Jeff Gadsden, Emily Lin, and Alicia L. Warlick
INTRODUCTION
Trauma is the leading cause of death in those aged 1–44 years and the third leading cause of death for all age groups. Trauma accounts for 30% of all life years lost in the United States — more than cancer, heart disease, and HIV combined. The economic burden of trauma exceeds $400 billion in the United States annually. This section aims to discuss the role of regional anesthesia within the overall framework of pain management in trauma, explore several examples of where regional anesthesia may affect outcomes in specific injuries, and briefly address the issue of acute compartment syndrome in the context of neuraxial and peripheral nerve block.
MANAGEMENT OF ACUTE PAIN IN PATIENT WITH TRAUMA
The management of pain in the acutely injured patient can be challenging. Resuscitation and the assessment and treatment of life-threatening injuries are the first priorities in the trauma patient, and provision of adequate analgesia must frequently be delayed until the patient is stable. However, there is mounting evidence that the pain associated with injury is often undertreated (oligoanalgesia). There are several barriers to effective analgesia for trauma patients. Physicians are often hesitant to administer pain medications (especially systemic opioids) to trauma patients for fear of causing hemodynamic instability or respiratory depression and airway compromise. Patients with head and/or spinal cord injury require frequent reassessments, which may be impaired or obscured with systemic opioids.
Opioid-induced delirium is also a concern, particularly in the elderly population. Trauma patients are frequently unable to communicate their pain intensity due to the need for sedation and mechanical ventilation, among other considerations, which can impair adequate pain assessment.
Analgesia is often unjustifiably delayed, even in patients with injuries that are not life threatening. In a study of 36 Australian emergency departments, patients who presented with hip fracture (n = 645) were found to have a mean time to first treatment of their fracture-related pain of 126 minutes.
Reported barriers included confusion/dementia, comorbidities such as head injury or hypotension, patient refusal, and language or communication problems. Notably, only 7% of these patients received a femoral nerve block. Another study of patients presenting to the emergency department predominantly with injuries of the extremities showed that while 91% had pain on admission (mean numeric rating scale rating 5.9), 86% still had pain upon discharge (mean numeric rating scale rating 5.0), and pain actually increased in 17% at the time of discharge. Of the 450 patients in this study, only 19% received any type of pharmacologic pain therapy.
Intravenous opioids are the most common approach to treating pain in trauma patients. While opioids are potent analgesics and a rational choice in patients with multiple injuries, they carry a significant burden of potential adverse effects, including the following:
- Nausea and vomiting
- Constipation
- Delirium
- Vasodilation and hypotension (especially in hypovolemia)
- Respiratory depression
- Pruritus
- Immunosuppression
- Increased staffing requirements to monitor the patient (primarily due to the risk of respiratory depression)
- Increased length of stay in emergency department or recovery room
Rather than single-drug therapy with opioids, a multimodal approach is increasingly becoming a standard approach for treating pain in a wide variety of elective surgical patients, where it has been shown to lead to a reduction in both opioid requirements and opioid-related adverse effects. Examples include nonsteroidal anti-inflammatory drugs (NSAIDs), acetaminophen, gabapentinoids, ketamine, and corticosteroids, as well as locoregional analgesia. One difference between the trauma and elective surgical populations is the frequent inability to utilize the oral route for medications in trauma patients due to sedation, neurologic impairment, or the presence of an airway device. Fortunately, many of the standard multimodal classes of drugs are available in parenteral form, including ketamine, acetaminophen, ketorolac, clonidine, and dexmedetomidine.
THE ROLE OF REGIONAL ANESTHESIA IN TRAUMA
Musculoskeletal injury is common in the trauma patient. While skeletal fractures and muscular injuries can occur anywhere on the body, the extremities are disproportionately affected. Approximately 60% of multiple-trauma patients with an injury severity score of ≥ 16 have an extremity injury of some type, and 18% have both lower and upper extremity injuries. Over 30% of the same population will have 2 or more extremity fractures. Since the majority of regional anesthesia procedures involve the extremities, its role in analgesia for trauma patients is particularly well suited.
Mechanism of injury is an important epidemiologic factor— for example, those in motor vehicle crashes (MVCs) have a significantly higher prevalence of extremity injury; similarly, due to improvements in battlefield medicine and body armor, modern military combatants have a dramatically reduced rate of fatal torso injury. As a result, more trauma victims are surviving with higher rates of extremity injury. While patients with isolated extremity injuries tend to have favorable outcomes, it has been shown that orthopedic and general health outcomes are significantly poorer if the same injury is present in a polytrauma patient.
Regional anesthetic techniques, and peripheral nerve blocks in particular, provide high-quality analgesia that is site specific and devoid of any systemic (especially opioid-related) side effects. Regional anesthesia may also confer several other advantages over systemic analgesic therapies for trauma patients, including decreased length of stay in the emergency department and critical care unit, improved ability to perform neurologic assessments, improved comfort and safety for transport, and cost savings compared to procedural sedation.
The persistence of pain well beyond the time of injury and the development of chronic pain is a significant problem following acute injury. Up to 77% of patients who incur severe musculoskeletal trauma will report posttraumatic chronic pain, defined as pain lasting greater than 3 months from the time of injury. There are multiple risk factors that contribute to the likelihood of transitioning from acute to chronic pain. These include age, comorbid medical conditions, depression or anxiety states, and alcohol and tobacco consumption. The risk factor that appears to be most predictive of eventual chronic pain is the intensity of acute pain at the time of injury (odds ratio between 2.4 and 11.2). Regional anesthesia has been shown to significantly reduce acute pain intensity in traumatic injury.
Despite this, the evidence supporting the preventive role of regional anesthesia in the development of chronic pain in trauma is very weak at present, and properly powered, randomized, controlled studies are needed. Regardless, there are multiple other benefits in providing high-quality analgesia in the acute setting with regional anesthesia and analgesia.
NYSORA Tips
- Up to 77% of patients with severe musculoskeletal trauma may develop chronic posttraumatic pain.
- The severity of the acute pain is predictive of development of chronic pain after trauma.
CONTINUOUS PERIPHERAL NERVE BLOCKS IN PATIENTS WITH TRAUMA
Single-injection techniques with bupivacaine or ropivacaine can be expected to provide 16–24 hours of analgesia, whereas continuous peripheral nerve block (CPNB) techniques can significantly prolong the duration of analgesia. The pain intensity associated with trauma is often severe and longstanding, making CPNBs useful. Catheters can be left in for days to weeks. Patients with complex injuries that require repeated debridement, fracture fixation, and/or skin grafting may benefit from indwelling catheters (Figure 1). The pumps can be programmed to deliver a background infusion of a low-concentration, long-acting local anesthetic (eg, 0.1%–0.2% ropivacaine) while on the ward or intensive care unit. The catheters can be manually bolused with a higher-concentration solution upon return to the operating room to provide surgical anesthesia.
