Kenneth D. Candido, Anthony R. Tharian, and Alon P. Winnie
INTRODUCTION
The technique of intravenous regional anesthesia (IVRA), or “Bier block,” was first introduced in 1908 by the German surgeon August Bier. A Bier block essentially consists of injecting local anesthetic solutions into the venous system of an upper or lower extremity that has been exsanguinated by compression or gravity and that has been isolated by means of a tourniquet from the central circulation. In Bier’s original technique, the local anesthetic procaine in concentrations of 0.25% to 0.5% was injected through an intravenous cannula, which had been placed between two Esmarch bandages utilized as tourniquets to divide the arm into proximal and distal components. After injecting the local anesthetic, Bier noted two distinct types of anesthesia: an almost-immediate onset of “direct” anesthesia between the two tourniquets and then, after a delay of 5 to 7 minutes, an “indirect” anesthesia distal to the distally placed tourniquet. By performing dissections of the venous system of the upper extremity in cadavers after injecting methylene blue, Bier was able to determine that the direct anesthesia was the result of local anesthesia bathing bare nerve endings in the tissues, whereas the indirect anesthesia was most probably due to local anesthesia being transported to the substance of the nerves via the vasa nervorum, where a typical conduction block occurs. Bier’s conclusion was that two mechanisms of anesthesia were associated with this technique: peripheral infiltration block and conduction block. The technique, as originally described by Bier, remains essentially unchanged in modern practice for the past 106 years, except for the introduction of the pneumatic-type double-tourniquet preparation used in current clinical practice (Figure 1).
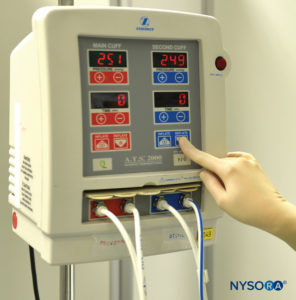
FIGURE 1.Double pneumatic tourniquet system for use in intravenous regional anesthesia of the upper or lower extremity.
A Bier block can be used for brief surgical procedures or manipulations of the upper or lower extremity. However, the technique has found its greatest acceptance for use for the upper extremity because tourniquet problems and other safety issues seem to arise more frequently when IVRA is used on the lower extremities. Bier block is also a procedure that has found utility as a treatment adjunct for patients suffering from complex regional pain syndromes (CRPSs) (formerly known as reflex sympathetic dystrophy, with sympathetically maintained pain) as an alternative to repeated sympathetic ganglion blocks. In this regard, IVRA has been shown to decrease neurogenic inflammation, a phenomenon possibly associated with CRPS, with little impairment of sensory function, at least when mepivacaine is the local anesthetic chosen for the block. Sensibility to cold is significantly decreased 10 to 30 minutes after the block, even with a reduction in the skin temperature on the blocked side.
Chemical sympathectomy using IVRA with agents such as guanethidine or bretylium may last up to 5 days, as compared with local anesthetic blocks, which typically provide analgesia lasting only several hours. Quantitative sensory testing (QSART, quantitative sudomotor axon reflex testing) before and after such blocks demonstrated that it is possible to predict which patients will have long-lasting pain alleviation using IVRA guanethidine blocks following traumatic injury or surgery.
Although IVRA is a safe and effective method of administering local anesthetics for extremity block both for surgery and for pain control, one large published survey noted that most thirdyear (CA-3) anesthesia residents had performed fewer than 10 such blocks during the entire course of their training.
ANATOMY
The only relevant anatomy is the location and distribution of the veins of the hand, of the antecubital fossa, and of the foot and ankle region.
INDICATIONS
Upper Extremity
Intravenous regional anesthesia using local anesthetic, most commonly lidocaine 0.5%–1% (prilocaine 1% in Europe), is appropriate for surgery and manipulation of the extremities requiring anesthesia of up to 1 hour’s duration. It is most suited for peripheral, soft tissue operations such as ganglionectomy, carpal tunnel release, Dupuytren’s contracture surgery, or reduction of fractures. However, the necessity of exsanguinating the extremity using an Esmarch bandage, a potentially painful maneuver, may preclude certain procedures from being undertaken with this technique (Figure 2 and Figure 3).
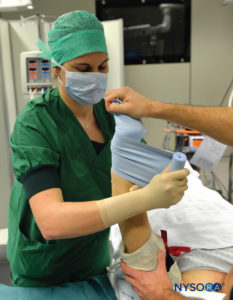
FIGURE 2.Beginning of the exsanguination process of the elevated left upper extremity using a tightly wrapped Esmarch bandage from the distal hand to the proximal upper extremity at the base of the distal tourniquet.
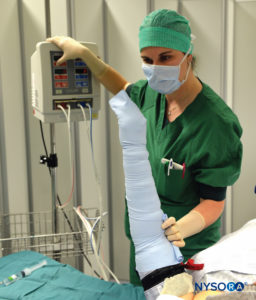
FIGURE 3. Keeping the Esmarch bandage tightly wrapped, first distal, then proximal tourniquets are to 50-100 mm Hg above the systolic arterial blood pressure.
