Jeff Gadsden
INTRODUCTION
The incidence of complications from general anesthesia has diminished substantially in recent decades, largely due to advances in monitoring of the respiratory and cardiovascular function during administration of anesthesia. The use of objective monitors such as pulse oximetry, capnography, electrocardiography, etc., allow practitioners to timely identify changing physiologic parameters, intervene rapidly and appropriately, and guide their therapeutic decisions.
The practice of regional anesthesia has traditionally suffered from a lack of objective monitors that aid the practitioner in more objectively monitoring the needle-nerve relationship and preventing neurologic injury. The practice of peripheral nerve blocks traditionally relied on subjective end points to gauge the potential risk to the patient. This is changing, however, with the introduction and adoption of standardized methods by which to safely perform peripheral nerve blocks with the minimal possible risk to the patient. For example, instead of relying on feeling “clicks,” “pops,” and “scratches” to identify needle-tip position, practitioners can now monitor the interaction at the needle–fascial layers using ultrasonography. Likewise, quantifying the minimal current intensity and resistance to injection can be used to gather additional data useful in clinical decision-making to minimize the risk of needle placement into an unwanted tissue plane, intravascularly, or into vulnerable anatomical structures intraneurally. Recent advances in monitoring therefore may reduce the three most feared complications of peripheral nerve block: nerve injury, local anesthetic toxicity, and inadvertent damage to adjacent structures (“needle misadventure”).
Objective monitoring, and the rationale for its use, is discussed in the first part of this section. The latter section focuses on documentation of nerve block procedures, which is logical record keeping of the objective information obtained by the monitors. Objective and robust documentation of how a nerve block was performed has obvious medicolegal implications and provides a useful database to guide advances in safety and efficacy.
MONITORING
Available Means for Monitoring Needle-Nerve Relationships
Monitors, as used in the medical practice, are devices that assess a specific physiologic state and warn the clinician of impending harm. The monitors discussed in this chapter include those for nerve stimulation, ultrasonography, and monitoring injection pressure. Each of these has its own distinct set of both advantages and limitations and each is best used in an additive, complementary fashion (Figure 1) to minimize the potential for patient injury, rather than just relying on the information provided by one monitor alone. There is sufficient evidence-based information that the combination of all three monitors is likely to produce the safest possible process during the practice of peripheral nerve blocks.
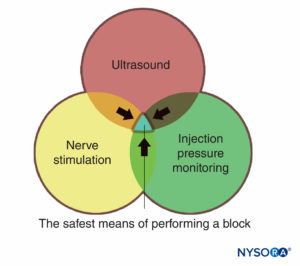
FIGURE 1. Three modes of monitoring peripheral nerve blocks for patient injury. The overlapping area of all three (blue area) represents the safest means of performing a block.
Another, pharmacologic monitor, that many clinicians utilize regularly is the use of epinephrine in the local anesthetic. There is some evidence to support this practice as a means of improving safety for most patients during peripheral nerve blocks. First, it acts as a marker of intravascular absorption. Intravenous injection of 10 to 15 μg epinephrine reliably increases the systolic blood pressure more than 15 mmHg, even in sedated patients or patients treated with β-blockers. The recognition of this increase may allow for early detection of intravascular injection and permits the clinician to promptly halt the injection and sharpen vigilance for signs of systemic toxicity. Second, epinephrine decreases the peak plasma level of local anesthetic, resulting in a lower risk for systemic toxicity. Concerns regarding the vascular effects of epinephrine, vasoconstriction and nerve ischemia have not been substantiated, and in fact concentrations of 2.5 μg/mL (1:400,000) have been associated with an increase in nerve blood flow, likely due to the predominance of the β-effect of the drug. Therefore, epinephrine can enhance safety during administration of larger doses of local anesthetics without documented risk of limb ischemia and neurologic demise.
Of note, the use of ultrasound guidance during peripheral nerve blocks has significantly decreased the risk of severe systemic toxicity of local anesthetics for several reasons. This is most likely because ultrasound guidance has allowed for decreased volume and dose of local anesthetic to accomplish most nerve block procedures by monitoring its spread. In addition, observation of the needle path on ultrasound, avoidance of intravascular placement, and confirmation of the spread of local anesthetic in the tissues all add to greater safety with ultrasound-guided regional anesthesia.
Nerve Stimulation
Neurostimulation largely replaced paresthesia as the primary means of nerve localization in the 1980s. However, its utility as a method of precisely locating nerves has been recently challenged by data of several studies that demonstrated that evoked motor response (EMR) may be absent despite intimate needle-nerve contact as confirmed by ultrasonography. Indeed, in a number of needle-nerve contacts or even intraneural needle placement, unexpectedly high current intensity may be required to elicit an EMR. For instance, in some instances, an EMR could be obtained only with a relative current intensity of more than 1 mA even with intraneural needle placement as seen on ultrasound.
There are probably multiple factors that contribute to the explanation for this phenomenon. The most important factor is most likely the shunting of the electrical current alongside the path of least resistance (impedance). In other words, even when the needle is in the immediate vicinity of the nerve, the electrical current may not necessary chose to travel toward the nerve but rather travels alongside the path of least resistance to exit via the skin electrode. An additional factor may include the nonuniform distribution of motor and sensory fibers in the compound nerve.
This, however, does not mean that electrical stimulation of peripheral nerves is obsolete in an era of ultrasound guidance. For instance, data from several animal and human studies suggested that the presence of a motor response at a very low current (ie, < 0.2 mA) is associated with intraneural needle-tip placement and intraneural inflammation after an injection in this condition (Table 1). Voelckel et al. reported that when local anesthetic was injected at currents between 0.3 and 0.5 mA, the resulting nerve tissue showed no signs of an inflammatory process, whereas injections at less than 0.2 mA resulted in lymphocytic and granulocytic infiltration in 50% of the nerves. Tsai et al. performed a similar study investigating the effect of distance to the nerve on current required; while a range of currents were recorded for a variety of distances, the only instances in which the motor response was obtained at less than 0.2 mA were when the needle tip was placed intraneurally.
TABLE 1. Summary of recent studies of nerve stimulation current and needle-tip position.