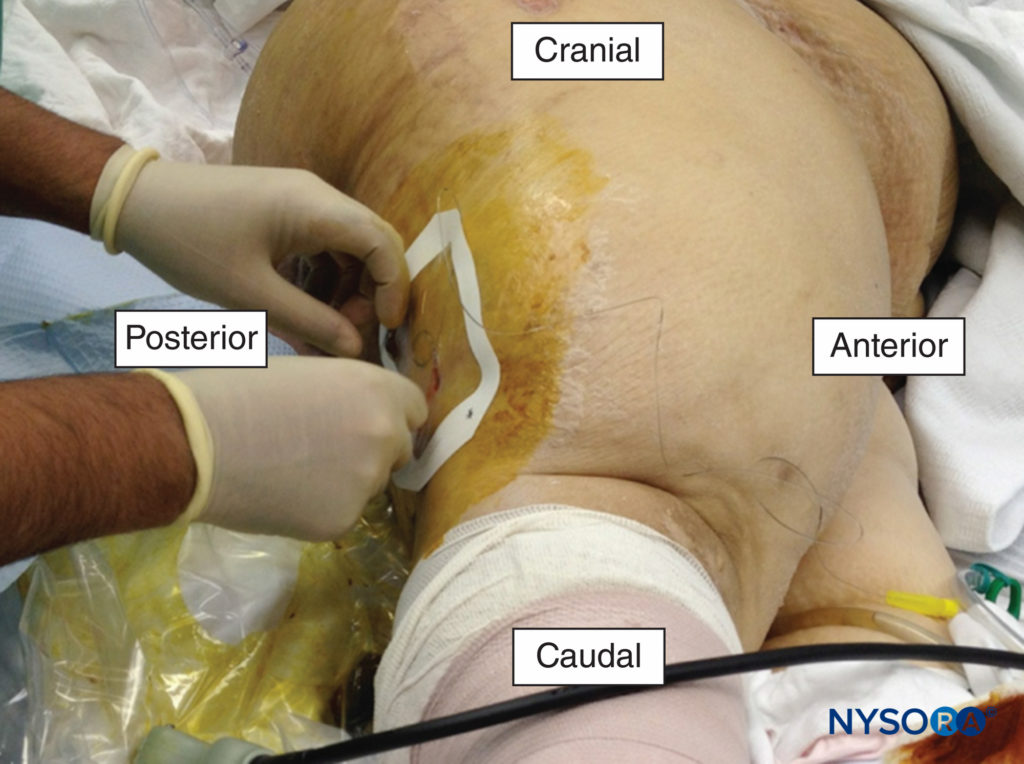
FIGURE 1. A popliteal catheter being placed for the management of pain following a traumatic amputation of the foot.
Buckenmaier et al. described a series of 187 patients injured in combat who were treated with CPNBs for a median of 8 days (range 1–33 days); catheter-specific complications were rare (3.7%) and included kinking, dislodgement, and superficial infection. It should be noted that these data reflect a specific patient population: healthy, fit, young soldiers. Catheter techniques in the elderly and unhealthy civilian trauma victims may have additional challenges, although there are limited data to suggest that these techniques have a lower safety profile in certain age or physical status subpopulations.
Colonization and infection of the catheter site is a concern when using an indwelling catheter in the trauma population, since these patients are at risk for bacteremia and sepsis, and procedures are often performed in less than ideal environments, such as the emergency room or intensive care unit (ICU). Capdevila et al. demonstrated that injured patients admitted to a trauma ICU were 5 times more likely to develop a CPNB catheter infection than elective surgical patients. Other factors reported to increase the risk of catheter infection include duration of catheter use greater than 48 hours, the use of prophylactic antibiotics, insertion at the femoral or axillary location, and frequent dressing changes. Catheter type may also play a role in the development of infection. Lai et al. reported a case series of 2 superficial and 4 deep infections, in which the deep infections requiring operative incision and drainage were associated with stimulating catheter use. The authors hypothesized that repetitive movements of a catheter with an internal metal coil could result in microhematoma formation, providing a rich culture medium for hematogenously spread bacteria. Despite these data, the overall incidence is still low, with only 0%–3% of all catheters showing evidence of infection.
Approximately 20% of polytrauma patients have both upper and lower extremity injuries; thus, the opportunity to use multiple catheters arises frequently. Plunkett and Buckenmaier placed bilateral sciatic nerve catheters and a single femoral nerve catheter in a patient with bilateral leg injuries who was receiving treatment doses of enoxaparin that precluded epidural analgesia. Care must be taken to consider the dose of local anesthetic that is being delivered in order to prevent toxic plasma levels; however, this is rarely an issue since the concentrations that are used clinically for catheters are low (eg, 0.1%–0.2% ropivacaine). One prospective study of 13 combat trauma patients receiving 0.2% ropivacaine infusions at 6–14 mL/h for a period of 4–25 days showed a median unbound plasma ropivacaine level over the duration of the study of 0.11 mg/L (range: undetectable–0.63 mg/L) with no reports of toxic events. The toxic unbound plasma concentration of ropivacaine is approximately 0.6 mg/L. However, two patients’ plasma levels neared this threshold after a large dose (60 mL bolus of 0.5% ropivacaine) prior to the determination of the plasma level. Taken together, these data suggest that long-term infusions of ropivacaine at low concentrations are safe in the trauma population. Notwithstanding, polytrauma patients frequently have two catheters infusing simultaneously, which may increase the risk of toxic plasma levels of local anesthetic. Common strategies to mitigate this risk include lowering the concentration of the local anesthetic infusate (eg, ropivacaine 0.1% or 0.15% rather than 0.2%) and/or relying more on periodic intermittent boluses than on a high-rate continuous background infusion.
REGIONAL ANALGESIA IN THE EMERGENCY DEPARTMENT AND PREHOSPITAL SETTINGS
Regional anesthesia has been used effectively in the emergency department for injured patients requiring analgesia for a variety of indications, including hip fracture, as well as during procedures such as reductions of fractures or dislocated joints and repair of lacerations. Compared to procedural sedation, upper extremity blocks confer several advantages. Interscalene block for shoulder reduction has been shown to reduce emergency department length of stay and the requirement for one-to-one monitoring. Patients with upper extremity fractures, dislocations, and/or abscesses who received supraclavicular block for their procedure experienced a shorter length of stay without any impact on patient safety or satisfaction. Ultrasound-guided intercostal blocks have been effective for chest drain placement following traumatic pneumothorax.
Clinical Pearl
Peripheral nerve blocks improve clinical flow and decrease the length of stay in the ED compared to procedural sedation for selected procedures.
Anesthesiologists are typically the physicians most qualified to perform nerve blocks. However, due to work demands and time constraints, anesthesiologists may not be able to attend to patients in the emergency department or critical care unit in a prompt manner, leading to significant delays in providing quality analgesia. Randall et al. reported the results of a successful initiative to train orthopedic nurses in the performance of fascia iliaca block. This creation of a “physician extender” improved patient access to effective pain control with the use of a simple and safe procedure that is easily taught. (The topic of regional and local anesthesia in the emergency department is covered in more detail in Complications and Prevention of Neurologic injury with Peripheral Nerve Blocks.)
It may also be safe and appropriate to provide regional analgesia in the field or during transport to the hospital. This decision has to be made in the context of the skill and experience of the doctors or medics attending to the patient, as well as the nature and severity of the injuries. In North America, where emergency medical services (EMS) teams are largely staffed by paramedics, emergency medical technicians (EMTs), or firefighters as first responders, there is a limited set of interventions available. In some parts of the world, physicians (eg, anesthesiologists in western Europe) highly trained in resuscitative and trauma medicine are dispatched by ambulance and helicopter to perform retrievals; these tend to be systems that benefit most from on-scene triaging, evaluation, and intervention.
Several studies have shown the fascia iliaca block to significantly reduce pain associated with femoral shaft or neck fractures when performed at the scene of the accident or injury. Advantages to the fascia iliaca block include minimal equipment required (a syringe and needle), a simple approach that does not rely on ultrasound or nerve stimulation, and a good safety profile with little chance of puncturing a vessel or nerve.
Femoral block has also been reported to be effective in prehospital care, but its success depends more on the experience and skill level of the operator.
Additional block techniques that have been reported to successfully reduce pain intensity prior to arriving at hospital include sciatic nerve block, interscalene nerve block, multiple nerve blocks about the elbow, and digital nerve block.
SPECIFIC INJURIES: HIP FRACTURES
Fracture of the femur at the hip joint is a very common injury and is associated with significant morbidity and mortality. Patients with hip fracture tend to be older and have multiple medical comorbidities, placing them at higher risk for complications, especially chest infection, delirium, and heart failure.
Over 95% of hip fractures are fall-related. Falls are the leading cause of death in adults over 64 years of age, with hip fracture being the most serious and costly injury resulting from a fall.
The reported pain intensity from a fractured hip can be moderate to severe. The pain resulting from these fractures is well suited to regional techniques due to the anatomical location of the fractures. In a systematic review of 83 studies addressing various analgesic options for hip fracture (including systemic analgesia, traction, multimodal pain management, and neurostimulation), the only intervention that was found to be effective at reducing acute pain was peripheral nerve block.