Likewise, manipulations of the ulnar, median or radial nerves may cause paresthesias, which may require the use of adjuvant parenteral analgesics or sedatives. A novel use of IVRA is for anesthetizing the hand prior to injecting botulinum toxin A (BTX-A) for the treatment of hyperhidrosis. BTX-A significantly reduces sweat production, as measured by Minor’s test and as quantified by corneometer analysis, but the injection is painful unless the hand is anesthetized beforehand; IVRA has been found to be suitable for this purpose. According to a recent study, there was no difference in the degree and duration of analgesia between IVRA and stellate ganglion block (SGB) using a combination of 70 mg of lidocaine and 30 μg of clonidine in patients with CRPS type 1 affecting the upper extremities. The study concluded that IVRA was preferable to SGB in this setting due to its lower risk of undesirable side effects and easier execution than SGB.
Upper extremity IVRA has been utilized occasionally for prolonged analgesia/anesthesia (ie, surgeries expected to persist for longer than 1 hour), with a mandatory tourniquet deflation period of at least 1 minute prior to reestablishing the anesthetized state.
Lower Extremity
Intravenous regional anesthesia may be used for brief surgical interventions of the lower extremity in a manner analogous to that described for upper extremity surgery. Surgical procedures that may be completed using this approach include excision of a mass; digital nerve repair; phalangeal fracture/dislocation surgery; and accessory navicular excision. Any foot, ankle, or distal lower extremity orthopedic procedure requiring approximately 45 minutes or less to complete may be amenable to this modality.
Although IVRA has been associated with an increased incidence of compartment syndrome when treating tibial shaft fractures and has therefore been deemed contraindicated in such cases, a study in volunteers showed no significant difference in tissue pressures before and after tourniquet inflation regardless of the volume of saline used (≤1.5 mL/kg) or as a function of time following saline injection during tourniquet inflation. The authors concluded that, in the normal atraumatic limb, simulated IVRA using normal saline (NS) does not increase tissue pressure within the anterior compartment of the leg.
Pediatrics
Intravenous regional anesthesia has been an acceptable choice in selected pediatric patients for the reduction of fractures of the upper extremity. A retrospective study comparing IVRA and conscious sedation for the reduction of pediatric forearm fractures found IVRA to be a safe, efficient, and cost-effective method of reducing pediatric forearm fractures. There were 600 patients in the IVRA group and 645 patients in the conscious sedation group. No patient experienced compartment syndrome or a need for readmission secondary to cast application. Some intervention to their cast because of tightness was needed by 28 patients (4.34%) in the procedural sedation group and 13 patients (2.16%) in the IVRA group.
CONTRAINDICATIONS
The only absolute contraindication to IVRA is patient refusal. Relative contraindications include the following:
• Crush injuries of an extremity
• Inability to locate peripheral veins
• Local skin infections
• Cellulitis
• Compound fractures
• Patients with convincing history of allergy to local anesthetics
• Patients with severe vascular injuries to the extremity
• Preexisting vascular arteriovenous shunts and patients in whom a tourniquet is unsuitable (ie, patients with severe peripheral vascular disease)
• Sickle cell disease
• Surgery planed for >1 hour are typically not good indication for IV regional anesthesia due to occurrence of Tourniquet pain.

From the Compendium of Regional Anesthesia: Intravenous regional anesthesia (IVRA, Bier block): Mechanism of action, indications, and contraindications infographic.
EQUIPMENT
Figure 1 to Figure 8 show equipment used in IVRA
1. Local anesthetic agents: lidocaine HCl, 0.25%–0.1% (alternative is prilocaine, 0.5%)
2. One rubber tourniquet (Penrose drain) 12–18 in. in length (30–45 cm) and 7/8-in. wide (2.3 cm) for use prior to placing the intravenous cannula
3. One 20- or 22-gauge intravenous extracatheter (catheter over needle) (Figure 5)
4. One 500-mL or 1-L bag of intravenous solution connected to an infusion set (vs. a hep lock) to be connected to the intravenous cannula to maintain its patency until the anesthetic solution is injected in the isolated extremity (may substitute a saline-flushed intravenous port instead)
5. Standard American Society of Anesthesiologists (ASA) monitors (electrocardiograph, blood pressure, pulse oximeter)
6. Resuscitation equipment (intravenous catheter, crystalloid solution, and infusion set for the contralateral upper extremity) (for upper extremity IVRA)
7. Two pneumatic tourniquets of appropriate size for the selected extremity (Figures 6 and 9)
8. One Esmarch bandage 60 in. in length (152 cm) and 4 in. wide (10 cm) for exsanguinating the arm (Figures 2, 3, and 7)
9. Sterile skin preparatory set
10. A 30- or 50-mL Luer lock syringe
11. One graduated measuring cup for the mixing of solution, preferably with a 100-mL capacity
12. Adhesive tape, various sizes
Learn more about Equipment for Regional Anesthesia.