| Study | Subject | Method | Findings |
|---|---|---|---|
| Voelckel et al (2005)11 | Pigs (n = 10) | • Posterior sciatic nerve blocks performed bilaterally • Two groups- Injection after EMR at 0.3–0.5 mA- Injection after EMR at < 0.2 mA • 6 hours postinjection, sciatic nerves harvested for histologic analysis | • Normal, healthy appearance of nerves in high-current group • 50% of nerves in low-current group showed evidence of lymphocyte and polymorphic granulocyte sub-, peri-. and intraneurally • One specimen in low-current group showed gross disruption of perineurium and multiple nerve fibers |
| Tsai et al (2008)12 | Pigs (n = 20) | • General anesthesia • Sciatic nerves exposed bilaterally • Current applied with needle at various distances from 2 cm away to intraneural • Two blinded observers agreed on minimal current required to obtain hoof twitch • 40 attempts at each distance | • Sciatic nerve twitches only obtainable 0.1 cm or closer • Wide range of currents required to elicit motor response • Only when intraneural did a motor response result from current < 0.2 mA |
| Bigeleisen et al (2009)13 | Patients for hand/wrist surgery (n = 55) | • Supraclavicular block • Minimum current (mA) recorded- With needle outside nerve trunk (but contacting nerve)- Inside trunk • “Intraneural” position sonographically confirmed with 5-mL injection of local anesthetic | • Median minimal current threshold outside nerve 0.60 mA ± 0.37 mA • Median minimal current threshold outside nerve was 0.30 ± 0.19 mA • No EMR observed at any time with < 0.2 mA when needle placed outside nerve |
| Wiesmann et al (2014)14 | Pigs (n = 6) | • Open brachial plexus model • Stimulation at three positions: intraneural, needle-nerve contact, and 1 mm away from nerve • 3 pulse durations tested (0.1, 0.3, and 1 ms) | • Current intensity cannot distinguish between intraneural and needle-nerve contact • Motor response < 0.2 mA (irrespective of pulse duration) indicated intraneural or needle-nerve contact |
Bigeleisen et al. studied 55 patients scheduled for upper limb surgery who received ultrasound-guided supraclavicular brachial plexus blocks. The authors set out to determine the minimum current threshold for motor response both inside and outside the first trunk encountered. They reported that the median minimum stimulation threshold was 0.60 mA outside the nerve and 0.3 mA inside the nerve. However, EMR was not observed with stimulation currents of 0.2 mA or less outside the nerve, whereas 36% of patients had an EMR twitch at currents less than 0.2 mA with the intraneural needle placement. To further refine this relationship, Wiesmann and colleagues applied an electrical current to the brachial plexus of pigs at three different positions (intraneural, with the needle contacting the epineurium, and at 1 mm from the nerve) while varying the pulse duration (0.1, 0.3, and 1.0 ms). The minimum threshold current to elicit a motor response was identical between the intraneural and needle-nerve contact positions, and both were significantly lower than the position 1 mm away. Pulse duration had no effect on the minimal threshold current. These authors concluded that a motor response at less than 0.2 mA (irrespective of pulse duration) indicated either intraneural or needle-nerve contact. This is important because it has been established that, even in the absence of puncture of the epineurium, even forceful needle-nerve (epineurium) contact results in inflammation and potential for nerve injury.
Taken together, the available data suggest that the sensitivity of a “low current” to elicit an EMR in a potentially dangerous needle-nerve relationship (intraneural/epineurial placement) is about 75%. However, the specificity of the EMR when present at less than 0.5 mA nears 100%. In other words, a motor response is elicited by a low-intensity stimulating current (eg, < 0.2 mA according to Voelckel et al), the tip is always intraneural or intimately related to the epineurium. Therefore, the utility of the nerve stimulator is obvious: Unexpected appearance of an EMR at 0.5 mA indicates a dangerous needle-nerve relationship (eg, needle-nerve contact) and may allow the operator to stop the needle advancement before the needle enters the nerve.
It is universally accepted that injection of local anesthetic into the nerve carries a a risk factor for nerve injury; therefore, extraneural deposition of local anesthetic constitutes safer practice. While unquestionably useful, ultrasonography is far from an infallible monitor of the needle-nerve relationship. Because injection into a fascicle carries a high risk of injury, addition of electrical monitoring of the needle-tip position is useful for safety, particularly in patients with challenging ultrasound anatomy when imaging proves to be difficult or the quality of an image is poor. If an EMR is elicited at currents below 0.5 mA, this indicates an intimate needle-nerve relationship that should prompt slight withdrawal of the needle and careful injection while avoiding an opening injection pressure greater than 15 psi. Overall, nerve stimulation adds little to the cost of a nerve block procedure, in terms of time or cost and can also serve as a useful functional confirmation of the anatomical image shown on the ultrasound screen (eg, “Is that the median or ulnar nerve?”). For these reasons, nerve stimulation should be used routinely in conjunction with ultrasound as a valuable additional monitor of the needle-tip position. Learn more about Electrical Nerve Stimulators and Localization of Peripheral Nerves.
Ultrasonography
Ultrasound has revolutionized the practice of regional anesthesia and allowed a substantial evolution of the subspecialty from an art practiced by a few to a science that is more reproducible. The advantages are that ultrasound makes it possible to see the needle in real time and therefore quickly and more accurately guide the needle toward the target. Ultrasound also allows for additional injection when the first attempt is not adequate and accurately depositing the injectate into tissue spaces for reproducible nerve block. Also, ultrasound allows nerve block to be performed even in patients who are paralyzed, amputees who do not have a limb for an EMR, and so on.
Ultrasound has the potential to improve the safety of peripheral nerve blocks for a number of reasons. Adjacent structures of importance can be seen and avoided. The resurgence in popularity of the supraclavicular block is a testament to this. Prior to ultrasound, this highly effective block was relatively unpopular as a means of anesthetizing the brachial plexus for fear of causing a pneumothorax, due to the proximity of the plexus and sight of needle placement to the pleura and chest cavity. However, because the brachial plexus, and more importantly the rib, pleura, and subclavian artery can be identified on ultrasound, supraclavicular block has become commonplace in clinical practice. Regardless, ultrasound should not be thought of as fail-safe because complications, including pneumothorax, still do occur with ultrasound guidance. Similarly, there are reports of intravascular and intraneural needle placement witnessed by (and despite the use of) ultrasound.
An important advantage of ultrasound screening is the ability to determine the distance from skin to target. This, coupled with needles that have depth markings etched on the side, confers an additional safety margin by warning the clinician of a “stop distance,” or a depth beyond which the clinician should stop advancing the needle to deeper tissue and reassess. Another important advantage that ultrasound is the ability to see the local anesthetic distribution in real time. (Figure 2). If corresponding tissue expansion is not seen when injection begins, then the needle tip may not be not where it is thought to be, and the clinician can halt injection and reassess the location of the needle tip. This is particularly important in vascular areas, as the lack of local anesthetic spread can signal intravascular needle placement. On the other hand, ultrasound monitoring can be used to diagnose intra-arterial needle-tip placement when an echogenic “blush” is noted in the lumen of the artery, decreasing the risk of systemic toxicity.
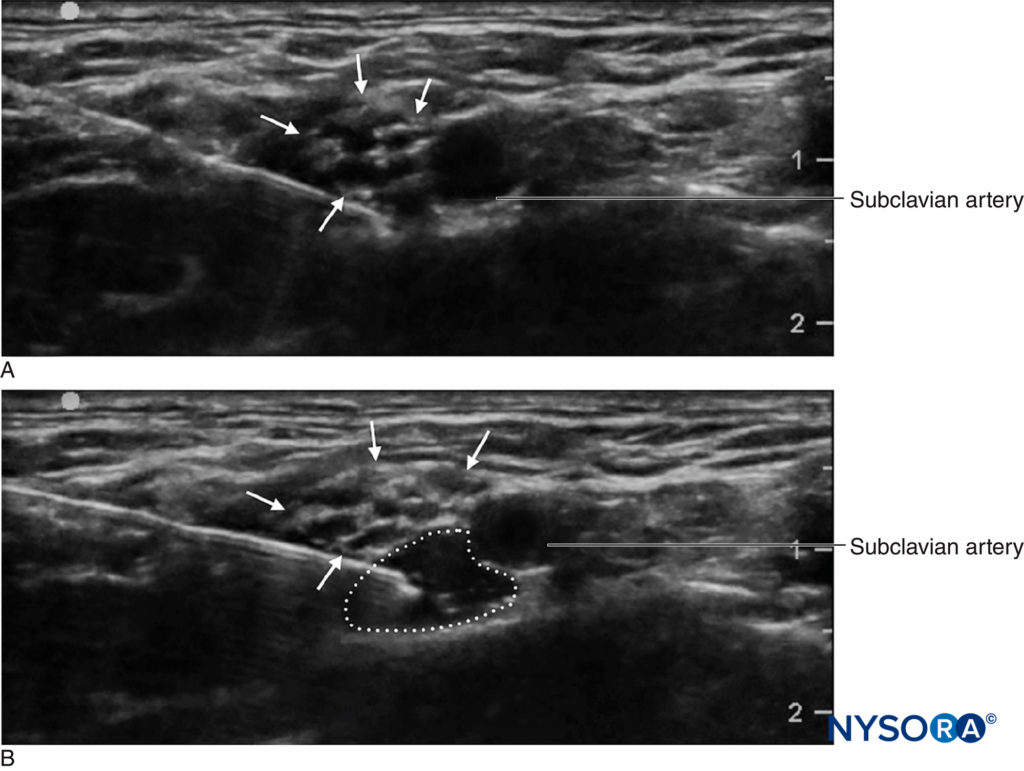
FIGURE 2. Supraclavicular brachial plexus block showing plexus (arrows) adjacent to subclavian artery (SA). A Before and B after deposition of 10 mL of local anesthetic (dotted outline).