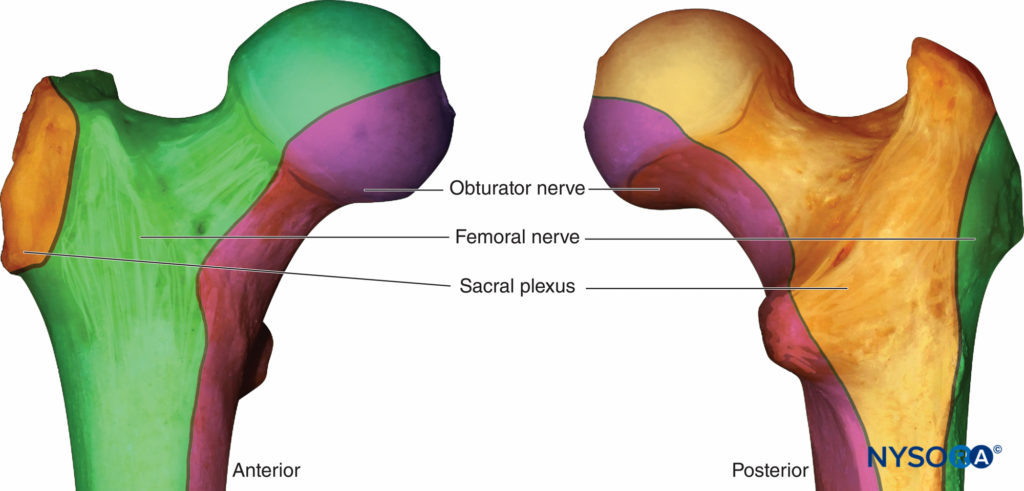
An understanding of the osteotomal innervation of the femur and hip joint is important in block planning. (Figure 2). Several studies have demonstrated that a femoral nerve block reduces pain intensity following hip fracture and is a valuable adjunct in this population, allowing patients to sit up, move in bed, breathe deeply, and cough with reduced pain while awaiting surgery. A Cochrane collaboration review of nerve blocks in patients with hip fracture concluded that femoral nerve block resulted in significant reductions in both pain intensity and opioid requirements both preoperatively and during surgery.
Several studies have found that fascia iliaca block reduced pain scores and opioid requirements in patients with hip fracture. Fascia iliaca block aims to block the femoral and lateral femoral cutaneous nerve (and possibly the obturator nerve) with one injection. The technique is less technically demanding than femoral nerve block, but when compared to femoral block, fascia iliaca may not provide the same degree of pain relief. This may be due to imprecise placement of the local anesthetic during what is traditionally a landmark technique, relying on the spread of a large (30–40 mL) volume for efficacy. Ultrasound guidance increases the frequency of sensory loss of all three nerves compared to the landmark technique. Obturator nerve block appears to also be an effective analgesic technique following hip fracture, which is not surprising given the proportion of the proximal femur and hip joint innervated by this nerve.
However, this technique is not as widely practiced as femoral block and even with ultrasound guidance is an intermediatelevel technique, limiting its widespread use. Patients with hip fracture benefit from regional analgesia immediately on admission to hospital, both to improve comfort and reduce the side effects of opioids. An increasing number of hospitals have a hip fracture clinical pathway that includes femoral nerve block placement in the emergency department. Catheter techniques are particularly valuable in this situation since patients with hip fracture may not receive their operative fixation for 48 hours or longer for various medical or logistical reasons. Pedersen et al. introduced a care pathway for hip fracture that replaced parenteral opioids with a continuous femoral nerve block in a retrospective cohort study; the nerve block group had a significantly reduced incidence of in-hospital complication (odds ratio 0.61, 95% CI 0.4–0.9, P = 0.002), as well as significantly reduced rates of confusion and pneumonia. Mortality was also decreased from 23% to 12%, although this trend was not present in patients who were admitted from nursing homes.
Confusion and delirium are common in the hospitalized elderly patient. Delirium is an independent risk factor for death, institutionalization, and dementia after hip fracture. Two factors that are known to substantially increase the likelihood of delirium are moderate to severe pain and opioids, both of which can be minimized with regional techniques. The impact of regional analgesia on the risk for developing perioperative delirium is unclear, and the absence of effect in some studies likely relates to the complex pathophysiology of delirium. However, there may be specific subpopulations of hip fracture patients who benefit from nerve blocks. Mouzopolous et al. risk-stratified hip fracture patients for delirium on admission using a validated instrument and investigated the effect of daily fascia iliaca block on delirium in intermediate- and high-risk patients. No difference was seen between high-risk patients who received the block with bupivacaine versus a sham block. However, intermediate-risk patients in the fascia iliaca block with bupivacaine group were significantly less likely (2%) to become delirious versus those in the sham block group (17%). Data from these studies support the idea that regional analgesic techniques should be initiated timely in hip fracture patients and continued until pain intensity is sufficiently low that oral nonopioid analgesics are all that are required for pain management.
Clinical Pearl
A clinical pathway including a femoral nerve block and/or catheter may reduce the incidence of confusion, delirium, pneumonia and opioid requirements for patients with hip fracture.
The best choice of anesthetic technique for operative fixation of hip fracture is still a matter of some controversy. Several recent large studies have focused on this question. Luger and colleagues conducted a meta-analysis of 34 randomized, controlled trials, 14 observational studies, and 8 reviews/meta-analysis publications (n = 18,715) and demonstrated that neuraxial anesthesia was associated with significantly reduced early mortality, fewer incidents of deep venous thrombosis, less postoperative confusion, and fewer overall pulmonary complications, including postoperative hypoxia, pneumonia, and fatal pulmonary embolism. There were no differences between groups in the rates of arrhythmias, myocardial events, congestive heart failure, intraoperative blood loss, renal failure, or stroke. Hypotension seemed to occur independent of anesthetic technique, although continuous spinal appeared to have an advantage over single-injection spinal in this regard. Geriatric patients are typically at low risk for postdural puncture headache, and the placement of a spinal catheter is usually free of side effects.
In 2012, Neuman and colleagues published a retrospective analysis of a prospectively collected database collected over 2 years from 126 New York State hospitals. Over 18,000 patients admitted for hip fracture from 126 hospitals were identified and the association between type of anesthesia and patient outcomes tested. Regional anesthesia in this study reduced the risk of in-hospital mortality relative to general anesthesia by 29% and the risk of pulmonary complications by 25%. There was no difference between groups with regard to cardiovascular morbidity.
More recently, White et al. reported the results of an observational audit of over 65,000 patients from the National Hip Fracture Database in the United Kingdom. The authors specifically looked at early mortality and found no difference between groups receiving general versus neuraxial anesthesia in terms of either 5-day or 30-day mortality. The authors suggest that with modern advances in pharmacotherapy and monitoring, as well as improved methods for optimizing patients prior to surgery, may have diminished any difference in this metric. They also suggest that our research efforts should now be focused on postoperative confusion, hypotension, pain, mobility, and respiratory complications.
On balance, it appears that data tend to show that there are morbidity advantages to regional anesthesia techniques, if not mortality advantages. Although regional anesthesia is not yet a standard of care, the burden of proof is increasingly on the anesthesia provider to demonstrate why it would be more appropriate to proceed with a general rather than regional anesthetic in this group of patients.
SPECIFIC INJURIES: FRACTURED RIBS
Rib fractures are the most common injury associated with chest trauma, with an incidence of 12% of all trauma admissions. The number of rib fractures is directly related to the associated mortality: 5% for 1–2 ribs, 15% for 3–5 ribs, and 34% for 6 or more ribs fractured. The cause of mortality is related primarily to pulmonary injury, such as lung contusion and pneumothorax, and delayed pulmonary processes, such as pneumonia and acute respiratory distress syndrome. Fractured ribs are a marker of injury severity, especially in young patients with compliant ribs cages where this injury is associated with more impact energy.
Fractured ribs and significant pain may limit the patient’s ability to breathe adequately. The lack of deep inspiratory sighs and shallow tidal breathing promote atelectasis, V/Q mismatching, and hypoxemia, increasing the risk of pneumonia and respiratory failure.
