Erika Cvetko, Marija Meznarič, and Tatjana Stopar Pintaric
INTRODUCTION
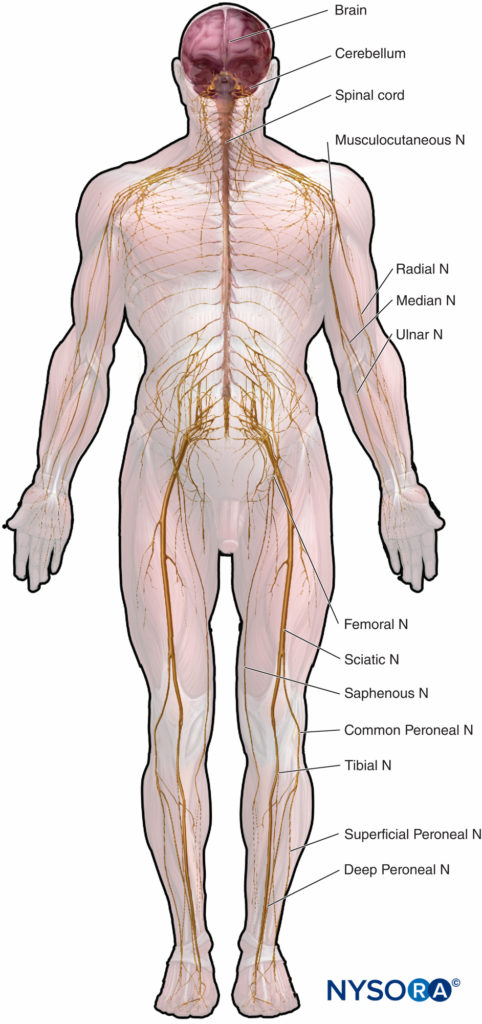
Microscopic anatomy that emphasizes structure-function relations is important to the clinical practice of regional anesthesia. This chapter provides basis for understanding of the structure, classification, and organization of the peripheral nerves and insight into how the characteristics of the peripheral nerves (Figure 1) relate to the clinical practice of regional anesthesia.
ORGANIZATION OF THE PERIPHERAL NERVOUS SYSTEM
The nervous system enables the body to respond to continuous changes in its external and internal environments. It controls and integrates the functional activities of the organs and organ systems.
Nervous system cells consist of neurons and neuroglia. Neurons transmit nerve impulses to and from the central nervous system (CNS), thereby integrating motor and sensory functions. Neuroglial cells support and protect the neurons. In the CNS, myelin is produced by oligodendrocytes and in the peripheral nervous system (PNS) by the Schwann cells. Although both Schwann cells and oligodendrocytes are in charge of axon myelination, they have distinct morphological and molecular properties and different embryonic origins, the neural crest and the neural tube, respectively.
The PNS consists of peripheral nerves (craniospinal, somatic, autonomic) with their associated ganglia and connective tissue investments. All lie peripheral to the pial covering of the CNS.
Peripheral nerves contain fascicles of nerve fibers consisting of axons. In peripheral nerve fibers, axons are ensheathed by Schwann cells, which may or may not form myelin around the axons, depending on their diameter. Nerve fibers are grouped into fascicles of variable numbers. The size, number, and pattern of fascicles vary in different nerves and at different levels along their paths. Generally, their number increases and their size decreases at some distance proximal to the branching point.
NEURONS
A neuron is the structural and functional unit of the nervous system. It includes the cell body, dendrites, and axon.
The cell body (perykarion) is the dilated region of the neuron that contains a large, euchromatic nucleus with a prominent nucleolus and surrounding perinuclear cytoplasm (Figure 2). The perinuclear cytoplasm contains abundant rough-surfaced endoplasmic reticulum and free ribosomes. On light microscopy, the rough endoplasmic reticulum with rosettes of free ribosomes appears as small bodies, called Nissl bodies. The perinuclear cytoplasm contains numerous mitochondria, a large perinuclear Golgi apparatus, liposomes, microtubules, neurofilaments, transport vesicles, and inclusions. The presence of the euchromatic nucleus, large nucleolus, prominent Golgi apparatus, and Nissl bodies indicates the high level of anabolic activity needed to maintain these large cells.
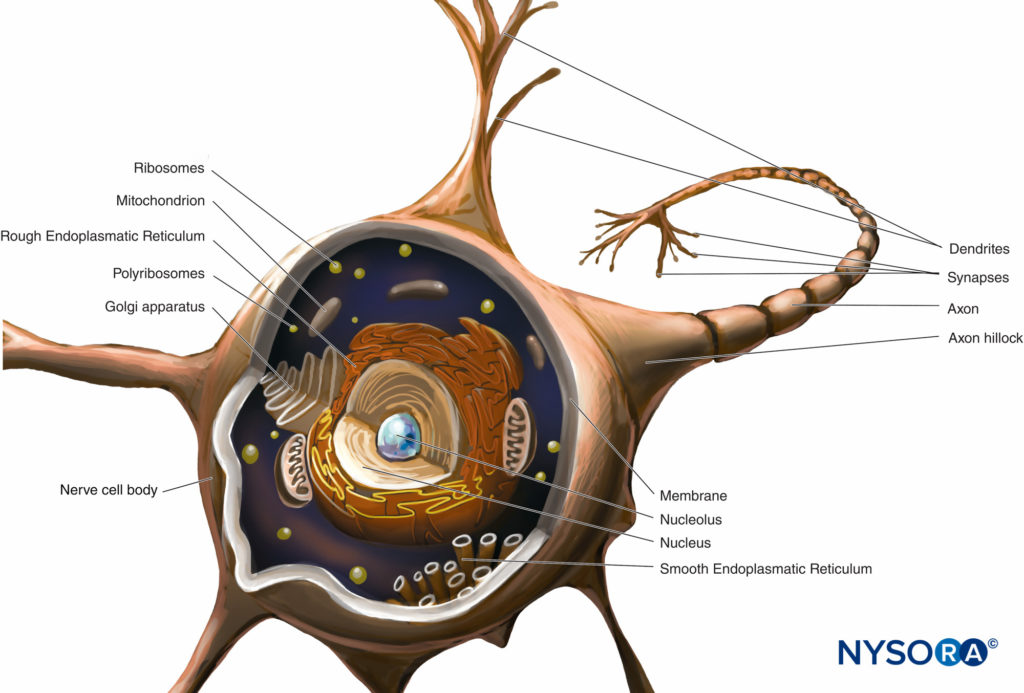
FIGURE 2. Diagram of a multipolar neuron. The nerve cell body, dendrites, and proximal part of the axon are within the CNS. The axons exiting the CNS distal to intervertebral foramina or foramina of the skull constitute the main part of the PNS.
Dendrites are elaborations of the receptive plasma membrane of the neuron. Most neurons possess multiple dendrites that typically arise from the cell body as single short trunks that ramify into smaller branches that taper at the ends. Dendrite-branching patterns are characteristic of each kind of neuron. The base of the dendrite contains the same organelles as the cell body, except the Golgi apparatus. Many organelles become sparse or absent toward the distal end of the dendrite. Dendrite branching results in several synaptic terminals and allows a neuron to receive and integrate multiple impulses.
The axon arises from the cell body as a single thin process, much longer than the dendrites. Its thickness is directly related to conduction velocity, which increases with axonal diameter. Some axons possess collateral branches. The portion of the axon between the cell body and the beginning of the myelin sheath is the initial segment. At the end of the axon, the ramifications may form many small branches. The axonal cytoplasm is called axoplasm.
Almost all of the structural and functional protein molecules are synthesized in the cell body and are transported to distant locations within a neuron in a process known as axonal transport. Crucial to the trophic relations within the axon, axonal transport serves as a mode of intracellular communication carrying molecules and information along microtubules and intermediate filaments from the neuronal cell body to the axon terminal (anterograde transport) or from the axon terminal to the neuronal cell body (retrograde transport). Neurons communicate with other neurons and with effector cells by synapses. These special junctions between neurons and effector cells facilitate the transmission of nerve impulses from one (presynaptic) neuron to another (postsynaptic) neuron or from axons to effector (target) cells, such as muscle and gland cells.