Ultrasonography appears to decrease the risk of local anesthetic systemic toxicity (LAST). In one analysis of a large, multicenter registry of peripheral nerve blocks (>25,000 peripheral nerve blocks), the risk of LAST was reduced by greater than 65% with the use of ultrasound guidance. The mechanism proposed by the authors was the ability to substantially reduce the volumes and doses of local anesthetic required to accomplish regional block. Indeed, decreasing the dose and volume of local anesthetic needed for success of regional anesthesia has been a consistent trend over the last decade. Numerous reports have documented substantial reductions in the volume required to effect an equivalent block as compared to pre-ultrasound–guided regional anesthesia techniques. For instance, brachial plexus blocks can be performed with as little as less than 10 mL of local anesthetic, without sacrifice in effectiveness of anesthesia or analgesia. Even if the entire volume of injectate is administered intravenously by accident, severe LAST resulting from, for example, a volume of 7 mL of 0.5% ropivacaine in an adult of average size is unlikely.In contrast, the use of ultrasound guidance during peripheral nerve blocks has not decreased the risk or incidence of nerve injury.
This disappointing observation was documented in several reports and is likely multifactorial. Ability to discern the needle-nerve relationship is user and anatomy dependent. In fact, studies have demonstrated that practitioners may miss approximately as much as one in six intraneural injections. Second, the current resolution of the ultrasound machine may not be adequate to discern between an intraversus extrafascicular needle-tip location. This difference is crucial, as an intraneural (but extrafascicular) injection is likely not associated with injury, whereas injection inside the fascicles themselves produces clinical and histological damage. Importantly, one cannot rely on the nerve swelling as warning of an intraneural injection because once this is noted on ultrasound, it may be too late to prevent injury. This is because even a miniscule amount of local anesthetic will produce damage if injected into the fascicule, yet such small quantities of local anesthetic (eg, 0.1–0.5 mL) may not be detected by ultrasound. Therefore, reliance on the visual confirmation of tissue expansion may result in damage before expansion is detected on the screen.
Injection Pressure Monitoring
An injection of lidocaine while the needle tip was in an intrafascicular position in canine sciatic nerves was associated with high opening pressure (>20 psi), followed by a return of injection pressure tracing to normal (ie, < 5 psi) after fascicular rupture. In contrast, perineural and intraneural extrafascicular injections yielded low opening and injection pressures. The limbs in which sciatic nerve injections were associated high opening injection pressures experienced clinical signs of neuropathy (muscle wasting, weakness) as well as histological evidence of neurologic injury (inflammation, disruption of the nerve architecture). The implication is that injection into a low-compliance compartment, such as within perineurium-bound fascicles, requires high opening injection pressure before the injection can be initiated.
The intraneural needle-tip position is also associated with high opening injection pressures in human cadavers. Orebaugh et al placed needles into cadaveric cervical roots using ultrasound and quantified the pressure over the course of a 5 mL injection of ropivacaine and ink over 15 s. In contrast to the control needles placed outside the roots (peak pressure < 20 psi), the intraneural injections resulted in a mean peak pressure of 49 psi (range 37–66 psi). Similarly, Krol et al performed ultrasound-guided injections intraneurally and perineurally in more distal nerves in fresh human cadavers (median, ulnar, and radial nerves) and also reported that the opening injection pressure was more than 15 psi intraneurally, while extraneural opening injection pressures were less than 10 psi.
In a clinical study of 16 patients undergoing shoulder surgery, needle-nerve contact during interscalene brachial plexus block was associated with an opening injection greater than 15 psi. In fact, at the needle-nerve contact and just before needle entry into the roots of the brachial plexus, the flow of injectate was not able to commence at pressures less than 15 psi. Halting an injection when the required opening injection pressure to commence an injection reached 15 psi allowed avoidance of injection in this hazardous position in 97% of subjects. In contrast, a needle position 1 mm away from the nerve was associated with the initiation of flow at opening pressures less than 15 psi. Therefore, as a monitor of needle-nerve contact, an opening injection pressure greater than 15 psi was far more sensitive than a minimum threshold current of either 0.5 or 0.2 mA or occurrence of paresthesia.
These data suggest that when the pressure in the system approaches 15 psi without the ability to commence flow of injectate, this high opening injection pressure may signal a dangerous needle-nerve relationship or needle placement in wrong tissue plane. Therefore, the injection should be halted and needle position reevaluated.
How should injection pressure be monitored? The use of “hand feel” to avoid high injection pressure is unfortunately not reliable. Studies of experienced practitioners blinded to the injection pressure and asked to perform a mock injection using standard equipment revealed wide variations in applied pressure, some grossly exceeding the established thresholds for safety. Similarly, anesthesiologists performed poorly when asked to distinguish between intraneural injection and injection into other tissues, such as muscle or tendon, in an animal model. Therefore, the only meaningful and reproducible monitoring is by using an objective and quantifiable method of monitoring opening injection pressure.
While the practice of injection pressure monitoring during peripheral nerve blocks is relatively new, there are several monitoring options. Tsui et al described a “compressed air injection technique” by which 10 mL of air is drawn into the syringe along with the local anesthetic. Holding the syringe upright, a maximum threshold of 1 atm (or 14.7 psi) can be avoided by allowing only the gas portion of the syringe contents to compress to half of its original volume, or 5 mL (Figure 3). This is based on Boyle’s law, which states that pressure × volume must be constant. A pressure of 20 psi or less is considered to be a safe threshold for initiating injection during peripheral nerve blocks. Boyle’s law has also been employed in another simple apparatus using a four-way stop-cock and a 1-mL syringe filled with air. If, during the initiation of injection, the fluid meniscus reaches the halfway point in the 1 mL syringe (ie, 0.5 mL), this is indicative of doubling of the pressure in the system (ie, another atmosphere or 14.7 psi). These are both inexpensive and ubiquitously available ways to limit injection pressure during peripheral nerve blocks. Practical limitations include the need either to hold the syringe upright or to periodically turn the stopcock to the 1-mL syringe off when aspirating so air is not allowed to enter the injection tubing.