Chest physiotherapy is usually either contraindicated or ineffective due to the chest wall pain. Effective analgesia for patients with rib fractures is the primary management goal for these patients, since operative fixation is not performed in most cases. There are several options available for pain management, and the management plan should be individualized, as there is no one best modality for all patients. The goal of therapy should be to minimize respiratory depression and optimize respiratory excursion, while minimizing the possible side effects and complications of the technical procedure, such as local anesthetic systemic toxicity (LAST) or iatrogenic pneumothorax.
Intravenous opioids are a common analgesic option but have the downside of causing sedation and respiratory depression; because of this, opioids may in fact promote respiratory complications at the same time that they reduce pain. NSAIDs can be effective for mild rib fracture pain but may potentiate bleeding in patients who have vascular injury or are taking anticoagulant medications. Acetaminophen is a fairly safe mild analgesic with few side effects, but its effect is limited in multiple rib fractures when pain intensity is high.
There are several regional analgesic options for relief of rib fracture pain. Thoracic epidural analgesia (TEA) is a very effective regional anesthetic technique for broken ribs, especially when injuries are bilateral. The Eastern Association for the Surgery of Trauma (EAST) has stated that epidural analgesia may improve clinically significant outcomes (Grade B recommendation) and that it should be considered the preferred analgesic modality (Grade A recommendation). Several studies have evaluated the effect of TEA on outcomes. Bulger et al. randomized 46 patients with 3 or more rib fractures to receive either TEA with bupivacaine or intravenous opioid therapy.
Despite a higher severity of pulmonary injury in the epidural group, the incidence of pneumonia was significantly higher in the opioid group (38% vs. 18%). When adjusted for the presence of direct pulmonary injury, the relative risk of pneumonia in the opioid group was 6-fold higher. In addition, randomization to epidural analgesia decreased the number of days requiring mechanical ventilation by half. This reduction in ventilator-dependent days has also been shown in other randomized, controlled studies. TEA also reduces the pain associated with coughing or deep breathing compared with intravenous opioids or intrapleural bupivacaine. In contrast, a retrospective review of 64 patients with rib fractures demonstrated that while TEA provided superior analgesia to intravenous patient-controlled morphine, hospital or ICU length of stay and major morbidity were unaffected. Furthermore, a meta-analysis of 8 studies (n = 232) also failed to show a difference in major outcomes such as mortality, hospital/ICU length of stay, and duration of mechanical ventilation with epidural analgesia, although the studies chosen were heterogeneous, with both lumbar and thoracic epidural sites included and various combinations of local anesthetic and/or epidural opioid in the infusate.
Although TEA may be effective at reducing morbidity and other outcomes in the setting of rib fractures, it is not appropriate for all patients. Contraindications include hypovolemia and hypotension, coagulopathy, head or spinal injury, and sepsis, conditions that are all relatively common in the trauma population. Thoracic epidural analgesia is performed infrequently in patients who are heavily sedated or under general anesthesia because of the traditional belief that the absence of patient feedback may put the patient at risk for a needle-related spinal cord injury. For this reason, the actual impact of TEA on the reduction in ventilator-dependent days may be limited, since these patients are likely to be sedated and mechanically ventilated prior to consultation for pain management.
Paravertebral nerve block (PVB) is an alternative regional anesthetic procedure that provides excellent unilateral (or bilateral, if performed on both sides) analgesia. A catheter technique is typically employed for fractured ribs, with the needle insertion at the midpoint of the rib levels. The block can then be manipulated to the desired level by the administration of increasing volumes of local anesthetic. In a randomized study of TEA versus thoracic PVB for unilateral multiple fractured ribs, both techniques were found to be equivalent with respect to pain relief, improvement in respiratory function, and incidence of pulmonary complications. The risks of the technique are generally small and include contralateral spread via the epidural space (1%), pneumothorax (0.5%), hypotension (5%), and vascular puncture (4%).
One unique advantage to PVB catheters over TEA is the ability to provide long-duration analgesia in the ambulatory setting. Murata and colleagues reported a case of a patient with multiple (T3–T8) unilateral rib fractures who was experiencing intense pain and respiratory distress. A paravertebral catheter provided rapid and long-lasting (60 h) relief and facilitated discharge home from the critical care unit the day after the block. In another example, Buckley et al. reported that an anesthesiology resident who was experiencing debilitating pain from multiple fractured ribs was able to continue clinical work opioid-free while receiving an infusion of local anesthetic through a paravertebral catheter for a total of 18 days.
NYSORA Tips
- Continuous paravertebral analgesia provides excellent relief from pain associated with rib fractures and facilitates improved respiratory mechanics.
- Selected patients can be safely discharged home with paravertebral catheters for days to weeks in order to prolong the high-quality analgesia.
Alternative regional techniques have been used but have not been shown to be as effective as either TEA or PVB. Intercostal blocks provide good initial relief but suffer from a limited duration of action and the need to repeat the procedure. In addition, the risk of pneumothorax with each level attempted is additive and thus increases the risk of this complication. Intrapleural block with local anesthetic is similarly limited in efficacy and carries a high risk for rapid systemic absorption of local anesthetic. Transdermal lidocaine patches placed over rib fracture sites have not been shown to significantly improve pain control in patients with traumatic rib fractures.
SPECIFIC INJURIES: DIGITAL REPLANTATION
Long-term graft function after digital replantation (Figure 3) is contingent on the grafted digits receiving an optimal blood supply and the avoidance of vasospasm and thrombosis. Continuous nerve blocks of the limbs facilitate these goals first and foremost by providing sympathetic block, which interrupts injury-induced vasospasm and allows maximal vasodilation.
The profound reduction in afferent input reduces the stress response, which both reduces the tendency toward hypercoagulability and potential thrombotic events and reduces circulating catecholamines, thereby promoting maximal vasodilation. Acral systolic blood pressure and flow are improved, and the muscle relaxation associated with a continuous nerve block helps to prevent inadvertent movement-related mishaps with the delicate anastomoses.
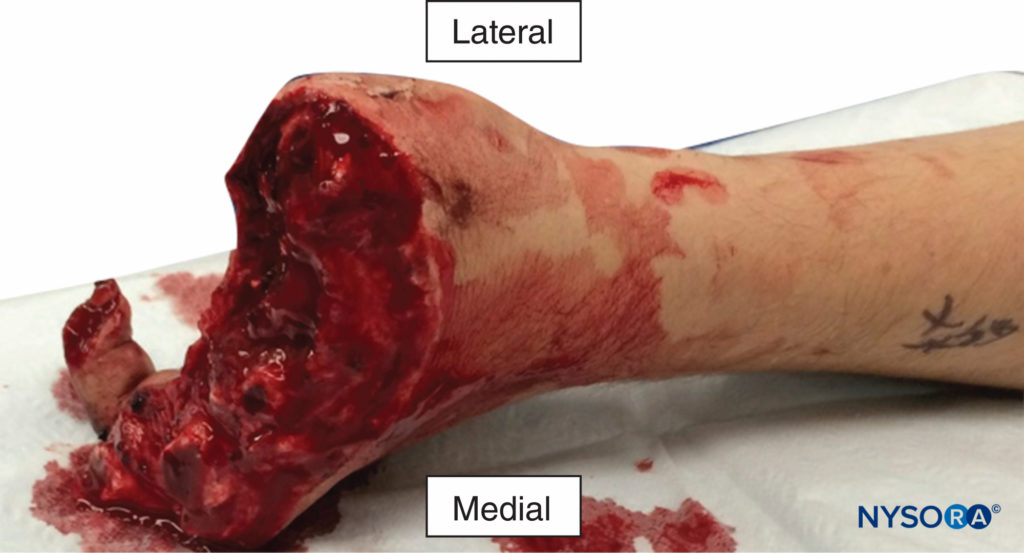
FIGURE 3. Multiple digital amputations resulting from an industrial accident. An infraclavicular catheter was placed preoperatively and local anesthetic infused for 6 days. All four fingers that were replanted survived with good function.
