ: Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am 2013;95(3):193–199.
- Memtsoudis SG, Sun X, Chiu YL, et al. Perioperative comparative effectiveness of anesthetic technique in orthopedic patients. Anesthesiology 2013;118(5):1046–1058.
- Stundner O, Chiu YL, Sun X, et al: Comparative perioperative outcomes associated with neuraxial versus general anesthesia for simultaneous bilateral total knee arthroplasty. Reg Anesth Pain Med 2012;37(6):638–644.
- Memtsoudis SG, Stundner O, Rasul R, et al: Sleep apnea and total joint arthroplasty under various types of anesthesia: A population-based study of perioperative outcomes. Reg Anesth Pain Med 2013;38(4):274–281.
- Liu J, Ma C, Elkassabany N, Fleisher LA, Neuman MD: Neuraxial anesthesia decreases postoperative systemic infection risk compared with general anesthesia in knee arthroplasty. Anesth Analg 2013;117(4):1010–1016.
- Rotter T, Kinsman L, James E, et al: Clinical pathways: Effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev 2010(3):CD006632.
- Shiboski S. Epidemiology and biostatistics. 2015. http://www.epibiostat.ucsf.edu/biostat/samplesize.html. Accessed June 30, 2015.
- Brant R. Sample size calculations. 2015. http://www.stat.ubc.ca/~rollin/stats/ssize/. Accessed June 30, 2015.
- Popping DM, Elia N, Marret E, Wenk M, Tramer MR: Opioids added to local anesthetics for single-shot intrathecal anesthesia in patients undergoing minor surgery: A meta-analysis of randomized trials. Pain 2012;153(4):784–793.
- Horlocker TT, Wedel DJ: Neuraxial block and low-molecular-weight heparin: Balancing perioperative analgesia and thromboprophylaxis. Reg Anesth Pain Med 1998;23(6 Suppl 2):164–177.
- Nightingale SL. From the Food and Drug Administration. JAMA 1999;282(1):19.
- Wysowski DK, Talarico L, Bacsanyi J, Botstein P: Spinal and epidural hematoma and low-molecular-weight heparin. N Engl J Med 1998;338(24):1774–1775.
- Bidstrup BP, Royston D, Sapsford RN, Taylor KM: Reduction in blood loss and blood use after cardiopulmonary bypass with high dose aprotinin (Trasylol). J Thorac Cardiovasc Surg 1989;97(3):364–372.
- Royston D, Bidstrup BP, Taylor KM, Sapsford RN: Effect of aprotinin on need for blood transfusion after repeat open-heart surgery. Lancet 1987;2(8571):1289–1291.
- Mahdy AM, Webster NR: Perioperative systemic haemostatic agents. Br J Anaesth 2004;93(6):842–858.
- Shiga T, Wajima Z, Inoue T, Sakamoto A: Aprotinin in major orthopedic surgery: A systematic review of randomized controlled trials. Anesth Analg 2005;101(6):1602–1607.
- Sedrakyan A, Treasure T, Elefteriades JA: Effect of aprotinin on clinical outcomes in coronary artery bypass graft surgery: A systematic review and meta-analysis of randomized clinical trials. J Thorac Cardiovasc Surg 2004;128(3):442-–448.
- Mangano DT, Tudor IC, Dietzel C, Multicenter Study of Perioperative Ischemia Research G, Ischemia R, Education F: The risk associated with aprotinin in cardiac surgery. N Engl J Med 2006;354(4):353–365.
- Shaw AD, Stafford-Smith M, White WD, et al: The effect of aprotinin on outcome after coronary-artery bypass grafting. N Engl J Med 2008;358(8):784–793.
- Mangano DT, Miao Y, Vuylsteke A, et al: Mortality associated with aprotinin during 5 years following coronary artery bypass graft surgery. JAMA 2007;297(5):471–479.
- Furnary AP, Wu Y, Hiratzka LF, Grunkemeier GL, Page US 3rd: Aprotinin does not increase the risk of renal failure in cardiac surgery patients. Circulation 2007;116(11 Suppl):I127–I133.
- Schroeder DR: Statistics: Detecting a rare adverse drug reaction using spontaneous reports. Reg Anesth Pain Med 1998;23(6 Suppl 2):183–189.
- Peersman G, Laskin R, Davis J, Peterson M: Infection in total knee replacement: A retrospective review of 6489 total knee replacements. Clin Orthop Relat Res 2001(392):15–23.