Neurons have greater variation in size and shape than any other group of cells in the body. They are classified morphologically into three major types according to their shape and the arrangement of their processes. The most common neuron type, multipolar, possesses a single axon with various arrangements of multiple dendrites emanating from the cell body. The majority of multipolar neurons (Figure 2 and Figure 3) are motor neurons. A second type of neuron, unipolar or pseudounipolar (Figure 3), possesses only one process, the axon emanating from the cell body and opening up into the peripheral and central branches shortly after leaving the cell body. The central branch enters the CNS, while the peripheral branch proceeds to its corresponding receptor in the body. Each of the two branches is morphologically axonal and can propagate nerve impulses, although the very distal part of the peripheral branch arborizes, indicating its receptor function. The majority of unipolar neurons are sensory neurons, whose cell bodies are situated in the dorsal root ganglia of spinal nerves and in the sensory ganglia of cranial nerves. The third type of neuron, bipolar, possesses two processes emanating from the cell body: a single dendrite and a single axon. They can only be found in some cranial nerves.
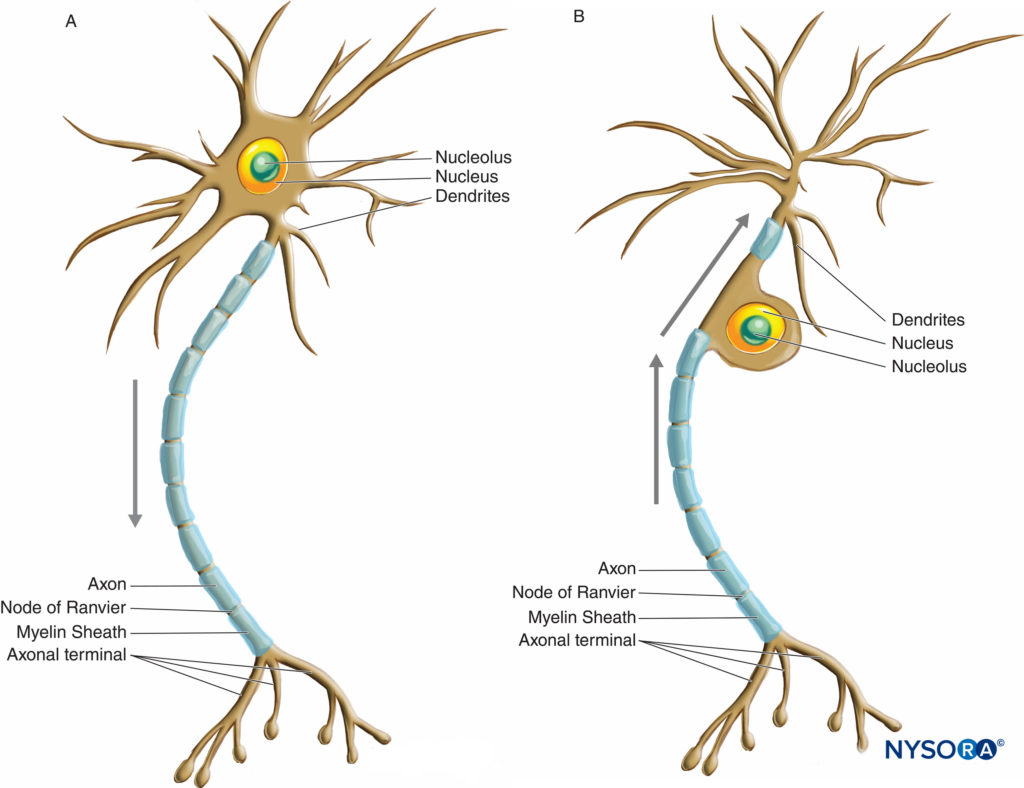
FIGURE 3. Diagram illustrating a multipolar (A) and unipolar or pseudounipolar (B) neuron. Arrows indicate the direction of nerve impulse propagation.
Functionally, the nervous system has somatic and autonomic components. Nerve fibers innervating tissues derived from somites (muscles and skin) are described as somatic; nerve fibers innervating endodermal or other mesodermal derivatives (internal organs) are visceral. The somatic nervous system controls functions that are under conscious voluntary control with the exception of the reflex arch. It provides sensory and motor innervation to all parts of the body except the viscera, smooth muscles, cardiac muscle, and glands. The autonomic nervous system provides efferent involuntary innervation to smooth and cardiac muscles and glands. It also provides the afferent sensory innervation of the viscera (pain and autonomic reflexes).
Efferent Axons
Efferent axons arise from either the somatic or the autonomic nervous system. Somatic efferent (motor) neurons innervate skeletal muscle and have cell bodies located in somatic motor nuclei of the brainstem (cranial nerves) or in the ventral horns of the spinal cord (spinal nerves).
Preganglionic visceral efferent neurons of the sympathetic part of the autonomic nervous system arise from the intermediolateral column of the spinal cord between levels T1 and L2 and synapse on paravertebral or prevertebral (preaortic) ganglia. Peripheral nerves thus contain both preganglionic and postganglionic sympathetic fibers. Preganglionic visceral efferent neurons of the parasympathetic part of the autonomic nervous system arise from the parasympathetic nuclei within the brainstem (cranial part of parasympathetic nervous system) or sacral spinal cord between the S2 and S4 segments (sacral part of the parasympathetic nervous system). Only preganglionic parasympathetic fibers travel along peripheral nerves to synapse on intramural ganglia in the wall of target organs.
Afferent Axons
Afferent axons are either somatic or visceral and have cell bodies either in the dorsal root ganglia of the spinal nerves or in the sensory ganglia of the cranial nerves. Somatic afferent (sensory) neurons transmit impulses from the receptors for touch, temperature, or pain (nociceptors) located in the body wall (skin) and from the proprioceptors in the skeletal muscles and joints. Visceral afferent neurons transmit information from viscera (interoceptors and nociceptors). The visceral afferent axons travel along the visceral efferent fibers and pass through the communicating branches and dorsal roots of the spinal nerves or along the vagus nerve to enter the CNS.
SCHWANN CELLS
Axons of peripheral nerves are ensheathed by Schwann cells. Their myelin sheath (modified plasmalemma) separates the axons from the endoneurium. Schwann cells are distributed along the axons in longitudinal chains depending on myelination along the axon. The coordinated differentiation of the axons and their myelinating cells requires close communication between neurons and glia. Signals provided by the axons regulate the proliferation, survival, and differentiation of glial cells. On the other hand, reciprocal glial signals affect axonal cytoskeleton and transport and are required for axonal survival and regeneration. Schwann cells also have a guiding function for growing axons, indicating that glia do more than provide support to the axon.
Schwann cell phenotypes are characterized by distinct morphologies and differential expression of myelin proteins, cell adhesion molecules, receptors, enzymes, intermediate filament proteins, ion channels, and extracellular matrix proteins. All Schwann cells are surrounded by basal lamina, whose extracellular matrix molecules, such as laminin, regulate key aspects of Schwann cell development.
CLASSIFICATION OF NERVE FIBERS
Nerve fibers are classified according to axonal diameter, conduction velocity, type of receptor, and myelin sheath thickness (Table 1). Conduction velocity is related to axonal diameter; that is, the larger the fiber is, the faster the conduction will be.
TABLE 1. Classification of peripheral nerve fibers according to axonal diameter, conduction velocity, type of receptor, and myelin sheath thickness (myelination).
| Axonal Diameter (µm) | Conduction Velocity (m/s) | Efferent Fibers | Afferent Fibersa (From Cutaneous Receptors) | Afferent Fibers From Skeletal Muscles, Tendons, and Joints | Myelination |
|---|---|---|---|---|---|
| 12–20 | 60–120 30–70 | Aα (to extrafusal muscle fibers) | Aα (from rapidly adapting mechanoreceptors) | Ia (from muscle spindles) Ib (from Golgi tendon organs) | Heavily myelinated |
| 6–12 | 25–70 | Aβ (from slowly adapting mechanoreceptors) | II (from joint proprioceptors) | Myelinated | |
| 3–8 | 15–30 | Aγ (to intrafusal muscle fibers) | Myelinated | ||
| 1–6 | 12–30 | Aδ (from thermal and mechanical nociceptors and thermoreceptors-cold only) | III (from joint proprioceptors and joint nociceptors) | Thinly myelinated | |
| 1–3 | 3–15 | B (preganglionic visceral) | Myelinated | ||
| 0.2–1.5 | 0.5–2 | C (postganglionic visceral) | C (from mechanical nociceptors and thermoreceptors—cold and warm, polymodal nociceptors) | IV (from joint nociceptors) | Unmyelinated |
aVisceral afferent fibers (from interoceptors) are classified as Aδ and C fibers.