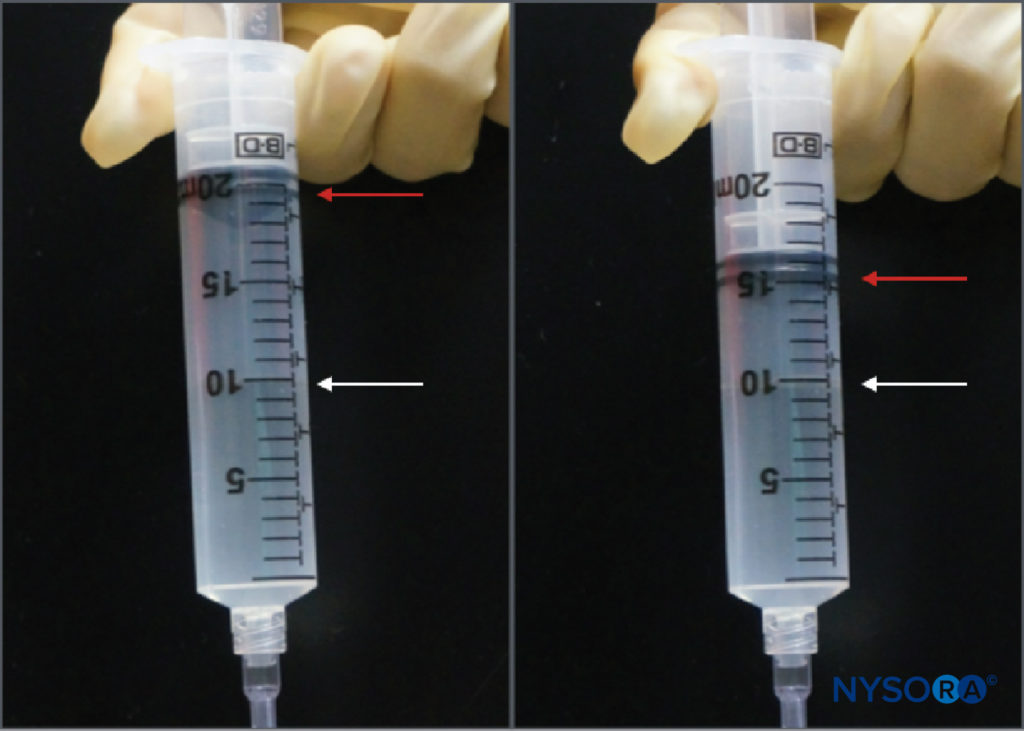
FIGURE 3. The compressed air injection technique. A 10-mL bubble of air is placed in the syringe of local anesthetic, which is then inverted. Compression of that bubble in a closed system to half of its original volume (ie, 5 mL) will increase the pressure in the system by 1 atm (14.7 psi).
Another option to monitor injection pressure is the use of inline, disposable pressure manometers specifically manufactured for this purpose. These devices bridge the syringe and needle tubing and, via a spring-loaded piston, allow the clinician to continuously monitoring the pressure in the syringe-tubing-needle system. On the shaft of the piston are markings delineating three different pressure thresholds: less than 15 psi, 15–20 psi, and more than 20 psi (Figure 4). An advantage of this method is the ease with which an assistant who is performing the injection can read and communicate the attained pressures. This method also allows objective documentation of the injection pressure during a peripheral nerve block procedure. Importantly, the opening pressure (pressure at which the flow begins) is independent of the size of the syringe, tubing, and needle or injection speed (Pascal’s law) (Figure 5). While greater pressure can be generated by a smaller syringe and higher injection pressure can be attained by fast injection, the opening pressure at which the flow begins is the same and independent of these variables for common syringe-tubing-needle sizes (ie, 18–25 gauge). When the injection begins, however, these factors will influence the attained injection pressure. Therefore, a slow, steady injection speed is suggested with all nerve block procedures (10–15 mL/min). The opening injection pressure becomes relevant with every needle reposition and consequent injection. Pressure monitoring may be a useful safety monitor in several other aspects of peripheral nerve blocks. In a study of patients receiving lumbar plexus blocks randomized to low (<15 psi) versus high (>20 psi) pressures, Gadsden et al demonstrated that 60% of patients in the high-pressure group experienced a bilateral epidural block.
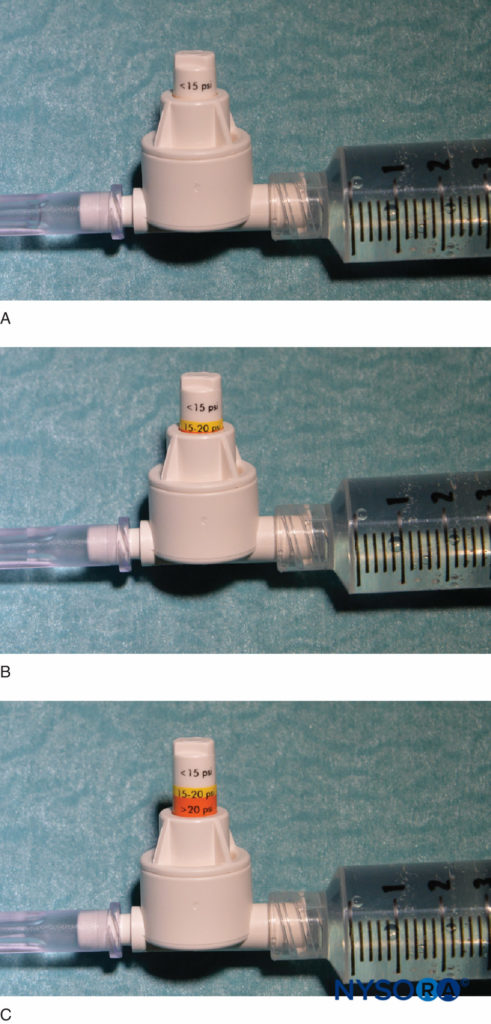
FIGURE 4. An example of a commercially available in-line pressure manometer (B-Smart, B. Braun Medical, Bethlehem, PA). As seen in A–C, respectively, the monitor displays pressure ranges in color on the movable piston: 0–15 psi (white), 15–20 psi (yellow), and more than 20 psi (orange). In clinical use, the exact opening injection pressure (OIP) is less important than prevention of exceeding the range of OIP associated with fascicular injury (>15 psi). Practically, this is avoided by aborting the injection with the appearance of any color on the piston throughout the injection cycle (>15 psi). Importantly, opening pressure (pressure at which the flow begins) is independent of the size of the syringe, tubing, and needle or injection speed (Pascal’s law).