- Zhou TJ, Tang J, White PF: Propacetamol versus ketorolac for treatment of acute postoperative pain after total hip or knee replacement. Anesth Analg 2001;92(6):1569–1575.
- Mishriky BM, Waldron NH, Habib AS: Impact of pregabalin on acute and persistent postoperative pain: A systematic review and meta-analysis. Br J Anaesth. 2015;114(1):10–31.
- Clarke H, Bonin RP, Orser BA, Englesakis M, Wijeysundera DN, Katz J: The prevention of chronic postsurgical pain using gabapentin and pregabalin: A combined systematic review and meta-analysis. Anesth Analg 2012;115(2):428–442.
- Sawan H, Chen AF, Viscusi ER, Parvizi J, Hozack WJ: Pregabalin reduces opioid consumption and improves outcome in chronic pain patients undergoing total knee arthroplasty. Phys Sportsmed 2014;42(2):10–18.
- Weingarten TN, Jacob AK, Njathi CW, Wilson GA, Sprung J: Multimodal analgesic protocol and postanesthesia respiratory depression during phase I recovery after total joint arthroplasty. Reg Anesth Pain Med 2015;40(4):330–336.
- Lin J, Zhang L, Yang H: Perioperative administration of selective cyclooxygenase-2 inhibitors for postoperative pain management in patients after total knee arthroplasty. J Arthroplasty. 2013;28(2):207–213 e2.
- Rothwell MP, Pearson D, Hunter JD, et al: Oral oxycodone offers equivalent analgesia to intravenous patient-controlled analgesia after total hip replacement: A randomized, single-centre, non-blinded, non-inferiority study. Br J Anaesth 2011;106(6):865–872.
- Pumberger M, Memtsoudis SG, Stundner O, et al: An analysis of the safety of epidural and spinal neuraxial anesthesia in more than 100,000 consecutive major lower extremity joint replacements. Reg Anesth Pain Med 2013;38(6):515–519.
- Bateman BT, Mhyre JM, Ehrenfeld J, et al: The risk and outcomes of epidural hematomas after perioperative and obstetric epidural catheterization: A report from the Multicenter Perioperative Outcomes Group Research Consortium. Anesth Analg 2013;116(6):1380–1385.
- Paul JE, Arya A, Hurlburt L, et al: Femoral nerve block improves analgesia outcomes after total knee arthroplasty: A meta-analysis of randomized controlled trials. Anesthesiology 2010;113(5):1144–1162.
- Carli F, Clemente A, Asenjo JF, et al: Analgesia and functional outcome after total knee arthroplasty: Periarticular infiltration vs continuous femoral nerve block. Br J Anaesth 2010;105(2):185–195.
- Neal JM, Barrington MJ, Brull R, et al: The second ASRA practice advisory on neurologic complications associated with regional anesthesia and pain medicine executive summary 2015. Reg Anesth Pain Med 2015;40(5):401–430.
- Jacob AK, Mantilla CB, Sviggum HP, Schroeder DR, Pagnano MW, Hebl JR: Perioperative nerve injury after total knee arthroplasty: Regional anesthesia risk during a 20-year cohort study. Anesthesiology 2011;114(2):311–317.
- Sato K, Adachi T, Shirai N, Naoi N: Continuous versus single-injection sciatic nerve block added to continuous femoral nerve block for analgesia after total knee arthroplasty: A prospective, randomized, double-blind study. Reg Anesth Pain Med 2014;39(3):225–229.
- 38. Cappelleri G, Ghisi D, Fanelli A, Albertin A, Somalvico F, Aldegheri G: Does continuous sciatic nerve block improve postoperative analgesia and early rehabilitation after total knee arthroplasty? A prospective, randomized, double-blinded study. Reg Anesth Pain Med 2011;36(5):489–492.
- Abdallah FW, Chan VW, Gandhi R, Koshkin A, Abbas S, Brull R: The analgesic effects of proximal, distal, or no sciatic nerve block on posterior knee pain after total knee arthroplasty: A double-blind placebo-controlled randomized trial. Anesthesiology 2014;121(6):1302–1310.
- Safa B, Gollish J, Haslam L, McCartney CJ: Comparing the effects of single shot sciatic nerve block versus posterior capsule local anesthetic infiltration on analgesia and functional outcome after total knee arthroplasty: a prospective, randomized, double-blinded, controlled trial. J Arthroplasty 2014;29(6):1149–1153.