Source: Modified with permission from Cramer GD, Darby S: Basic and Clinical Anatomy of the Spine, Spinal Cord, and ANS, 2nd ed. Philadelphia: Elsevier/Mosby; 2005.
NYSORA Tips
The larger the fiber, the more concentrated the local anesthetic must be to affect neural block.
MYELINATED NERVE FIBERS
Myelinated nerve fibers are ensheathed by myelin, greatly extended and modified plasmalemma of the Schwann cells (Figures 4 and 5). Myelin formation begins with extension of the Schwann cell cytoplasm and development of the inner mesaxon, which wraps around the axon several times. During the wrapping process, the cytoplasm is nearly extruded between the plasmalemma. Apposing extracellular faces of plasmalemma become “the major dense line,” and apposing cytoplasmic faces form an “intraperiod line” of myelin. The proposed molecular structure of myelin fits the concept of plasmalemma as a lipid bilayer with integral and peripheral membrane proteins attached to the extracellular or to the cytoplasmic side of plasmalemma. In contrast to most biological membranes, myelin has a high ratio of lipid to protein (70%– 85% lipid, 15%–30% protein), where the latter serve as structural proteins, enzymes, voltage channels, and signal transducers.
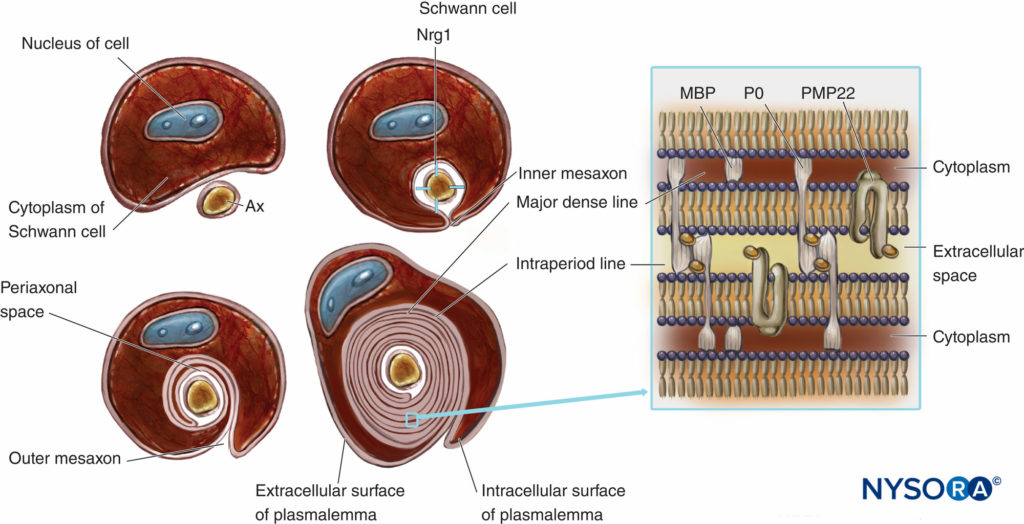
FIGURE 4. Schematic presentation of myelin formation and simplified scheme of its molecular organization. For simplification, the basal lamina of Schwann cells is not drawn. Nrg1 = neuregulin; MPB = myelin basic protein; P0 = protein zero; PMP22 = peripheral membrane protein of 22 kDa; Ax = axon. (Modified with permission from Ross M, Pawlina W: Histology: A Text and Atlas With Correlated Cell and Molecular Biology, 6th ed. Philadelphia: Wolters Kluwer; Lippincott Williams & Wilkins; 2011.)
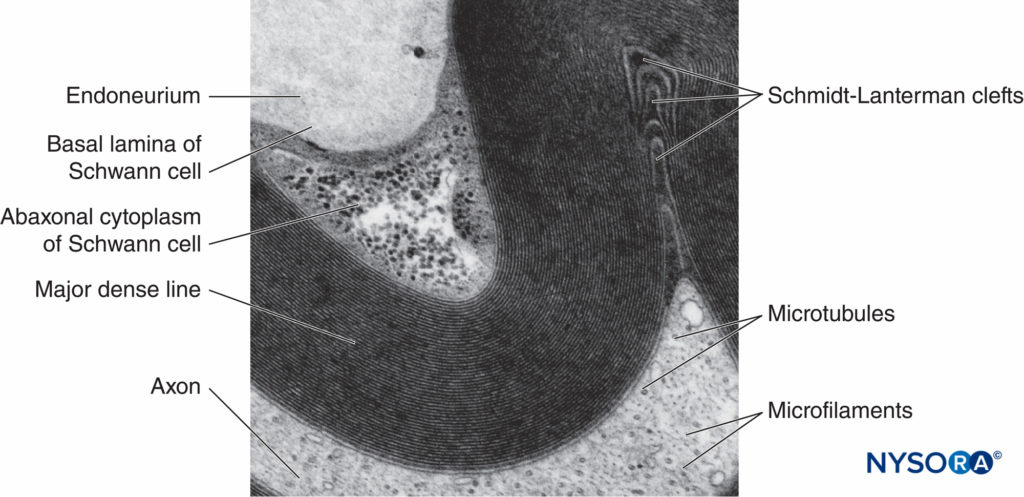
FIGURE 5. Electron micrograph of the myelinated fiber. Myelin is visualized as a series of alternating dark and less-dark lines. Biopsy of human sural nerve.
Myelin sheath wraps the axon in segments. Areas of the axon covered by concentric lamellae of myelin and a single myelin-producing Schwann cell are called internodes and range in length from 200 to 1000 µm. Interruptions, which occur in the myelin sheath at regular intervals along the length of axons and expose the axon, are called nodes of Ranvier (Figure 6). Each node indicates an interface between the myelin sheaths of two different Schwann cells located along the axon.
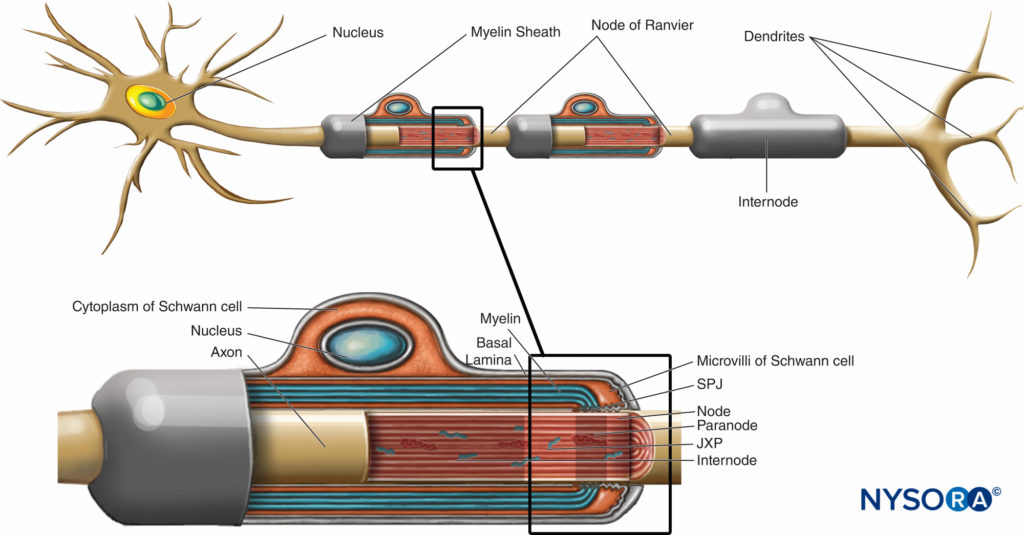
FIGURE 6. Distinct domains of the nodal region. The region occupied by distinct proteins located in the nodal axolemma is schematically depicted in black over axon. SPJ = septate like junctions; JXP = juxtaparanode. (Modified with permission from Poliak S, Peles E. The local differentiation of myelinated axons at nodes of Ranvier. Nat Rev Neurosci. 2003 Dec;4(12):968-980.)