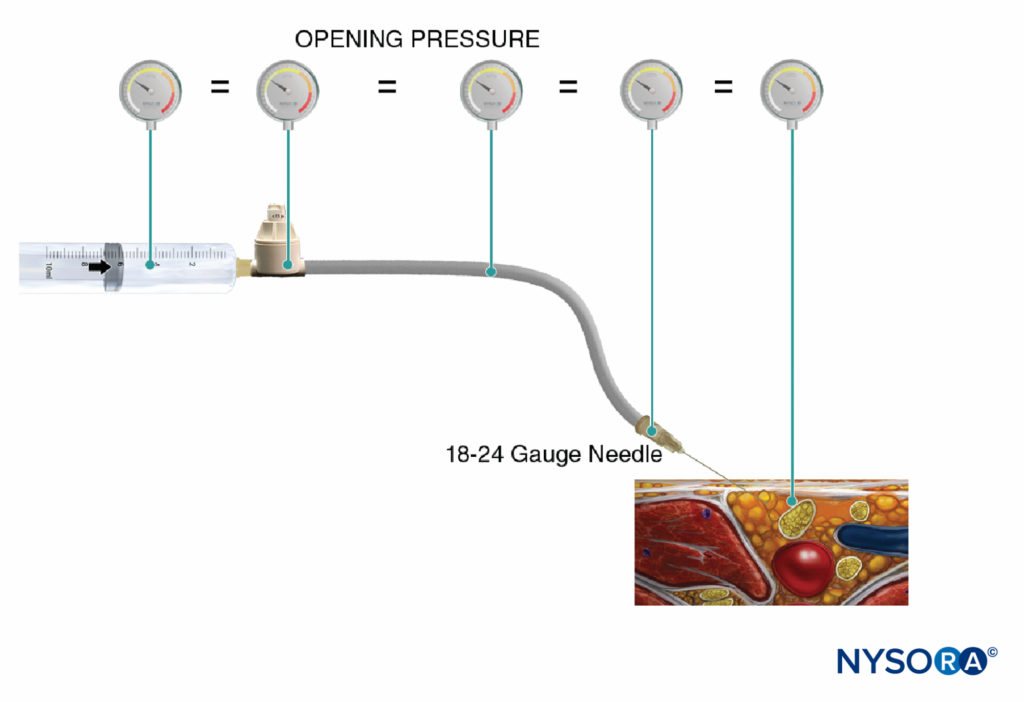
FIGURE 5. Opening injection pressure (pressure at which the flow begins) is independent of the size of the syringe, tubing, and needle or injection speed, and it is equal throughout the injection system (Pascal’s law).
Furthermore, 50% of the patients in the same group developed an epidural block in the thoracic distribution, whereas no patient in the low-pressure group experienced bilateral or epidural block. Similarly, Gautier et al demonstrated that when volunteers were randomized to low (<15 psi) versus high (>20 psi) pressures during interscalene brachial plexus block, cervical epidural spread occurred in 11% of high-pressure injections (vs 0% in the low-pressure group). In addition, all subjects requested that the injection be halted in the high-pressure condition due to discomfort, but not during the low-pressure injection. These data suggest that monitoring opening injection pressure is important for several aspects of safety and patient comfort during the practice of peripheral nerve blocks.
Summary
Regional anesthesia has been making a transition from art to science, as more rigorous and precise means of locating nerves are developed. The same process should be expected for monitoring peripheral block. The use of neurostimulation, ultrasonography, and injection pressure monitoring together provides a complementary package of objective data that can guide clinicians to perform the safest blocks possible.
Figure 6 is a flowchart outlining how these monitors are used in our practice.
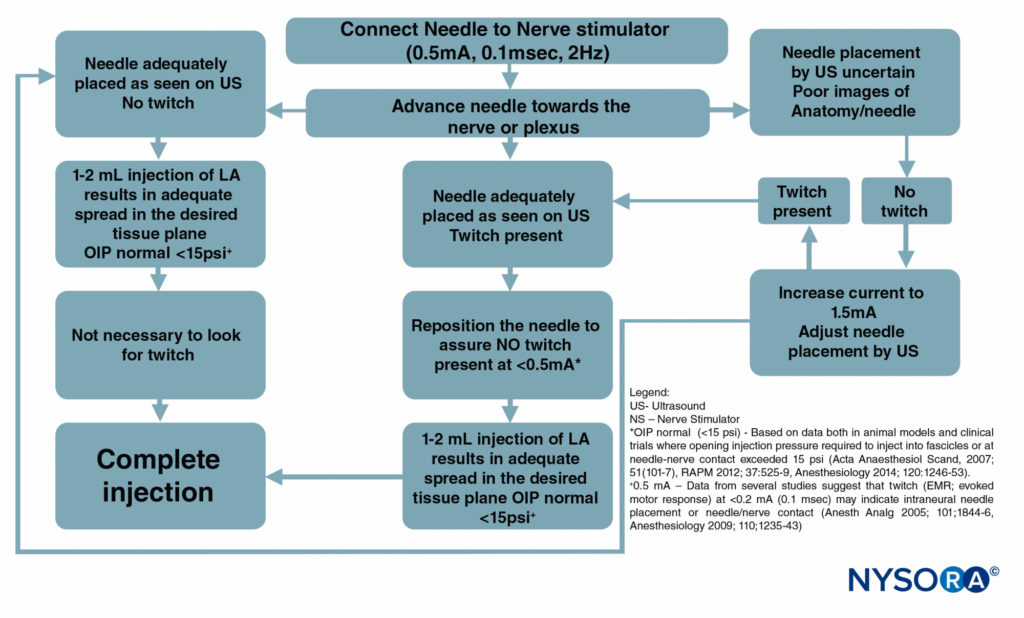
FIGURE 6. A flowchart depicting the order of correctly monitoring nerve block procedures by combining ultrasound (US), nerve stimulation (NS), and injection pressure monitoring. LA = local anesthetic.
DOCUMENTATION
Block Procedure Notes
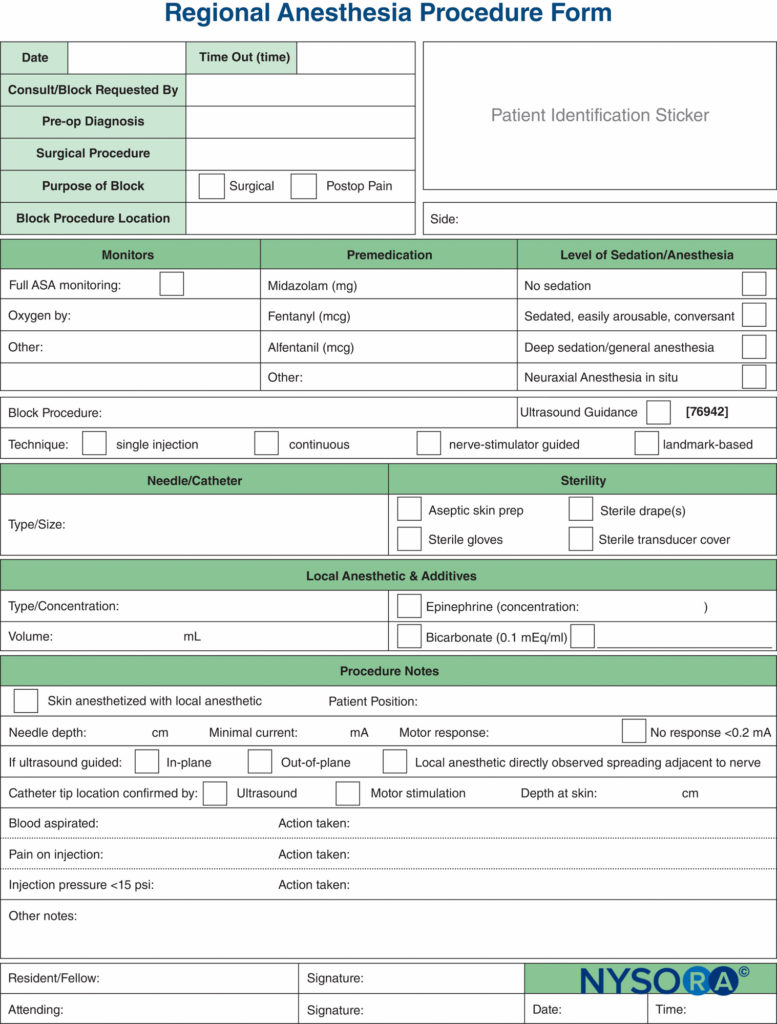
Documentation of nerve block procedures has, lagged behind the documentation of general anesthesia, and it is often relegated to a few scribbled lines in the corner of the anesthetic record. Increasing pressure from legal, billing, and regulatory sources has provoked an effort to improve the documentation for peripheral nerve blocks. Samples of a peripheral nerve block documentation form that incorporates all of the monitoring elements mentioned previously in this chapter are shown in Figures 7 and 8. These can be adopted and modified to suit individual practices. The forms have a number of features that should be considered by institutions attempting to formulate their own procedure note. These include the following:
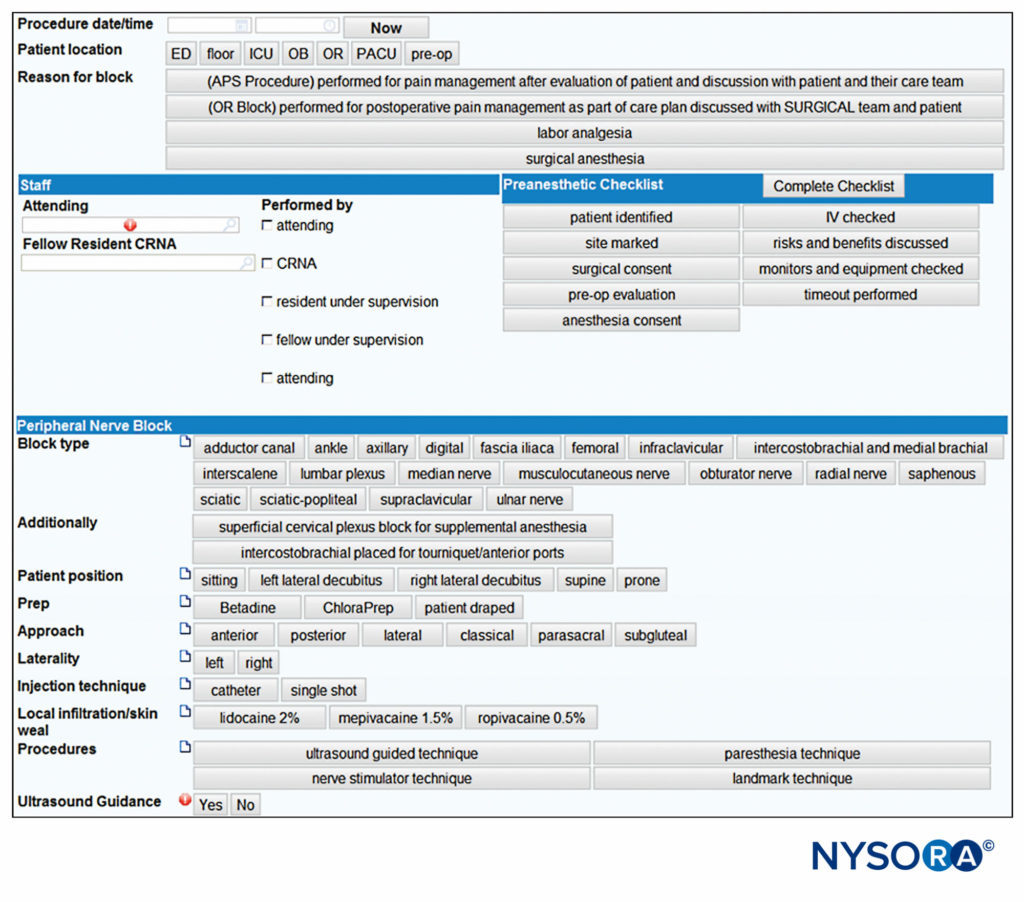
Paper records are increasingly being replaced with electronic medical record-keeping systems. Block documentation is simple with computerized systems such as these, as the block variables can be selected quickly from a list by indicating relevant documentation items, whereas any narrative elements can be rapidly typed using a keyboard (Figure 8). Legibility and ability to correct errors are advantages to the e-block note.
Another useful aspect of peripheral nerve block documentation is the recording of an ultrasound image or video clip, to be stored either as a hard copy in the patient’s chart or as a digital copy in the electronic health record (EHR) or separate secure hard drive. This not only is good practice from a medicolegal point of view but also is a required step that must be taken if the clinician wishes to bill for the use of ultrasound guidance. Any hard copies should have a patient identification sticker attached, the date recorded, and any pertinent findings high-lighted with a marker, such as local anesthetic spreading around nerve. Additional examples of highly practical documentation of essential nerve block and spinal anesthesia procedures are shown in Figures 9 and 10, respectively.
| Useful Features of a Peripheral Nerve Block Procedure Note | Example |
|---|---|
| Elements that guide the practitioner to meet a given standard of care | A space to indicate the use of epinephrine in the local anesthetic solution or if none was used, why not |
| A compromise between efficiency and ability to individualize | Information recorded using both tick boxes and blank line spaces |
| Documentation to safeguard against common medicolegal challenges | Practitioner must indicate patient’s level of consciousness |
| Documentation of compliance with regulatory agencies (eg, Joint Commission) | Tick boxes indicate laterality |
| Elements to facilitate successful billing | Language required by many insurance companies indicating block specifically requested by surgeon |
| Documentation of clinicians involved and in what capacity | Was the attending the individual performing the block, or was he or she medically directing a resident? |
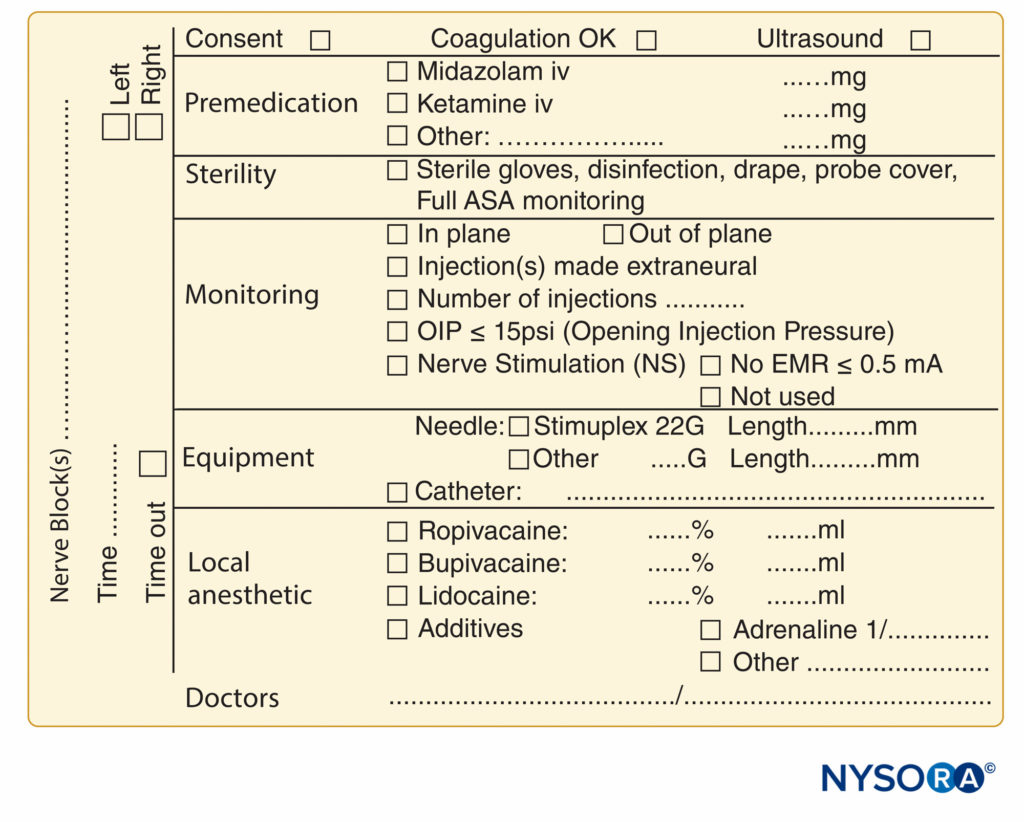
FIGURE 9. Essential elements of documentation of peripheral nerve block procedures used at NYSORA-Europe CREER (Center for Research, Education, and Enhanced Recovery From Orthopedic Surgery) at ZOL (Ziekenhuis Oost-Limburg), Genk, Belgium.