Improvements in outcomes with continuous brachial plexus block have been demonstrated in several studies. In one study that randomized patients to continuous supraclavicular block versus parenteral opioids for digit transfer and/or replantation, reoperation rates due to vascular insufficiency were 0% vs. 29%, respectively. Skin temperature, a marker of tissue perfusion, is consistently elevated in patients with brachial plexus blocks. Pain scores have also been shown to be improved, as well as the incidence of vasospasm. On the other hand, one study failed to show a difference in overall graft survival at 6 months when continuous brachial plexus block was used.
However, the retrospective nature of this study limits the strength of its conclusions. Additional prospective, randomized studies are needed to clarify the extent of the impact of these techniques on outcomes.
REGIONAL ANESTHESIA FOR SHOULDER REDUCTION
Reduction of a dislocated shoulder is a common procedure performed in the emergency department. Intravenous procedural sedation using propofol, ketamine, or etomidate is commonly employed to produce sufficient muscle relaxation to reduce the joint. However, procedural sedation is often not ideal for such short and limited procedures. Approximately 6 hours of fasting is required to reduce the risk of gastric aspiration, a condition not commonly met in trauma patients presenting to the emergency room. Hypotension and respiratory compromise are real risks, especially with the use of such potent cardiopulmonary depressive agents as propofol. These risks mandate close monitoring and one-on-one care in the emergency room that can occupy nursing resources.
Regional anesthesia, particularly interscalene brachial plexus block (ISB), offers an attractive alternative that eases the requirements for performing shoulder dislocation reduction. ISB provides profound shoulder girdle muscle relaxation by anesthetizing the superior trunk of the brachial plexus. ISB does not require sedation, and, although cardiorespiratory monitoring is still required, the risk of apnea or hypotension is virtually nonexistent. Blaivas et al. demonstrated that length of stay in the emergency department and the need for one-on-one care are reduced in patients receiving ISB versus procedural sedation for shoulder reduction.
REGIONAL ANESTHESIA FOR BURNS
Early management of burn injuries should focus on (1) an airway with a low threshold for intubation; (2) breathing with the availability of high-flow 100% oxygen administration; and (3) aggressive fluid resuscitation. Standard protocols suggest 2-4 mL/kg of crystalloid for each 1% body surface area (BSA) affected. This applies to large burns only (ie >20% total BSA) given this population’s increased vascular permeability and fluid requirement.
Pain related to burn injuries can range from mild to debilitating, depending on the area involved and the depth of the burn. Skin nociceptors that are not destroyed transmit pain immediately after injury, and the perception of pain is complicated by both primary and secondary hyperalgesia, which occur at the wound and spinal level, respectively. Patients with extensive burns often experience more postoperative pain at the split-thickness skin donor site than in the grafted wound itself. Single-injection nerve blocks have provided much success in the harvesting of these donor sites. Burn patients, however, commonly require repeated visits to the operating room and painful procedures such as physical therapy and dressing changes in the burn unit. This pattern of brief, intense painful procedures superimposed on moderate background pain makes effective analgesia challenging in these patients. Such procedures can occasionally be severe enough to require general anesthesia or deep sedation in the ICU or operating room. This is disadvantageous for a number of reasons, not least of which is the frequent interruption of enteral nutrition to keep patients fasted at a time when anything by mouth (nil per os, NPO) at a time when their metabolic demand is supranormal.
Therefore, the use of continuous peripheral or neuraxial catheter may be favored over single-injection techniques whenever appropriate. Given the greater risk of infection from the loss of a protective barrier and altered immune response in burn patients, the decision to use catheters should be made carefully. Catheters should not be placed through burned skin. Burns result in a hypercoagulable state, and deep blocks or neuraxial analgesic techniques are generally safe, unless the patient develops coagulation abnormalities from sepsis or profound blood loss without factor replacement.
Multiple peripheral nerve catheters can aid in covering extensive burn injuries. While the principal concern with continuous infusions is systemic toxicity, evidence has shown this to be rare with clinically relevant dosing regimens and primarily a theoretical concern (see discussion above under heading “Continuous Peripheral Nerve block in Patients with Trauma”). Moreover, plasma levels of alpha-1 acid glycoprotein (AAG), the plasma protein and acute phase reactant that binds local anesthetics, are known to be significantly elevated in burns (and trauma in general) for at least 20 days, which may help to provide an increased margin of safety in these patients.
Much of the morbidity in burn patients is due to the significant stress response that results, with its attendant effects on metabolism, wound healing, and immune function. Neural block of a burned area can substantially reduce this profound stress response via its inhibition of nociceptive input to the central nervous system. It has been demonstrated that neural block reduces the incidence of hyperalgesia following thermal injury. Moreover, regional anesthesia results in reduced vasospasm and local thrombosis during skin grafting procedures, effects that are detrimental to graft function.
ACUTE COMPARTMENT SYNDROME CONSIDERATIONS
Acute compartment syndrome (ACS) is a serious soft tissue injury of an extremity that can occur following trauma. This pathologic syndrome arises when the pressure within a closed compartment rises above a capillary perfusion pressure, compromising the circulation and tissue function within that space.
This is typically the result of a high-energy injury to soft tissue but has also been reported with crush or reperfusion injury, exercise, arterial puncture, circumferential dressings, burns, and snake bites. Over one-third of all cases of ACS are associated with tibial fracture, particularly the proximal and middle thirds of the diaphysis (due to the bulkier muscle mass compared with the ankle). Fractures of the forearm are also common injuries that may lead to ACS.
NYSORA Tips
- ACS occurs in areas packed with muscles, such as the proximal leg or forearm.
- The risk of ACS is highest in proximal tibial fractures, occurring at a rate of approximately 6%–10%.
Following capillary collapse, flow into the venous system ceases, leading to tissue hypoxia and the release of vascular mediators. The resultant leakage of fluid through capillary and muscle membranes increases edema and worsens the intracompartmental pressure, leading to a vicious cycle of increased pressure → ischemia → leakage → increased pressure. Tissue pressure in muscular compartments is usually 0–10 mm Hg, and capillary filling pressure is equivalent to diastolic arterial pressure. When the gradient between tissue pressure and diastolic blood pressure falls to within 30 mm Hg, the risk for capillary collapse and the development of ACS rises significantly.
Emergent fasciotomy is required to release the tense muscles from the inelastic osteofascial compartments (Figure 4); if not performed within 3–6 hours of the onset of ischemia, myonecrosis occurs, followed by rhabdomyolysis, myoglobinuria, acute tubular necrosis, and hyperkalemia. ACS can be a fatal complication. Regional anesthesia and analgesia in the presence of injuries at high risk for ACS remain controversial. Many anesthesiologists and orthopedic surgeons agree to avoid regional techniques for fear that the neural block may mask the developing syndrome, since ACS is traditionally diagnosed on the basis of pain out of proportion to the injury (especially on passive stretch) and paresthesia.
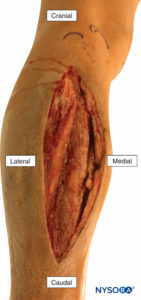
FIGURE 4. Fasciotomy of the anterior and lateral compartments of the leg to relieve acute compartment syndrome following a tibial fracture.
However, these clinical signs and symptoms appear to have a sensitivity and positive predictive value of only 11%–19%, whereas the specificity and negative predictive value for lower leg injuries are 97%–98%. In other words, the classic clinical findings are more likely to be present in an injured patient without ACS than in a patient with the syndrome. While the absence of clinical signs and symptoms appears to be a reassuring sign, it is unlikely that a patient who has a sufficiently serious injury to be at risk for ACS would be free of pain, therefore calling into question the utility of the high negative predictive value. In addition, these signs are probably even less useful in the sedated or neurologically impaired patient.
A handful of case reports have been published relating specifically to peripheral nerve blocks and ACS. However, in all but one of these cases, a nerve block actually facilitated the early detection and prompt treatment of the ischemic limb by the development of new-onset breakthrough pain, alerting the clinicians to a change in the status. One case report did assert that a femoral block was responsible for a missed anterior compartment syndrome of the leg following intramedullary nailing.