The nodal region and its surroundings can be further subdivided into several domains (Figure 6) that contain a unique set of ion channels, cell adhesion molecules, and cytoplasmic adaptor proteins. In the PNS, the node is in contact with Schwann cell microvilli and covered by its basal lamina (Figure 6). An important characteristic of the nodal axolemma is its high density of voltagegated Na+ channels as compared to the juxtaparanodal axolemma, which typically contains a high density of K+ channels. Na+ channels potentiate the nerve impulse in a saltatory manner (Figure 7) along the myelinated fibers. When the membrane at the node is excited, the local circuit that is generated cannot flow through the high-resistance myelin sheath. It therefore flows out and depolarizes the membrane at the next node, which may be 1 mm or farther away. The low capacitance of the sheath means that little energy is required to depolarize the remaining membrane between the nodes, resulting in increased speed of local circuit spreading.
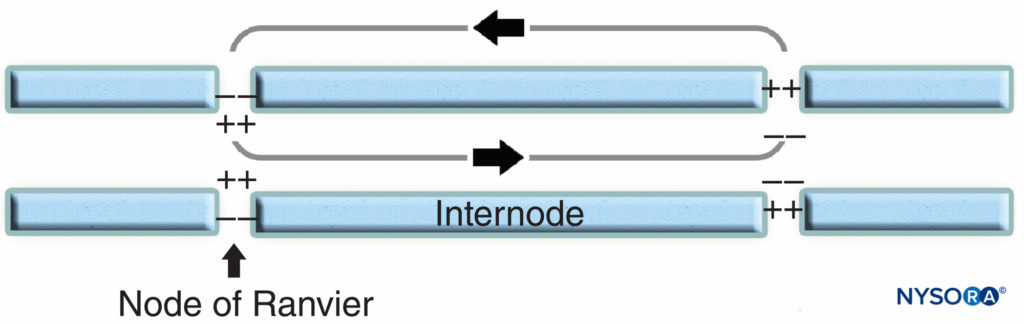
FIGURE 7. Saltatory conduction in myelinated nerve fiber. Na+ channels, located at nodal axolemma, potentiate the nerve impulse in a saltatory manner along myelinated nerve fiber.
Myelination is an example of cell-to-cell communication in which axons interact with Schwann cells. The number of myelin layers is determined by the axon and not by the Schwann cell. Myelin sheath thickness is regulated by a growth factor called neuregulin 1 (Nrg1). The compaction of myelin sheath is associated with the expression of transmembrane myelin-specific proteins such as protein 0 (P0), a peripheral myelin protein of 22 kilodaltons (PMP22), and a myelin basic protein (MBP). The absence of proteins that regulate myelin sheath formation might result in severe hypomyelination or dismyelination in humans and experimental animals.
UNMYELINATED NERVE FIBERS
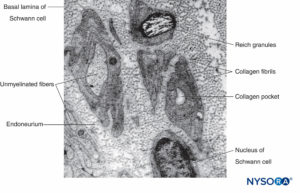
Unmyelinated axons are also enveloped by Schwann cells and their basal lamina. An individual Schwann cell can ensheath a single or several unmyelinated axons (Figures 8 and 9). Unmyelinated fibers predominate in human cutaneous spinal nerves, where the average ratio of unmyelinated to myelinated fiber density is 3.7:1. In unmyelinated fibers, conduction velocity is proportional to the square root of fiber diameter and is much slower compared to saltatory conduction in myelinated fibers (Table 1).
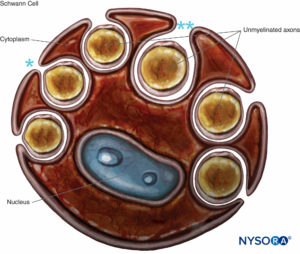
FIGURE 8. Schwann cell that engulfs several unmyelinated axons. The lips of the groove of cytoplasm can be closed (*), forming the mesaxon, or may be opened (**). Basal lamina of the Schwann cell is not drawn.
CONNECTIVE TISSUE INVESTMENTS OF PERIPHERAL NERVES
In a peripheral nerve, nerve fibers and their supporting Schwann cells are held together by connective tissue organized into three distinctive components that have specific morphological and functional characteristics. The epineurium forms the outermost connective tissue of the peripheral nerve, the perineurium surrounds each nerve fascicle separately, while the individual nerve fibers are embedded in the endoneurium (Figures 10 to 13).
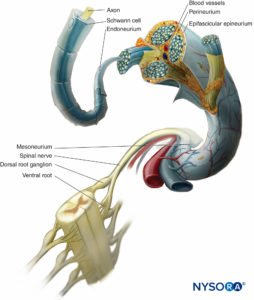
FIGURE 10. Connective tissue investments of peripheral nerve. The diagram demonstrates the arrangement of the peripheral nerve. A segment of the spinal nerve is enlarged to show the relation of the nerve fibers to the surrounding connective tissue (endoneurium, perineurium, and epineurium).
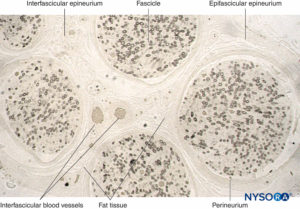
FIGURE 11. Semithin section of human sural nerve fixed in osmium tetroxide. The myelin sheaths are preserved and stained black.
Perineurium surrounds the nerve fascicle. Streaks of connective tissue originate from epifascicular epineurium inside the nerve as
interfascicular epineurium. Fat tissue and blood vessels are localized in interfascicular epineurium.
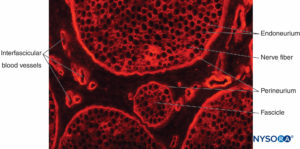
FIGURE 12. Transverse section of pig sciatic nerve. Immunohistochemical staining for collagen. Blood vessels course through the
interfascicular epineurium, which fills the space around the perineurium and fascicles.
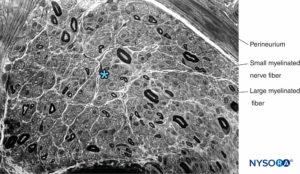
FIGURE 13. Semithin section of human sural nerve stained by cresyl violet. Axonal neuropathy with the predominant loss of large myelinated fibers. * Intrafascicular space between myelinated fibers (occupied by endoneurium, Schwann cell nuclei, and unmyelinated fibers).
Epineurium
The epineurium is a condensation of a loose areolar connective tissue that surrounds a peripheral nerve and binds its fascicles into a common bundle (Figure 10 and Figure 11).
Epineurium that extends between the fascicles is the interfascicular or inner epineurium, while epineurium that surrounds the entire nerve trunk is the epifascicular or external epineurium called the epineurium comprises 30%–75% of the nerve cross-sectional area but varies along the nerve. It is the thickest where continuous with the dura covering the CNS and more abundant in nerves adjacent to the joints, where nerves are subject to pressure. Susceptibility to compression injury is therefore likely to be greater in unifascicular than in multifascicular nerves because the latter have greater amount of epineurium. As the peripheral nerve divides and the number of fascicles is reduced, the epineurium becomes progressively thinner and eventually disappears around monofascicular nerves.
The epineurium contains collagen, fibroblasts, mast cells, and fat cells. Collagen bundles have a predominant longitudinal orientation; however, an electron microscopy study found epineural collagen in bundles 10–20 µm in width are arrayed obliquely around the circumference of the nerve. Elastic fibers are also present, particularly adjacent to the perineurium, which are mainly oriented longitudinally. Collagen and elastic fibers are aligned and oriented to prevent damage by overstretching of the nerve bundle, suggesting that the epineurium is designed to accommodate the stretch.