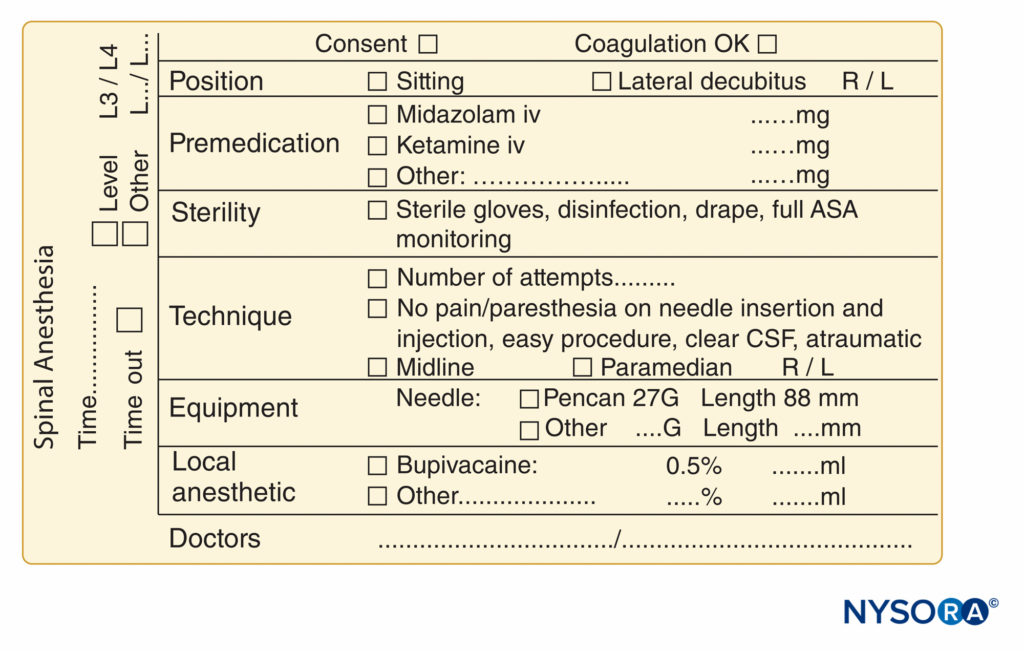
FIGURE 10. Essential elements of documentation of spinal anesthesia procedures used at NYSORA-Europe CREER (Center for Research, Education, and Enhanced Recovery From Orthopedic Surgery) at ZOL (Ziekenhuis Oost-Limburg), Genk, Belgium.
Informed Consent
Documentation of informed consent is an important aspect of regional anesthesia practice. Practice patterns vary widely on this issue, and specific written consent for nerve block procedures is often not obtained. However, the written documentation of this process can be important for a number of reasons:
- Patients are often distracted and anxious on the day of surgery (when many consents are obtained) and may not remember the details of a discussion with their anesthesiologist. Studies have shown that a written record of the informed consent process improves patient recall of risks and benefits.
- A written consent establishes that a discussion of risks and benefits occurred between the patient and the physician.
- A specific document for regional anesthesia can be tailored to include all the common and serious risks; this allows the physician to explain them to the patient as a matter of routine and reduce the chance of omitting important risks.
The following tips can be utilized to maximize the consent process:
| Suggestions for improving the consent process. |
|---|
| Be brief. A simple, short explanation helps recall of the risks and benefits more than lengthy paragraphs. |
| Include not only serious and major risks but also benefits and expected results of the proposed regional anesthetic procedure. It is difficult for patients to make an informed choice if only risks are discussed. |
| Use the consent process as a means to educate the patient simultaneously. |
| Offer a copy of the form to the patient. This has been shown to aid in recall of consent-related information. |
REFERENCES
- Buhre W, Rossaint R: Perioperative management and monitoring in anaesthesia. Lancet 2003;362:1839–1846.
- Guinard JP, Mulroy MF, Carpenter RL, Knopes KD: Test doses: optimal epinephrine content with and without acute beta-adrenergic block. Anesthesiology 1990;73:386–392.
- Tanaka M, Sato M, Kimura T, Nishikawa T: The efficacy of simulated intravascular test dose in sedated patients. Anesth Analg 2001;93: 1612–1617, table of contents.
- Karmakar MK, Ho AM-H, Law BK, Wong ASY, Shafer SL, Gin T: Arterial and venous pharmacokinetics of ropivacaine with and without epinephrine after thoracic paravertebral block. Anesthesiology 2005;103:704–711.
- Van Obbergh LJ, Roelants FA, Veyckemans F, Verbeeck RK: In children, the addition of epinephrine modifies the pharmacokinetics of ropivacaine injected caudally. Can J Anaesth 2003;50:593–598.
- Neal JM: Effects of epinephrine in local anesthetics on the central and peripheral nervous systems: neurotoxicity and neural blood flow. Reg Anesth Pain Med 2003;28:124–134.
- Sites BD, Taenzer AH, Herrick MD, et al: Incidence of local anesthetic systemic toxicity and postoperative neurologic symptoms associated with 12,668 ultrasound-guided nerve blocks: an analysis from a prospective clinical registry. Reg Anesth Pain Med 2012;37(5):478–482.
- Perlas A, Niazi A, McCartney C, Chan V, Xu D, Abbas S: The sensitivity of motor response to nerve stimulation and paresthesia for nerve localization as evaluated by ultrasound. Reg Anesth Pain Med 2006;31:445–450.
- Chan VWS, Brull R, McCartney CJL, Xu D, Abbas S, Shannon P: An ultrasonographic and histological study of intraneural injection and electrical stimulation in pigs. Anesth Analg 2007;104:1281–1284, table of contents.
- Robards C, Hadzic A, Somasundaram L, et al: Intraneural injection with low-current stimulation during popliteal sciatic nerve block. Anesth Analg 2009;109:673–677.
- Voelckel WG, Klima G, Krismer AC, et al: Signs of inflammation after sciatic nerve block in pigs. Anesth Analg 2005;101:1844–1846.
- Tsai TP, Vuckovic I, Dilberovic F, et al: Intensity of the stimulating current may not be a reliable indicator of intraneural needle placement. Reg Anesth Pain Med 2008;33:207–210.
- Bigeleisen PE, Moayeri N, Groen GJ: Extraneural versus intraneural stimulation thresholds during ultrasound-guided supraclavicular block. Anesthesiology 2009;110:1235–1243.
- Wiesmann T, Bornträger A, Vassiliou T, et al: Minimal current intensity to elicit an evoked motor response cannot discern between needle-nerve contact and intraneural needle insertion. Anesth Analg 2014;118:681–686.