However, the anterior compartment is supplied by the deep peroneal nerve, making femoral block a very unlikely contributing factor. In contrast to peripheral nerve block, epidural analgesia has been implicated in at least 3 reports of ACS when dense motor block has been present. This finding highlights the need to use dilute solutions of local anesthetics when placing peripheral nerve blocks in trauma patients. Catheter techniques are particularly effective and safe, as the concentration of the local anesthetic can be adjusted to match the intervention (surgical procedure vs. postoperative pain), and the infusion can be stopped entirely if required. Catheters can be placed at any time during the hospital course and left “dry” (or with a small infusion of saline to prevent clotting) and bolused when appropriate.
Clinical Pearl
To date, there are no published cases of regional anesthesia delaying the diagnosis of ACS. In contrast, there are several reports of pain breaking through a block, which has facilitated the early diagnosis of a developing ACS.
Since randomized controlled trials are unlikely to be forthcoming due to ethical issues, hard data on the safety of nerve blocks is unavailable. Furthermore, it is possible that the diagnosis of ACS in the setting of peripheral nerve blocks is underreported or simply avoided in high-risk patients. Rather than focusing on whether or not to perform a PNB, our attention might better be directed toward careful monitoring of analgesic consumption and breakthrough pain and the use of compartment pressure monitoring for high-risk patients. Vigilance by both surgical and anesthetic teams involved in the patient’s care is the key to the early detection of ACS.
SUMMARY
Patients with acute trauma frequently require complex management, with coexisting, often competing, priorities. High-quality analgesia in this population must also be addressed. In addition to improving patient comfort, peripheral nerve and neuraxial block significantly reduce the requirement for systemic opioid analgesia and the adverse effects associated with opioid use. This is often critical in the multiply injured patient who suffers from neurologic, cardiovascular, and/or pulmonary impairment. In addition, the early use of regional anesthetic techniques in selected trauma patients appears to improve outcomes such as pulmonary morbidity, delirium, and mortality and facilitates reductions in length of stay in both the emergency room and hospital overall.
Additional research is required to clarify the impact of peripheral nerve blocks and neuraxial analgesia on outcomes such as the development of delirium, mobility, chronic posttraumatic pain and posttraumatic stress disorder. While peripheral nerve blocks may not delay the diagnosis of acute compartment syndrome, prudent use of regional techniques in trauma patients should be combined with a multidisciplinary approach, astute clinical judgment, and vigilance.
REFERENCES
- Centers for Disease Control and Prevention. FastStats: All Injuries. http://www.cdc.gov/nchs/fastats/injury.htm. Accessed March 16, 2015.
- Holdgate A, Shepherd SA, Huckson S: Patterns of analgesia for fractured neck of femur in Australian emergency departments. Emerg Med Australas 2010;22:3–8. doi: 10.1111/j.1742-6723.2009.01246.x.
- Berben SAA, Meijs THJM, van Dongen RTM, et al: Pain prevalence and pain relief in trauma patients in the accident & emergency department. Injury 2008;39:578–585. doi: 10.1016/j.injury.2007.04.013.
- Choi JJ, Lin E, Gadsden J: Regional anesthesia for trauma outside the operating theatre. Curr Opin Anaesthesiol 2013;26:495–500. doi: 10.1097/ACO.0b013e3283625ce3.
- Elvir-Lazo OL, White PF: The role of multimodal analgesia in pain management after ambulatory surgery. 2010;23:697–703. doi: 10.1097/ACO.0b013e32833fad0a.
- Kehlet H, Dahl JB: The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth Analg 1993;77:1048–1056.
- Banerjee M, Bouillon B, Shafizadeh S, et al: Epidemiology of extremity injuries in multiple trauma patients. Injury 2013;44:1015–1021. doi: 10.1016/j.injury.2012.12.007.
- Gallay SH, Hupel TM, Beaton DE, Schemitsch EH, McKee MD: Functional outcome of acromioclavicular joint injury in polytrauma patients. J Orthop Trauma 1998;12:159–163.
- Stone MB, Wang R, Price DD: Ultrasound-guided supraclavicular brachial plexus nerve block vs procedural sedation for the treatment of upper extremity emergencies. Am J Emerg Med 2008;26:706–710. doi: 10.1016/j.ajem.2007.09.011.
- Blaivas M, Adhikari S, Lander L: A prospective comparison of procedural sedation and ultrasound-guided interscalene nerve block for shoulder reduction in the emergency department. Acad Emerg Med 2011;18:
922–927. doi: 10.1111/j.1553-2712.2011.01140.x. - Edwards D, Bowden M, Aldington DJ: Pain management at role 4. J R Army Med Corps 2009;155:58–61.
- Hughes S, Birt D: Continuous peripheral nerve block on OP HERRICK 9. J R Army Med Corps 2009;155:57–58.
- Miller SL, Cleeman E, Auerbach J, Flatow EL: Comparison of intra-articular lidocaine and intravenous sedation for reduction of shoulder dislocations: a randomized, prospective study. J Bone Joint Surg Am 2002; 84A:2135–2139.
- Radresa O, Chauny J-M, Lavigne G, Piette E, Paquet J, Daoust R: Current views on acute to chronic pain transition in post-traumatic patients: risk factors and potential for pre-emptive treatments. J Trauma Acute Care Surg 2014;76:1142–1150. doi: 10.1097/TA.0000000000000188.
- Clay FJ, Watson WL, Newstead SV, McClure RJ: A systematic review of early prognostic factors for persisting pain following acute orthopedic trauma. Pain Res Manag 2012;17:35–44.
- Macrae WA: Chronic post-surgical pain: 10 years on. Br J Anaesth 2008; 101:77–86. doi: 10.1093/bja/aen099.
- Buckenmaier CC3 rd, Rupprecht C, McKnight G, et al: Pain following battlefield injury and evacuation: a survey of 110 casualties from the wars in Iraq and Afghanistan. Pain Med 2009;10:1487–1496. doi: 10.1111/j.1526-4637.2009.00731.x.
- Buckenmaier CC 3rd, Shields CH, Auton AA, et al: Continuous peripheral nerve block in combat casualties receiving low-molecular weight heparin. Br J Anaesth 2006;97:874–877. doi: 10.1093/bja/ael269.
- Capdevila X, Bringuier S, Borgeat A: Infectious risk of continuous peripheral nerve blocks. Anesthesiology 2009;110:182–188. doi: 10.1097/ALN.0b013e318190bd5b.
- Neuburger M, Büttner J, Blumenthal S, Breitbarth J, Borgeat A: Inflammation and infection complications of 2285 perineural catheters: a prospective study. Acta Anaesthesiol Scand 2007;51:108–114. doi: 10.1111/j.1399-6576.2006.01173.x.
- Morin AM, Kerwat KM, Klotz M, et al: Risk factors for bacterial catheter colonization in regional anaesthesia. BMC Anesthesiol 2005;5:1. doi: 10.1186/1471-2253-5-1.
- Cuvillon P, Ripart J, Lalourcey L, et al: The continuous femoral nerve block catheter for postoperative analgesia: bacterial colonization, infectious rate and adverse effects. Anesth Analg 2001;93:1045–1049.
- Capdevila X, Pirat P, Bringuier S, et al: Continuous peripheral nerve blocks in hospital wards after orthopedic surgery: a multicenter prospective analysis of the quality of postoperative analgesia and complications in 1,416 patients. Anesthesiology 2005;103:1035–1045.