Human epineurium is constructed predominantly of type I and type III collagen, with the type I predominating. The diameter of the collagen fibrils averages 60–110 nm.
Adipose tissue inside a nerve surrounds the fascicles and forms adipose sheaths that separate the fascicles from each other. The thickness of adipose sheaths varies from one fascicle to another and is greater in larger nerve trunks, highlighting its protective function in cushioning the fascicles against damage by compression. Loss of epineural fat may present a risk factor for pressure-caused palsies in emaciated, bedridden patients. In contrast, excessive adipose tissue can also delay the diffusion of local anesthetic injected near a nerve, thus interfering with the anesthetic block. Epineurium is continuous with the connective tissue called adventitia or mesoneurium that surrounds the nerve when passing through, underneath, or between the muscle fascia, serving as (1) a conduit for the injected local anesthetic, (2) a path allowing for nerve gliding, and (3) a layer of protection against nerve trauma. Because their attachment is loose, nerves are relatively mobile except where tethered by entering vessels or exiting nerve branches.
Perineurium
The perineurium is a specialized connective tissue surrounding individual nerve fascicles (Figures 10 and 12). This protective cellular layer is thinner than the epineurium and separates the endoneurium from the epineurium. The perineurium consists of alternating layers of flattened polygonal cells, which are thought to be derived from fibroblasts, and collagenous connective tissue, the formation of which is controlled by the Schwann cells. The flattened polygonal cells, which constitute the lamellae, are specialized to function as a diffusion barrier. The number of lamellae varies, depending mainly on the diameter of the fascicle; the larger the fascicle is, the greater the number of lamellae. In mammalian nerve trunks, the perineurium contains 15–20 cell layers. Contiguous cells in each layer interdigitate along extensive tight junctions. The cells may branch and give rise to processes and contribute to the adjacent lamellae. Each layer of cells, enclosed by basal lamina, can reach a thickness of up to 0.5 µm in human nerves.
Collagen fibers originate in a lattice-like arrangement, in which bundles are circular, longitudinal, and obliquely arranged. The innermost perineural cell layer adheres to a distinct boundary layer of densely woven collagen fibers and subperineurial fibroblasts that mechanically links the perineurium to the endoneurial contents. Collagen fibers are predominantly type III, although type I collagen fibers are also present. The diameter of the collagen fibrils is substantially smaller than that of the epineural fibrils, with an average of 52 nm in the rat sural nerve. The basal lamina of polygonal cells is composed of collagens IV and V, fibronectin, heparan sulfate proteoglycan, and laminin. The ubiquitous presence of pinocytotic vesicles rich in phosphorylating enzymes underlie the assumption that the perineurium functions as a metabolically active diffusion barrier, playing an essential role in maintaining the osmotic milieu and fluid pressure within the endoneurium. For instance, in one of our studies, inflammatory cells accumulated between the nerve fascicles in piglets after exposure of the nerve to ultrasound gel did not penetrate the perineurium. Because of its tightly adherent cellular structure and more longitudinally oriented collagen, the perineurium is less tolerant to elongation than the epineurium. In the rabbit, mechanical failure during elongation coincided with a disruption of the perineurium while the epineurium remained intact. The integrity of the diffusion barrier was maintained after 2 hours of 15% elongation, while 27% elongation caused acute perineural disruption.
Endoneurium
The endoneurium comprises loose intrafascicular connective tissue that does not include the perineural partitions that subdivide the fascicles and surrounds Schwann cells (Figure 12). Approximately 40%–50% of the intrafascicular space is occupied by nonneural elements (ie, other than axon and Schwann cells), of which the endoneurial fluid and connective tissue matrix occupy 20%–30%. There are substantial variations among nerves in different species and age groups.
The endoneurium is composed of collagen fibers (produced by the underlying Schwann cells and fibroblasts); cellular components are bathed in endoneurial fluid, contained in substantial intrafascicular spaces. The nerve fibers tend to be grouped into small bundles with intervening clefts. Endoneurial fluid pressure is slightly higher than that of the surrounding epineurium. It is believed that this pressure gradient minimizes endoneurial contamination by toxic substances external to the nerve bundle.
Endoneurial collagen fibrils are smaller than those in the epineurium and range between 30 and 65 nm in diameter in humans. The fibrils run parallel to and around the nerve fibers, binding them into fascicles or bundles. They show condensations around capillaries and nerve fibers. Near the distal terminus of the axon, the endoneurium is reduced to a few reticular fibers surrounding the basal lamina of the Schwann cells. Types I, II, and III collagen are present in endoneurium.
Cellular constituents of endoneurium are fibroblasts, endothelial cells of capillaries, mast cells, and macrophages. Mast cells occur in varying numbers, being especially numerous along blood vessels. Macrophages account for 2%–4% of the intrafascicular nuclei in rat peripheral nerve and are the primary antigen-presenting cells of peripheral nerve. They scavenge extracellular proteins and present them to T cells emerging from circulation. The macrophages mediate immunologic surveillance and participate in nerve tissue repair. Following nerve injury, they proliferate and actively phagocytose myelin debris.
The extracellular matrix is rich in glycoproteins, glycosaminoglycans, and proteoglycans. The best characterized of these include the glycoprotein fibronectin, tenascin C, trombospondin, and the chondroitin sulfate proteoglycans versican and decorin. Expression of these molecules changes after nerve injury, so they are potentially relevant during nerve regeneration.
From a hydrodynamic point of view, the various tissues that comprise a peripheral nerve can be divided into the loose, high-compliance, expansible connective tissue of the epineurium and the low-compliance, disruptable fascicles and fascicular bundles, densely packed within the perineurium. These anatomical differences between connective tissues and fascicles or their bundles explain why an injection into fascicles requires more force (pressure) than injection into the loose connective tissue of the epineurium.
NYSORA Tips
- The perineurium is a tough and resistant tissue, that tends to escape slowly advancing a blunt, short-bevel needle during nerve block procedure.
- Higher force (pressure) is required for an injection into a low-compliant fascicle as opposed to the high-compliant epineurium.
- In the interscalene and supraclavicular regions of the brachial plexus, the nerves are more densely packed and
- oligofascicular, while more distally, they are polyfascicular with a larger amount of stromal tissue.
- Multifascicular nerves are less susceptible to injury as compared to unifascicular due to a reduced fascicular diameter and an increased epineurial protection.
- The abundance of loose epineurial tissue offers an explanation as to why most intraneural injections (intraneural, but extrafascicular) do not result in overt nerve injury.
THE CENTRAL-PERIPHERAL TRANSITION REGION
The transition between the CNS and the PNS in cranial and spinal nerve roots is referred to as the central-peripheral transition region or CNS-PNS border (Figure 14). It represents an abrupt change in the type of myelin, supporting elements, and vascularization. The main glial components in the CNS are astrocytes and oligodendrocytes, while in the PNS the main components are the Schwann cells. The nerve roots of spinal nerves are bathed in cerebrospinal fluid. The transition region is the length of the rootlet that contains both central and peripheral nervous tissue. Transition details distinguishing ensheathment of the spinal roots with the meninges and connective tissue investments of the peripheral nerves have not been fully clarified. Their structural arrangements however, are well documented in electron microscopic studies.