- Steinfeldt T, Graf J, Schneider J, et al: Histological consequences of needle-nerve contact following nerve stimulation in a pig model. Anesthesiol Res Pract 2011;2011:591851.
- Steinfeldt T, Poeschl S, Nimphius W, et al: Forced needle advancement during needle-nerve contact in a porcine model: histological outcome. Anesth Analg 2011;113:417–420.
- Gadsden J, Latmore M, Levine DM, Robinson A: High opening injection pressure is associated with needle-nerve and needle-fascia contact during femoral nerve block. Reg Anesth Pain Med 2016;41(1):50–55.
- Hogan QH: Pathophysiology of peripheral nerve injury during regional anesthesia. Reg Anesth Pain Med 2008;33:435–441.
- Whitlock EL, Brenner MJ, Fox IK, Moradzadeh A, Hunter DA, Mackinnon SE: Ropivacaine-induced peripheral nerve injection injury in the rodent model. Anesth Analg 2010;111(1):214–220.
- Sala-Blanch X, Ribalta T, Rivas E, et al: Structural injury to the human sciatic nerve after intraneural needle insertion. Reg Anesth Pain Med 2009;34:201–205.
- Hadzic A, Dilberovic F, Shah S, et al: Combination of intraneural injection and high injection pressure leads to fascicular injury and neurologic deficits in dogs. Reg Anesth Pain Med 2004;29:417–423.
- Gauss A, Tugtekin I, Georgieff M, Dinse-Lambracht A, Keipke D, Gorsewski G: Incidence of clinically symptomatic pneumothorax in ultrasound-guided infraclavicular and supraclavicular brachial plexus block. Anaesthesia 2014;69:327–336.
- Russon K, Blanco R: Accidental intraneural injection into the musculocutaneous nerve visualized with ultrasound. Anesth Analg 2007;105:1504–1505, table of contents.
- Schafhalter-Zoppoth I, Zeitz ID, Gray AT: Inadvertent femoral nerve impalement and intraneural injection visualized by ultrasound. Anesth Analg 2004;99:627–628.
- Loubert C, Williams SR, Hélie F, Arcand G: Complication during ultrasound-guided regional block: accidental intravascular injection of local anesthetic. Anesthesiology 2008;108:759–760.
- Vadeboncouer T, Weinberg G, Oswald S, Angelov F: Early detection of intravascular injection during ultrasound-guided supraclavicular brachial plexus block. Reg Anesth Pain Med 2008;33:278–279.
- Martínez Navas A, DE LA Tabla González RO: Ultrasound-guided technique allowed early detection of intravascular injection during an infraclavicular brachial plexus block. Acta Anaesthesiol Scand 2009;53:968–970.
- Barrington MJ, Kluger R: Ultrasound guidance reduces the risk of local anesthetic systemic toxicity following peripheral nerve block. Reg Anesth Pain Med 2013;38:289–297.
- Orebaugh SL, Kentor ML, Williams BA: Adverse outcomes associated with nerve stimulator-guided and ultrasound-guided peripheral nerve blocks by supervised trainees: update of a single-site database. Reg Anesth Pain Med 2012;37:577–582.
- Casati A, Baciarello M, Di Cianni S, et al: Effects of ultrasound guidance on the minimum effective anaesthetic volume required to block the femoral nerve. Br J Anaesth 2007;98:823–827.
- Sandhu NS, Bahniwal CS, Capan LM: Feasibility of an infraclavicular block with a reduced volume of lidocaine with sonographic guidance. J Ultrasound Med 2006;25:51–56.
- Vandepitte C, Gautier P, Xu D, Salviz EA, Hadzic A: Effective volume of ropivacaine 0.75% through a catheter required for interscalene brachial plexus block. Anesthesiology 2013;118:863–867.
- Riazi S, Carmichael N, Awad I, Holtby RM, McCartney CJL: Effect of local anaesthetic volume (20 vs 5 ml) on the efficacy and respiratory consequences of ultrasound-guided interscalene brachial plexus block. Br J Anaesth 2008;101:549–556.
- O’Donnell B, Riordan J, Ahmad I, Iohom G: Brief reports: a clinical eval-uation of block characteristics using one milliliter 2% lidocaine in ultrasound- guided axillary brachial plexus block. Anesth Analg 2010;111:808–810.
- Krediet AC, Moayeri N, Bleys RLAW, Groen GJ: Intraneural or extraneural: diagnostic accuracy of ultrasound assessment for localizing low-volume injection. Reg Anesth Pain Med 2014;39:409–413.
- Liu SS, YaDeau JT, Shaw PM, Wilfred S, Shetty T, Gordon M: Incidence of unintentional intraneural injection and postoperative neurological complications with ultrasound-guided interscalene and supraclavicular nerve blocks. Anaesthesia 2011;66:168–174.
- Hara K, Sakura S, Yokokawa N, Tadenuma S: Incidence and effects of unintentional intraneural injection during ultrasound-guided subgluteal sciatic nerve block. Reg Anesth Pain Med 2012;37:289–293.
- Bigeleisen PE: Nerve puncture and apparent intraneural injection during ultrasound-guided axillary block does not invariably result in neurologic injury. Anesthesiology 2006;105:779–783.
- Selander D, Dhunér KG, Lundborg G: Peripheral nerve injury due to injection needles used for regional anesthesia. An experimental study of the acute effects of needle point trauma. Acta Anaesthesiol Scand 1977;21:182–188.
- Orebaugh SL, Mukalel JJ, Krediet AC, et al: Brachial plexus root injection in a human cadaver model: injectate distribution and effects on the neuraxis. Reg Anesth Pain Med 2012;37:525–529.
- Krol A, Szarko M, Vala A, De Andres J: Pressure monitoring of intraneural and perineural injections into the median, radial and ulnar nerves: lessons from a cadaveric study. Anesth Pain Med 2015;5:e22723.
- Gadsden JC, Choi JJ, Lin E, Robinson A: Opening injection pressure consistently detects needle–nerve contact during ultrasound-guided interscalene brachial plexus block. Anesthesiology 2014;120: 1246–1253.
- Claudio R, Hadzic A, Shih H, et al: Injection pressures by anesthesiologists during simulated peripheral nerve block. Reg Anesth Pain Med 2004;29:201–205.
- Theron PS, Mackay Z, Gonzalez JG, Donaldson N, Blanco R: An animal model of “syringe feel” during peripheral nerve block. Reg Anesth Pain Med 2009;34:330–332.
- Tsui BCH, Knezevich MP, Pillay JJ: Reduced injection pressures using a compressed air injection technique (CAIT): an in vitro study. Reg Anesth Pain Med 2008;33:168–173.
- Patil J, Ankireddy H, Wilkes A, Williams D, Lim M: An improvised pressure gauge for regional nerve block/anesthesia injections: an initial study. J Clin Monit Comput 2015. doi:10.1007/s10877- 015-9701-z.
- Gadsden JC, Lindenmuth DM, Hadzic A, Xu D, Somasundarum L, Flisinski KA: Lumbar plexus block using high-pressure injection leads to contralateral and epidural spread. Anesthesiology 2008;109: 683–688.
- Gautier P, Vandepitte C, Schaub I, et al: The disposition of radiocontrast in the interscalene space in healthy volunteers. Anesth Analg 2015;120:1138–1141.
- Gerancher JC, Grice SC, Dewan DM, Eisenach J: An evaluation of informed consent prior to epidural analgesia for labor and delivery. Int J Obstet Anesth 2000;9:168–173.