- Wegener JT, van Ooij B, van Dijk CN, et al: Long-term pain and functional disability after total knee arthroplasty with and without single-injection or continuous sciatic nerve block in addition to continuous femoral nerve block: A prospective, 1-year follow-up of a randomized controlled trial. Reg Anesth Pain Med 2013;38(1):58–63.
- Sinha SK, Abrams JH, Arumugam S, et al: Femoral nerve block with selective tibial nerve block provides effective analgesia without foot drop after total knee arthroplasty: A prospective, randomized, observer-blinded study. Anesth Analg 2012;115(1):202–206.
- Jaeger P, Zaric D, Fomsgaard JS, et al: Adductor canal block versus femoral nerve block for analgesia after total knee arthroplasty: A randomized, double-blind study. Reg Anesth Pain Med 2013;38(6):526–532.
- Jaeger P, Grevstad U, Henningsen MH, Gottschau B, Mathiesen O, Dahl JB: Effect of adductor-canal-block on established, severe post-operative pain after total knee arthroplasty: A randomised study. Acta Anaesth Scand 2012;56(8):1013–1019.
- Andersen LO, Kehlet H: Analgesic efficacy of local infiltration analgesia in hip and knee arthroplasty: A systematic review. Br J Anaesth 2014;113(3):360–374.
- Tsukada S, Wakui M, Hoshino A: Postoperative epidural analgesia compared with intraoperative periarticular injection for pain control following total knee arthroplasty under spinal anesthesia: A randomized controlled trial. J Bone Joint Surg Am 2014;96(17):1433–1438.
- Bingham AE, Fu R, Horn JL, Abrahams MS: Continuous peripheral nerve block compared with single-injection peripheral nerve block: A systematic review and meta-analysis of randomized controlled trials. Reg Anesth Pain Med 2012;37(6):583–594.
- Schroer WC, Diesfeld PG, LeMarr AR, Morton DJ, Reedy ME: Does extended-release liposomal bupivacaine better control pain than bupivacaine after TKA? A prospective, randomized clinical trial. J Arthroplasty 2015;30(9 Suppl):64–67.
- Ilfeld BM, Viscusi ER, Hadzic A, et al: Safety and side effect profile of liposome bupivacaine (Exparel) in peripheral nerve blocks. Reg Anesth Pain Med 2015;40(5):572–582.
- Memtsoudis SG, Sun X, Chiu YL, et al: Utilization of critical care services among patients undergoing total hip and knee arthroplasty: Epidemiology and risk factors. Anesthesiology 2012;117(1):107–116.
- Fleischut PM, Eskreis-Winkler JM, Gaber-Baylis LK,et al: Variability in anesthetic care for total knee arthroplasty: An analysis from the anesthesia quality institute. Am J Med Qual 2015;30(2):172–179.
- Backes JR, Bentley JC, Politi JR, Chambers BT: Dexamethasone reduces length of hospitalization and improves postoperative pain and nausea after total joint arthroplasty: A prospective, randomized controlled trial. J Arthroplasty 2013;28(8 Suppl):11–17.
- Goodnough LT: Risks of blood transfusion. Crit Care Med 2003;31(12 Suppl):S678–S686.
- Fitzgibbons PG, Digiovanni C, Hares S, Akelman E: Safe tourniquet use: A review of the evidence. J Am Acad Orthop Surg 2012;20(5):310–319.
- Nitz AJ, Dobner JJ, Matulionis DH: Pneumatic tourniquet application and nerve integrity: Motor function and electrophysiology. Exp Neurol 1986;94(2):264–279.
- Kornbluth ID, Freedman MK, Sher L, Frederick RW: Femoral, saphenous nerve palsy after tourniquet use: A case report. Arch Phys Med Rehab 2003;84(6):909–911.
- Weingarden SI, Louis DL, Waylonis GW: Electromyographic changes in postmeniscectomy patients. Role of the pneumatic tourniquet. JAMA 1979;241(12):1248–1250.
- Saunders KC, Louis DL, Weingarden SI, Waylonis GW: Effect of tourniquet time on postoperative quadriceps function. Clin Orthop Relat Res 1979(143):194–199.
- Kagoma YK, Crowther MA, Douketis J, Bhandari M, Eikelboom J, Lim W: Use of antifibrinolytic therapy to reduce transfusion in patients undergoing orthopedic surgery: A systematic review of randomized trials. Thrombosis Res 2009;123(5):687–696.
- Murkin JM, Falter F, Granton J, Young B, Burt C, Chu M: High-dose tranexamic acid is associated with nonischemic clinical seizures in cardiac surgical patients. Anesth Analg 2010;110(2):350–353.