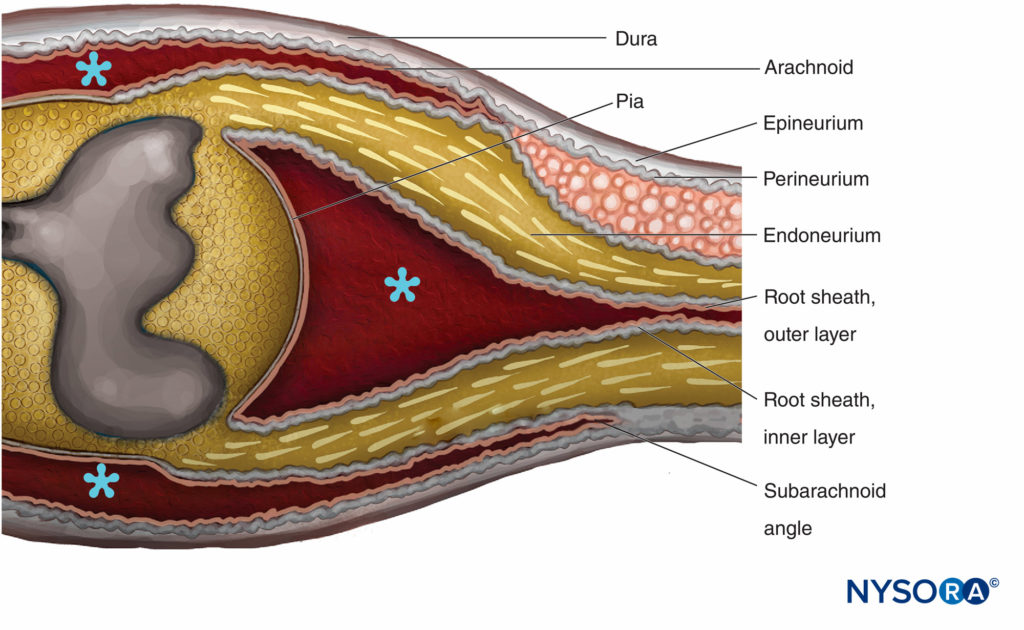
FIGURE 14. Central-peripheral transition region. The epineurium becomes continuous with the dura mater. The arachnoid is reflected over the roots at the subarachnoid angle and becomes continuous with the outer layer of the root sheath. At the junction with the spinal cord, the outer layer becomes continuous with the pia mater. The perineurium divides in two layers at the subarachnoid angle: The outer layer separates from the nerve root and runs between the dura and the arachnoid; the inner layer is adherent with spinal roots and constitutes the inner layer of the root sheath. Spinal ganglion is embedded in the perineurium. * Subarachnoid space. (Reproduced with permission from Haller FR, Low FN. The fine structure of the peripheral nerve root sheath in the subarachnoid space in the rat and other laboratory animals. Am J Anat. 1971 May;131(1):1-19.
The cellular components of the endoneurium in the spinal roots resemble those of peripheral nerves. The quantity of collagen is substantially less and is not organized in sheaths around the nerve fibers. The region in which the spinal roots attach to the spinal cord is characterized by an irregularly designed transition from the peripheral nerve to the CNS, the Ober-steiner-Redlich zone where Schwann cells are replaced by oligodendrocytes. The central portion of the root is limited at its periphery by marginal glia, composed of astrocytes covered by basal lamina.
The spinal roots traverse the subarachnoid space covered by a multicellular root sheath and penetrate the dura at the sub-arachnoid angle (Figure 14). External to the subarachnoid angle, the nerve roots possess epineurium, perineurium, and endoneurium as in the peripheral nerve trunks. The epineurium is the continuation of the spinal dura, while the endoneurium is developed distal to the junction of the roots with the central nervous tissue. Perineurium ensheaths the spinal ganglia and is proximal to it. It is divided in the outer layers that pass between the dura and the arachnoid to form the “dural mesothelium,” while the inner layers of perineurium continue over the roots as the “inner layer of the root sheath.”
The root sheath is composed of cellular and fibrous lamellae divided into two layers. The outer layer consists of loosely associated cells bordering on the subarachnoid space. Where the roots become attached to the spinal cord, the cells of the outer layer of the root sheath become continuous with the pia. At the subarachnoid angle, the outer layer becomes reflected to the external meningeal investments of the spinal cord (arachnoidea attached to the inner layer of spinal dura). The inner layer of the root sheath consists of flattened cells that are closely associated with each other, are intermittently invested with a basal lamina, and resemble the perineurium but are not classifiable as perineural cells. It becomes continuous with the perineurium peripherally.
The subarachnoid space opens into a lateral recess that extends between the ventral and dorsal roots and may constitute a communication between the subarachnoid and endoneurial spaces. This communication is clinically relevant because it allows inflammation to spread from the subarachnoid space to the endoneurium in the case of polyradiculoneuritides.
NYSORA Tips
- Local anesthetic injection within the epineurial cuff during performance of interscalene or lumbar plexus blocks may lead to spinal anesthesia due to dural cuff extension beyond the intervertebral foramen.
- During performance of lumbar plexus block, epidural spread of local anesthetic is observed particularly when high injection pressure (force) is used during the injection process.
VASCULAR SUPPLY OF PERIPHERAL NERVES
The peripheral nerve is a well-vascularized structure, supplied by vessels that originate from the nearby large arteries and veins as well as from smaller adjacent muscular and periostal blood vessels (Figure 12). Peripheral nerves have two separate, functionally independent, vascular systems: an extrinsic system (regional nutritive vessels and epineural vessels) and an intrinsic system (microvessels in the endoneurium). There are rich anastomoses between the two systems, resulting in considerable overlap between the territories of the segmental arteries.
The epineurium is characterized by a predominantly longitudinal vascular plexus. Transperineural arterioles, 10–25 µm in diameter, pass from the epineurium to the endoneurium through sleeves of perineural tissue. Their course through the perineurium is oblique, rendering them potentially susceptible to changes in intra- or extrafascicular pressure. Epineurial and perineurial vessels have a rich perivascular plexus of peptidergic, serotoninergic, and adrenergic nerves that play an important role in the neurogenic control of endoneurial blood flow.
The endoneurial vasculature is noted for its anatomical dissimilarities from a conventional capillary bed, although, physiologically, it serves similar metabolic functions. Transperineurial arterioles gradually lose their continuous muscle coat and become postarteriolar capillaries. Endoneurial capillaries have atypically greater diameter and intercapillary distances than those in many other tissues. Such angioarchitecture suggests a lower exchange capacity. Endoneurial arterioles have a poorly developed smooth muscle layer and thus limited capacity for autoregulation. The density of endoneurial microvessels varies significantly throughout peripheral nerves; these variations correlate with susceptibility to ischemic neuropathy. This unique pattern of vessels, together with the high basal blood flow relative to metabolic requirements of the nerve, confer a high degree of resistance to ischemia so that nerve dysfunction does not occur during acute ischemia until blood flow is almost zero. The outstanding characteristic of the peripheral neurovascular system is its flexibility. Peripheral nerves may be surgically mobilized, severing their nutrition vessels without clinical consequences, to a surprising degree. However, the distribution of the circulation within the endoneurium is exquisitely sensitive to physical and chemical manipulation.
NYSORA Tips
- Peripheral nerves are relatively resistant to ischemia because nerve dysfunction can only occur when the blood flow is almost zero.
- Local anesthetics have the ability to constrict vasculature and decrease the blood flow to the nerves.
AGE-RELATED CHANGES IN PERIPHERAL NERVES
An intact aged PNS is characterized by several extensive structural, functional, and biochemical changes, which have been documented in both myelinated and unmyelinated fibers. In the elderly, myelinated fiber density decreases. A regular relation between internodal length and fiber diameter becomes less precise with aging. This is associated with segmental demyelination and remyelination and the axonal degeneration and regeneration clinically evident as mild peripheral neuropathy.
In unmyelinated fibers, regressive changes attributed to aging have been reported. In unmyelinated fiber complexes of aging nerves, the proportion of Schwann cell bands devoid of axons increases (so-called collagen pockets; see Figure 9). An early age-related change appears to be the budding of Schwann cell processes into numerous flattened tongues, which usually occur in groups. The perineurial index (ratio of perineurium thickness to fascicle diameter) shows a tendency to increase with age, most probably reflecting age-related loss of nerve fibers.
Aging is associated with a decreased number of endoneurial capillaries and an increase in the thickness of capillary walls and the perineurium. The rate of axonal regeneration becomes slower as density and number of regenerating axons decreases. Aging also impairs terminal sprouting of regenerated axons and collateral sprouting of intact adjacent axons, further limiting target reinnervation and functional recovery.
The cause of changes related to aging is uncertain. It is not yet established whether they are the result of neuronal aging, giving rise to distal axonal degeneration and secondary demyelination, or local factors in the nerves, such as ischemia or the consequences of repeated minor trauma. Nevertheless, age-related changes in peripheral nerves probably result from the cumulative, lifelong effect of various pathogenic factors modified by genetic determinants and by a gradual decrease in regenerative capacity.