- Lai TT, Jaeger L, Jones BL, Kaderbek EW, Malchow RJ: Continuous peripheral nerve block catheter infections in combat-related injuries: a case report of five soldiers from Operation Enduring Freedom/Operation
Iraqi Freedom. Pain Med 2011;12:1676–1681. doi: 10.1111/ j.1526-4637.2011.01251.x. - Plunkett AR, Buckenmaier CC 3rd: Safety of multiple, simultaneous continuous peripheral nerve block catheters in a patient receiving therapeutic low-molecular-weight heparin. Pain Med 2008;9:624–627.
doi: 10.1111/j.1526-4637.2008.00418.x. - Bleckner LL, Bina S, Kwon KH, McKnight G, Dragovich A, Buckenmaier CC 3rd: Serum ropivacaine concentrations and systemic local anesthetic toxicity in trauma patients receiving long-term continuous peripheral
nerve block catheters. Anesth Analg 2010;110:630–634. doi: 10.1213/ ANE.0b013e3181c76a33. - Knudsen K, Beckman Suurküla M, Blomberg S, Sjövall J, Edvardsson N: Central nervous and cardiovascular effects of i.v. infusions of ropivacaine, bupivacaine and placebo in volunteers. Br J Anaesth 1997;78:507–514.
- Stone MB, Carnell J, Fischer JWJ, Herring AA, Nagdev A: Ultrasoundguided intercostal nerve block for traumatic pneumothorax requiring tube thoracostomy. Am J Emerg Med 2011;29:697.e1–2. doi: 10.1016/j.
ajem.2010.06.014. - Randall A, Grigg L, Obideyi A, Srikantharajah I: Fascia iliaca compartment block: a nurse-led initiative for preoperative pain management in patients with a fractured neck of femur. J Orthop Nurs 2008;12:69–74.
- Minville V, Gozlan C, Asehnoune K, Zetlaoui P, Chassery C, Benhamou D: Fascia-iliaca compartment block for femoral bone fracture in prehospital medicine in a 6-yr-old child. Eur J Anaesthesiol 2006;23:
715–716. doi: 10.1017/S0265021506271126. - Gozlan C, Minville V, Asehnoune K, Raynal P, Zetlaoui P, Benhamou D: [Fascia iliaca block for femoral bone fractures in prehospital medicine]. Ann Fr Anesth Reanim 2005;24:617–620. doi: 10.1016/j. annfar.2005.03.030.
- Lopez S, Gros T, Bernard N, Plasse C, Capdevila X: Fascia iliaca compartment block for femoral bone fractures in prehospital care. Reg Anesth Pain Med 2003;28:203–207. doi: 10.1053/rapm.2003.50134.
- McRae PJ, Bendall JC, Madigan V, Middleton PM: Paramedic-performed fascia iliaca compartment block for femoral fractures: a controlled trial. J Emerg Med 2015;48:581–589. doi: 10.1016/j.jemermed.2014.12.016.
- Gros T, Amaru P, Basuko C, Dareau S, Eledjam JJ: [Sciatic nerve block in prehospital care]. Ann Fr Anesth Reanim 2010;29:162–164. doi: 10.1016/j.annfar.2009.11.006.
- Gros T, Delire V, Dareau S, Sebbane M, Eledjam JJ: [Interscalene brachial plexus block in prehospital medicine]. Ann Fr Anesth Reanim 2008;27: 859–860. doi: 10.1016/j.annfar.2008.09.002.
- Lopez S, Gros T, Deblock N, Capdevila X, Eledjam JJ: [Multitruncular block at the elbow for a major hand trauma for prehospital care]. Ann Fr Anesth Reanim. 2002;21(10):816-819.
- Simpson PM, McCabe B, Bendall JC, Cone DC, Middleton PM: Paramedic-performed digital nerve block to facilitate field reduction of a dislocated finger. Prehosp Emerg Care 2012;16:415–417. doi: 10.3109/10903127.2012.670690.
- Roche JJW, Wenn RT, Sahota O, Moran CG: Effect of comorbidities and postoperative complications on mortality after hip fracture in elderly people: prospective observational cohort study. BMJ 2005;331:1374. doi: 10.1136/bmj.38643.663843.55.
- Roudsari BS, Ebel BE, Corso PS, Molinari N-AM, Koepsell TD: The acute medical care costs of fall-related injuries among the U.S. older adults. Injury 2005;36:1316–1322. doi: 10.1016/j.injury.2005.05.024.
- Abou-Setta AM, Beaupre LA, Rashiq S, et al: Comparative effectiveness of pain management interventions for hip fracture: a systematic review. Ann Intern Med 2011;155:234–245. doi:10.7326/0003-4819-155-4-201108160-00346.
- Beaudoin FL, Nagdev A, Merchant RC, Becker BM: Ultrasound-guided femoral nerve blocks in elderly patients with hip fractures. Am J Emerg Med 2010;28:76–81. doi: 10.1016/j.ajem.2008.09.015.
- Watson MJ, Walker E, Rowell S, et al: Femoral nerve block for pain relief in hip fracture: a dose finding study. Anaesthesia 2014;69:683–686. doi: 10.1111/anae.12683.
- Temelkovska-Stevanovska M, Durnev V, Jovanovski-Srceva M, Mojsova-Mijovska M, Trpeski S: Continuous femoral nerve block versus fascia iliaca compartment block as postoperative analgesia in patients with hip fracture. Prilozi 2014;35:85–94.
- Parker MJ, Handoll HHG, Griffiths R: Anaesthesia for hip fracture surgery in adults. Cochrane Database Syst Rev. 2004;(4):CD000521. doi: 10.1002/14651858.CD000521.pub2.
- Newman B, McCarthy L, Thomas PW, May P, Layzell M, Horn K: A comparison of pre-operative nerve stimulator-guided femoral nerve block and fascia iliaca compartment block in patients with a femoral neck fracture. Anaesthesia 2013;68:899–903. doi: 10.1111/anae.12321.
- Dolan J, Williams A, Murney E, Smith M, Kenny GNC: Ultrasound guided fascia iliaca block: a comparison with the loss of resistance technique. Reg Anesth Pain Med 2008;33:526–531.
- Rashiq S, Vandermeer B, Abou-Setta AM, Beaupre LA, Jones CA, Dryden DM: Efficacy of supplemental peripheral nerve block for hip fracture surgery: multiple treatment comparison. Can J Anaesth 2013;60:230– 243. doi: 10.1007/s12630-012-9880-8.
- Pedersen SJ, Borgbjerg FM, Schousboe B, et al: A comprehensive hip fracture program reduces complication rates and mortality. J Am Geriatr Soc 2008;56:1831–1838. doi: 10.1111/j.1532-5415.2008.01945.x.
- Marcantonio ER, Flacker JM, Michaels M, Resnick NM: Delirium is
independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc 2000;48:618–624. - Mouzopoulos G, Vasiliadis G, Lasanianos N, Nikolaras G, Morakis E, Kaminaris M: Fascia iliaca block prophylaxis for hip fracture patients at risk for delirium: a randomized placebo-controlled study. J Orthop
Traumatol 2009;10:127–133. doi: 10.1007/s10195-009-0062-6. - Luger TJ, Kammerlander C, Gosch M, et al: Neuroaxial versus general anaesthesia in geriatric patients for hip fracture surgery: does it matter? Osteoporos Int 2010;21(Suppl 4):S555–S572. doi: 10.1007/s00198-010-1399-7.
- Neuman MD, Silber JH, Elkassabany NM, Ludwig JM, Fleisher LA: Comparative effectiveness of regional versus general anesthesia for hip fracture surgery in adults. Anesthesiology 2012;117:72–92. doi: 10.1097/ALN.0b013e3182545e7c.
- White SM, Moppett IK, Griffiths R: Outcome by mode of anaesthesia for hip fracture surgery. An observational audit of 65 535 patients in a national dataset. Anaesthesia 2014;69:224–230. doi: 10.1111/anae.12542.
- Sharma OP, Oswanski MF, Jolly S, Lauer SK, Dressel R, Stombaugh HA: Perils of rib fractures. Am Surg 2008;74:310–314.
- Flagel BT, Luchette FA, Reed RL, et al: Half-a-dozen ribs: the breakpoint for mortality. Surgery 2005;138:717–723; discussion 723–725. doi: 10.1016/j.surg.2005.07.022.
- Simon BJ, Cushman J, Barraco R, et al: Pain management guidelines for blunt thoracic trauma. J Trauma 2005;59:1256–1267.