NYSORA Tips
- Due to age-related nerve degeneration, less local anes- thetic at lower concentration may be needed for nerve block.
- Age-related changes of the peripheral nerve may be responsible for the typically poorer ultrasonographic images of the peripheral nerves in the elderly as compared to younger subjects.
RESPONSE OF PERIPHERAL NERVE TO INJURY
Injuries to the peripheral nerves result in loss of motor, sensory, and autonomic functions in the denervated segments of the body due to the interruption of axons, degeneration of distal nerve fibers, and eventual death of axotomized neurons. Functional deficits caused by nerve injuries can be compensated by reinnervation of denervated targets by regenerating injured axons or by collateral branching of undamaged axons and remodeling of nervous system circuitry related to the lost functions. Nerve regeneration is possible if the cut ends remain near each other otherwise, regeneration may not be complete or successful.
After an injury, the neuron attempts to repair the damage, regenerate the process, and restore function by initiating a series of structural and metabolic events called axon reaction. The reactions to the trauma are localized in three regions of the neuron: at the site of damage (local changes), distal to the site of damage (anterograde changes), and proximal to the site of damage (retrograde changes). Local reaction to injury involves removal of debris by neuroglial cells. The portion of the axon distal to an injury undergoes degeneration and is phagocytosed. The proximal portion of the injured axon undergoes degeneration followed by sprouting of a new axon whose growth is directed by Schwann cells.
NYSORA Tips
- The electrical stimulation threshold for a motor response of the sciatic nerve is increased in patients with diabetic foot gangrene, which may affect nerve identification.
- Many postprocedure nerve injuries occur in nerves with preexisting pathology.
SUMMARY
The knowledge that neural anatomy is unique at different ana- tomical sites is essential for a safe and effective practice of regional anesthesia. Understanding the peripheral nerve structure and its implication while using state-of-the-art monitors, including ultrasonography, nerve stimulation, and injection pressure monitoring, is helpful in minimizing the potential for patient injury.
REFERENCES
- Jessen KR, Mirsky R: The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci 2005;6(9):671–682.
- Williams P: Gray s Anatomy, 38th ed. Churchill Livingstone, 1995.
- Gartner L, Hiatt J: Color Textbook of Histology, 2nd ed. Saunders, 2001.
- Ross M, Pawlina W: Histology: A Text and Atlas With Correlated Cell and Molecular Biology, 6th ed. Wolters Kluwer Lippincott Williams & Wilkins, 2011.
- Thomas P, Berthold C, Ochoa J: Microscopic anatomy of the peripheral nervous system. In Dyck P, Thomas P (eds): Peripheral Neuropathy, 3rd ed. Saunders, 1993, pp 28–91.
- Brushart T: Nerve Repair. Oxford University Press, 2011.
- Palay SL, Sotelo C, Peters A, Orkand PM: The axon hillock and the initial segment. J Cell Biol 1968;38(1):193–201.
- Poliak S, Peles E: The local differentiation of myelinated axons at nodes of Ranvier. Nat Rev Neurosci 2003;4(12):968–980.
- Colognato H, Baron W, Avellana-Adalid V, et al: CNS integrins switch growth factor signalling to promote target-dependentsurvival. Nat Cell Biol 2002;4(11):833–841.
- Fernandez PA, Tang DG, Cheng L, Prochiantz A, Mudge AW, Raff MC: Evidence that axon-derived neuregulin promotes oligodendrocyte survival in the developing rat optic nerve. Neuron 2000;28(1):81–90.
- de Waegh SM, Lee VM, Brady ST. Local modulation of neurofilament phosphorylation, axonal caliber, and slow axonal transport by myelinating Schwann cells. Cell 1992;68(3):451–463.
- Griffiths I, Klugmann M, Anderson T, et al: Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science 1998;280(5369):1610–1613.
- Lappe-Siefke C, Goebbels S, Gravel M, et al: Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat Genet 2003;33(3):366–374.
- Nadim W, Anderson PN, Turmaine M: The role of Schwann cells and basal lamina tubes in the regeneration of axons through long lengths of freeze-killed nerve grafts. Neuropathol Appl Neurobiol 1990;16(5): 411–421.
- Noakes PG, Bennett MR: Growth of axons into developing muscles of the chick forelimb is preceded by cells that stain with Schwann cell antibodies. J Comp Neurol 1987;259(3):330–347.
- Court FA, Wrabetz L, Feltri ML: Basal lamina: Schwann cells wrap to the rhythm of space-time. Curr Opin Neurobiol 2006;16(5):501–507.
- Darby S, Frysztak R: Neuroanatomy of the spinal cord. In Cramer GD, Darby S (eds): Basic and Clinical Anatomy of the Spine, Spinal Cord, and ANS, 2nd ed. Elsevier Mosby, 2005, pp 339–410.
- Morell P, Quarles R: Molecular architecture of myelin. In Siegel G, Agranoff B, Albers R (eds): Basic Neurochemistry: Molecular, Cellular and Medical Aspects, 6th ed. Lippincott-Raven, 1999, pp 51–71.
- Ritchie JM, Rogart RB: Density of sodium channels in mammalian myelinated nerve fibers and nature of the axonal membrane under the myelin sheath. Proc Natl Acad Sci U S A 1977;74(1):211–215.
- Wang H, Kunkel DD, Martin TM, Schwartzkroin PA, Tempel BL: Heteromultimeric K+ channels in terminal and juxtaparanodal regions of neurons. Nature 1993;365(6441):75–79.
- Thaxton C, Pillai AM, Pribisko AL, Dupree JL, Bhat MA: Nodes of Ranvier act as barriers to restrict invasion of flanking paranodal domains in myelinated axons. Neuron 2011;69(2):244–257.
- Waxman SG, Ritchie JM: Molecular dissection of the myelinated axon. Ann Neurol 1993;33(2):121–136.
- Ochoa J, Mair WG: The normal sural nerve in man. I. Ultrastructure and numbers of fibres and cells. Acta Neuropathol 1969;13(3):197–216.
- Millesi H, Terzis J: Nomenclature in peripheral nerve surgery. In Terzis J (ed): Microreconstruction of Nerve Injuries. Saunders, 1987, pp 3–13.
- Sala-Blanch X, Vandepitte C, Laur JJ, et al: A practical review of perineural versus intraneural injections: A call for standard nomenclature. Int Anesthesiol Clin 2011;49(4):1–12.
- Sunderland S, Bradley KC: The perineurium of peripheral nerves. Anat Rec 1952;113(2):125–141.
- Sunderland S: The connective tissues of peripheral nerves. Brain 1965;88(4):841–854.
- Ushiki T, Ide C: Three-dimensional organization of the collagen fibrils in the rat sciatic nerve as revealed by transmission- and scanning electron microscopy. Cell Tissue Res 1990;260(1):175–184.
- Thomas PK. The connective tissue of peripheral nerve: an electron microscope study. J Anat 1963;97:35–44.
- Thomas PK, Bhagat S: The effect of extraction of the intrafascicular contents of peripheral nerve trunks on perineurial structure. Acta Neuropathol 1978;43(1–2):135–141.
- Lorimier P, Mezin P, Labat Moleur F, Pinel N, Peyrol S, Stoebner P: Ultrastructural localization of the major components of the extracellular matrix in normal rat nerve. J Histochem Cytochem 1992;40(6):859–868.
- Reina MA, Lopez A, De Andres JA: [Adipose tissue within peripheral nerves. Study of the human sciatic nerve]. Rev Esp Anestesiol Reanim 2002;49(8):397–402.
- Mirsky R, Parmantier E, McMahon AP, Jessen KR: Schwann cell-derived desert hedgehog signals nerve sheath formation. Ann N Y Acad Sci 1999;883:196–202.
- Shanthaveerappa TR, Bourne GH: Perineural epithelium: A new concept of its role in the integrity of the peripheral nervous system. Science 1966;154(3755):1464–1467.
- Lehmann HJ: [Structure and function of the perineural diffusion barrier]. Z Zellforsch Mikrosk Anat 1957;46(2):232–241.
- Eldridge CF, Sanes JR, Chiu AY, Bunge RP, Cornbrooks CJ: Basal laminaassociated heparan sulphate proteoglycan in the rat PNS: Characterization and localization using monoclonal antibodies. J Neurocytol 1986;15(1):37–51.
- Paetau A, Mellstrom K, Vaheri A, Haltia M: Distribution of a major connective tissue protein, fibronectin, in normal and neoplastic human nervous tissue. Acta Neuropathol 1980;51(1):47–51.
- Stopar-Pintaric T, Cvetko E, Strbec M, et al: Intraneural and perineural inflammatory changes in piglets after injection of ultrasound gel, endotoxin, 0.9% NaCl or needle insertion without injection. Anaesth Analg 2014;115:1–6.
- Rydevik BL, Kwan MK, Myers RR, et al: An in vitro mechanical and histological study of acute stretching on rabbit tibial nerve. J Orthop Res 1990;8(5):694–701.
- Olsson Y: Microenvironment of the peripheral nervous system under normal and pathological conditions. Crit Rev Neurobiol 1990;5(3): 265–311.
- Jacobs JM, Love S: Qualitative and quantitative morphology of human sural nerve at different ages. Brain 1985;108(Pt 4):897–924.
- Powell HC, Myers RR, Costello ML, Lampert PW: Endoneurial fluid pressure in wallerian degeneration. Ann Neurol 1979;5(6):550–557.
- Gamble HJ, Eames RA. An electron microscope study of the connective tissues of human peripheral nerve. J Anat 1964;98:655–663.
- Salonen V, Roytta M, Peltonen J: The effects of nerve transection on the endoneurial collagen fibril sheaths. Acta Neuropathol 1987;74(1):13–21.
- 45. Oldfors A: Macrophages in peripheral nerves. An ultrastructural and enzyme histochemical study on rats. Acta Neuropathol 1980;49(1): 43–49.
- 46. Braunewell KH, Martini R, LeBaron R, et al: Up-regulation of a chondroitin sulphate epitope during regeneration of mouse sciatic nerve: Evidence that the immunoreactive molecules are related to the chondroitin sulphate proteoglycans decorin and versican. Eur J Neurosci 1995;7(4): 792–804.
- Kapur E, Vuckovic I, Dilberovic F, et al: Neurologic and histologic outcome after intraneural injections of lidocaine in canine sciatic nerves. Acta Anaesthesiol Scand 2007;51(1):101–107.
- Jeng CL, Rosenblatt MA: Intraneural injections and regional anesthesia: The known and the unknown. Minerva Anestesiol 2011;77(1):54–58.
- Orebaugh SL, Mukalel JJ, Krediet AC, et al: Brachial plexus root injection in a human cadaver model: Injectate distribution and effects on the neuraxis. Reg Anesth Pain Med 2012;37(5):525–529.
- Moayeri N, Bigeleisen PE, Groen GJ: Quantitative architecture of the brachial plexus and surrounding compartments, and their possible significance for plexus blocks. Anesthesiology 2008;108(2):299–304.
- Sala Blanch X, Lopez AM, Carazo J, et al: Intraneural injection during nerve stimulator-guided sciatic nerve block at the popliteal fossa. Br J Anaesth 2009;102(6):855–861.
- Berthold CH, Carlstedt T. Observations on the morphology at the transition between the peripheral and the central nervous system in the cat. III. Myelinated fibres in S1 dorsal rootlets. Acta Physiol Scand Suppl 1977;446:43–60.
- Andres KH: [The fine structure of the olfactory region of macrosmatic animals]. Z Zellforsch Mikrosk Anat 1966;69:140–154.
- Haller FR, Low FN: The fine structure of the peripheral nerve root sheath in the subarachnoid space in the rat and other laboratory animals. Am J Anat 1971;131(1):11–19.
- Himango WA, Low FN: The fine structure of a lateral recess of the subarachnoid space in the rat. Anat Rec 1971;171(1):1–19.
- McCabe JS, Low FN: The subarachnoid angle: an area of transition in peripheral nerve. Anat Rec 1969;164(1):15–33.
- Waggener JD, Beggs J: The membranous coverings of neural tissues: An electron microscopy study. J Neuropathol Exp Neurol 1967;26(3): 412–426.
- Pease DC, Schultz RL: Electron microscopy of rat cranial meninges. Am J Anat 1958;102(2):301–321.
- Nabeshima S, Reese TS, Landis DM, Brightman MW: Junctions in the meninges and marginal glia. J Comp Neurol 1975;164(2):127–169.
- Evans PJ, Lloyd JW, Wood GJ: Accidental intrathecal injection of bupivacaine and dextran. Anaesthesia 1981;36(7):685–687.
- Gadsden JC, Lindenmuth DM, Hadzic A, Xu D, Somasundarum L, Flisinski KA: Lumbar plexus block using high-pressure injection leads to contralateral and epidural spread. Anesthesiology 2008;109(4):683–688.
- Lundborg G. Intraneural microcirculation. Orthop Clin North Am 1988;19(1):1–12.
- McManis PG, Schmelzer JD, Zollman PJ, Low PA: Blood flow and autoregulation in somatic and autonomic ganglia. Comparison with sciatic nerve. Brain 1997;120(Pt 3):445–449.
- Beggs J, Johnson PC, Olafsen A, Watkins CJ, Cleary C: Transperineurial arterioles in human sural nerve. J Neuropathol Exp Neurol 1991;50(6): 704–718.
- Bell MA, Weddell AG: A morphometric study of intrafascicular vessels of mammalian sciatic nerve. Muscle Nerve 1984;7(7):524–534.
- Smith DR, Kobrine AI, Rizzoli HV: Blood flow in peripheral nerves. Normal and post severance flow rates. J Neurol Sci 1977;33(3): 341–346.
- Kozu H, Tamura E, Parry GJ: Endoneurial blood supply to peripheral nerves is not uniform. J Neurol Sci 1992;111(2):204–208.
- Perez-Castro R, Patel S, Garavito-Aguilar ZV, et al: Cytotoxicity of local anesthetics in human neuronal cells. Anesth Analg 2009;108(3):997–1007.
- Drac H, Babiuch M, Wisniewska W: Morphological and biochemical changes in peripheral nerves with aging. Neuropatol Pol 1991;29(1–2–): 49–67.
- Lehmann J: [Age-related changes in peripheral nerves]. Zentralbl Allg Pathol 1986;131(3):219–227.
- Arnold N, Harriman DG: The incidence of abnormality in control human peripheral nerves studied by single axon dissection. J Neurol Neurosurg Psychiatry 1970;33(1):55–61.
- Tohgi H, Tsukagoshi H, Toyokura Y: Quantitative changes with age in normal sural nerves. Acta Neuropathol 1977;38(3):213–220.
- Vizoso AD: The relationship between internodal length and growth in human nerves. J Anat 1950;84(4):342–353.
- Ochoa J, Mair WG: The normal sural nerve in man. II. Changes in the axons and Schwann cells due to ageing. Acta Neuropathol 1969;13(3): 217–239.
- Ochoa J: Recognition of unmyelinated fiber disease: morphologic criteria. Muscle Nerve 1978;1(5):375–387.
- Kovacic U, Sketelj J, Bajrovic FF: Chapter 26: Age-related differences in the reinnervation after peripheral nerve injury. Int Rev Neurobiol 2009;87:465–482.
- Li X, Karmakar MK, Lee A, Kwok WH, Critchley LA, Gin T: Quantitative evaluation of the echo intensity of the median nerve and flexor muscles of the forearm in the young and the elderly. Br J Radiol 2012;85(1014):e140–e145.
- Navarro X, Vivo M, Valero-Cabre A: Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol 2007;82(4):163–201.
- Keyl C, Held T, Albiez G, Schmack A, Wiesenack C: Increased electrical nerve stimulation threshold of the sciatic nerve in patients with diabetic foot gangrene: A prospective parallel cohort study. Eur J Anaesthesiol 2013;30(7):435–440.
- Borgeat A, Ekatodramis G, Kalberer F, Benz C: Acute and nonacute complications associated with interscalene block and shoulder surgery: A prospective study. Anesthesiology 2001;95(4):875–880.