- Bulger EM, Edwards T, Klotz P, Jurkovich GJ: Epidural analgesia improves outcome after multiple rib fractures. Surgery 2004;136:426–430. doi: 10.1016/j.surg.2004.05.019.
- Ullman DA, Fortune JB, Greenhouse BB, Wimpy RE, Kennedy TM: The
treatment of patients with multiple rib fractures using continuous thoracic epidural narcotic infusion. Reg Anesth 1989;14:43–47. - Sahin S, Uckunkaya N, Soyal S, et al: The role of epidural continuous pain treatment on duration of intubation, ventilation and ICU stay in flail chest patients. Agri Dergisi 1993;5:18–20.
- Pierre E, Martin P, Frohock J, et al: Lumbar epidural morphine versus. Patient-controlled analgesia morphine in patients with multiple rib fractures. Anesthesiology 2005;103:A289.
- Luchette FA, Radafshar SM, Kaiser R, Flynn W, Hassett JM: Prospective evaluation of epidural versus intrapleural catheters for analgesia in chest wall trauma. J Trauma 1994;36:865–869; discussion 869–870.
- Moon MR, Luchette FA, Gibson SW, et al: Prospective, randomized comparison of epidural versus parenteral opioid analgesia in thoracic trauma. Ann Surg 1999;229:684–691; discussion 691–692.
- Wu CL, Jani ND, Perkins FM, Barquist E: Thoracic epidural analgesia versus intravenous patient-controlled analgesia for the treatment of rib fracture pain after motor vehicle crash. J Trauma 1999;47:564–567.
- Carrier FM, Turgeon AF, Nicole PC, et al: Effect of epidural analgesia in patients with traumatic rib fractures: a systematic review and metaanalysis of randomized controlled trials. Can J Anaesth 2009;56:230–242. doi: 10.1007/s12630-009-9052-7.
- Gadsden J, Kwofie K, Shastri U: Continuous intercostal versus paravertebral block for multiple fractured ribs. J Trauma Acute Care Surg 2012;73:293–294; author reply 294. doi: 10.1097/TA.0b013e31825aaeb5.
- Mohta M, Verma P, Saxena AK, Sethi AK, Tyagi A, Girotra G: Prospective, randomized comparison of continuous thoracic epidural and thoracic paravertebral infusion in patients with unilateral multiple fractured ribs—a pilot study. J Trauma 2009;66:1096–1101. doi: 10.1097/
TA.0b013e318166d76d. - Karmakar MK: Thoracic paravertebral block. Anesthesiology 2001;95:771–780.
- Murata H, Salviz EA, Chen S, Vandepitte C, Hadzic A: Case report: ultrasound-guided continuous thoracic paravertebral block for outpatient acute pain management of multilevel unilateral rib fractures. Anesth Analg 2013;116:255–257. doi: 10.1213/ANE.0b013e31826f5e25.
- Buckley M, Edwards H, Buckenmaier CC 3rd, Plunkett AR: Continuous thoracic paravertebral nerve block in a working anesthesia resident-when opioids are not an option. Mil Med 2011;176:578–580.
- Hwang EG, Lee Y: Effectiveness of intercostal nerve block for management of pain in rib fracture patients. J Exerc Rehabil 2014;10:241–244. doi: 10.12965/jer.140137.
- Ho AM-H, Karmakar MK, Critchley LAH. Acute pain management of patients with multiple fractured ribs: a focus on regional techniques. Curr Opin Crit Care 2011;17:323–327. doi: 10.1097/MCC.0b013e328348bf6f.
- Ingalls NK, Horton ZA, Bettendorf M, Frye I, Rodriguez C: Randomized, double-blind, placebo-controlled trial using lidocaine patch 5% in traumatic rib fractures. J Am Coll Surg 2010;210:205–209. doi: 10.1016/j.jamcollsurg.2009.10.020.
- Shanahan PT: Replantation anesthesia. Anesth Analg 1984;63:785–786.
- Kurt E, Ozturk S, Isik S, Zor F: Continuous brachial plexus block for digital replantations and toe-to-hand transfers. Ann Plast Surg 2005;54:24–27.
- Berger A, Tizian C, Zenz M: Continuous plexus block for improved circulation in microvascular surgery. Ann Plast Surg 1985;14:16–19.
- Su H-H, Lui P-W, Yu C-L, et al: The effects of continuous axillary brachial plexus block with ropivacaine infusion on skin temperature and survival of crushed fingers after microsurgical replantation. Chang Gung Med J 2005;28:567–574.
- Taras JS, Behrman MJ: Continuous peripheral nerve block in replantation and revascularization. J Reconstr Microsurg 1998;14:17–21. doi: 10.1055/s-2007-1006896.
- Niazi AU, El-Beheiry H, Ramlogan R, Graham B, von Schroeder HP, Tumber PS: Continuous infraclavicular brachial plexus block: effect on survival of replanted digits. Hand Surg 2013;18:325–330. doi: 10.1142/S0218810413500342.
- Vinson DR, Hoehn CL: Sedation-assisted orthopedic reduction in emergency medicine: the safety and success of a one physician/one nurse model. West J Emerg Med 2013;14:47–54. doi: 10.5811/westjem.2012.4.12455.
- Gadsden J: Regional Anesthesia in Trauma. Cambridge: Cambridge University Press, 2012.
- Shteynberg A, Riina LH, Glickman LT, Meringolo JN, Simpson RL: Ultrasound guided lateral femoral cutaneous nerve (LFCN) block: safe and simple anesthesia for harvesting skin grafts. Burns 2013;39:146–149. doi:10.1016/j.burns.2012.02.015.
- Gupta A, Bhandari PS, Shrivastava P: A study of regional nerve blocks and local anesthetic creams (Prilox) for donor sites in burn patients. Burns 2007;33:87–91. doi: 10.1016/j.burns.2006.04.019.
- Cuignet O, Pirson J, Boughrouph J, Duville D: The efficacy of continuous fascia iliaca compartment block for pain management in burn patients undergoing skin grafting procedures. Anesth Analg 2004;98:
1077–1081, table of contents. - Pedersen JL, Crawford ME, Dahl JB, Brennum J, Kehlet H: Effect of preemptive nerve block on inflammation and hyperalgesia after human thermal injury. Anesthesiology 1996;84:1020–1026.
- Mar GJ, Barrington MJ, McGuirk BR: Acute compartment syndrome of the lower limb and the effect of postoperative analgesia on diagnosis. Br J Anaesth 2009;102:3–11. doi: 10.1093/bja/aen330.
- Ulmer T: The clinical diagnosis of compartment syndrome of the lower leg: are clinical findings predictive of the disorder? J Orthop Trauma 2002;16:572–577.
- Walker BJ, Noonan KJ, Bosenberg AT: Evolving compartment syndrome not masked by a continuous peripheral nerve block: evidence-based case management. Reg Anesth Pain Med 2012;37:393–397. doi: 10.1097/AAP.0b013e31824df1ac.
- Cometa MA, Esch AT, Boezaart AP: Did continuous femoral and sciatic nerve block obscure the diagnosis or delay the treatment of acute lower leg compartment syndrome? A case report. Pain Med 2011;12:823–828. doi: 10.1111/j.1526-4637.2011.01109.x.
- Uzel A-P, Steinmann G: Thigh compartment syndrome after intramedullary femoral nailing: possible femoral nerve block influence on diagnosis timing. Orthop Traumatol Surg Res 2009;95:309–313. doi: 10.1016/j.otsr.2009.03.014.
- Noorpuri BS, Shahane SA, Getty CJ: Acute compartment syndrome following revisional arthroplasty of the forefoot: the dangers of ankle-block. Foot Ankle Int 2000;21:680–682.
- Kucera TJ, Boezaart AP: Regional anesthesia does not consistently block ischemic pain: two further cases and a review of the literature. Pain Med 2014;15:316–319. doi: 10.1111/pme.12235.
- Hyder N, Kessler S, Jennings AG, De Boer PG: Compartment syndrome in tibial shaft fracture missed because of a local nerve block. J Bone Joint Surg Br 1996;78:499–500.