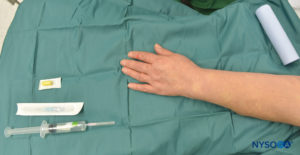
FIGURE 4. Equipment for IVRA consists of Esmarch bandage, local anesthetic vials, rubber tourniquet, intravenous (IV) extracatheter (catheter over needle), alcohol swabs, and a syringe to draw up local anesthetic.
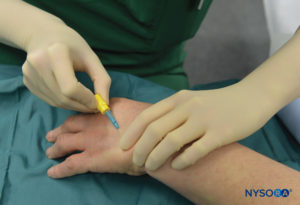
FIGURE 5.Intravenous cannula and Hep-Lock placed in a distal vein of the hand in preparation for IVRA.
PATIENT PREPARATION
The patient lies in the dorsal recumbent position as long as the vein selected for placement is readily accessible. The resuscitative equipment is checked, and the pneumatic tourniquets are tested and prepared for use. For surgery on the elbow, the needle will be placed in the forearm or antecubital fossa. For procedures on the hand or forearm, a vein in the dorsum of the hand is best selected (Figure 5).
For lower extremity procedures, a vein on the foot, ankle, or lower leg is chosen. After obtaining intravenous access in a nonoperated extremity (alternatively, a central venous access may be secured), a full complement of ASA monitors is applied, and baseline vital signs are assessed. If the patient is in severe pain, small aliquots of intravenous analgesics may now be administered (ie, fentanyl 1–2 μg/kg) to facilitate the exsanguination process. Because total patient cooperation is not essential to be successful, small doses of water-soluble benzodiazepine (ie, midazolam 15–25 μg/kg) may alternatively be administered for anxiolysis. An important benefit to choosing a benzodiazepine is the suppression of the convulsant response associated with local anesthetic toxicity, a valid concern in the patient undergoing IVRA due to the large volume of the agent being directly administered into the vascular system.
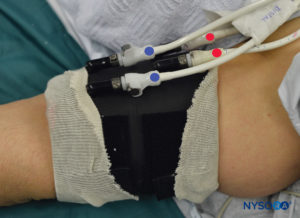
FIGURE 6. Clearly labeled proximal (RED) and distal (BLUE) tourniquet of the double tourniquet system. The tourniquet is always inflated in the following order: Distal, Proximal. Once the functionality is checked, the distal tourniquet (BLUE) is deflated.
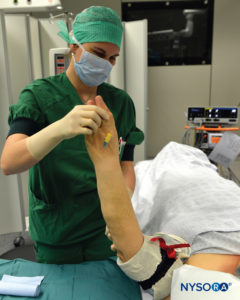
FIGURE 7. Elevation of the extremity to allow passive exsanguination.
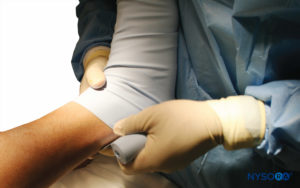
FIGURE 8. The elevated right lower extremity is wrapped with a tightly wound Esmarch bandage to the tourniquet.
TECHNIQUE

From the Compendium of Regional Anesthesia: Intravenous regional anesthesia (IVRA, Bier block): Technique infographic.
Upper Extremity IVRA
The following is the technique for IVRA for upper extremity procedures:
1. An indwelling plastic catheter is inserted into a peripheral vein as far distally as possible under strict aseptic precautions (Figure 5).
2. A double-pneumatic tourniquet is placed on the proximal cuff high on the upper arm (Figures 6 and 7). While by convention the tourniquets are placed on the biceps area, one study found that the dosage of lidocaine could be almost halved when the tourniquet was placed in the forearm instead of the upper arm. Twenty patients undergoing forearm and hand surgeries received IVRA with a combination of lidocaine 1.5 mg/kg and ketorolac 0.15 mg/kg with a tourniquet placed on the forearm. Another 20 patients undergoing similar procedures received IVRA with double the dose of the same medications and with the tourniquet placed on the upper arm. Surgical anesthesia was assessed as excellent in all 20 patients in the upper arm tourniquet group, while it was rated as excellent in 19/20 patients in the forearm tourniquet group. Onset as well as regression of sensory block were similar in both groups. A recent study comparing forearm tourniquet placement (n = 28) with 8 mL of 2% lidocaine and 10 mg ketorolac and upper arm tourniquet placement (n = 28) with 15 mL of 2% lidocaine and 20 mg ketorolac found that patients in the forearm tourniquet group experienced less discomfort, fewer sedation interventions, and a greater likelihood of bypassing the postanesthesia care unit (PACU) when compared with the group with the upper arm tourniquet.
3. The entire arm is elevated to allow passive exsanguination (Figure 7), and a rubber Esmarch bandage is wound around the arm spirally from the fingertips of the hand to the distal cuff of the double tourniquet to exsanguinate the arm (Figures 2 and 3).
4. The axillary artery is digitally occluded, and while pressure is maintained on it, the proximal pneumatic cuff is inflated to 50–100 mm Hg above the systolic arterial blood pressure, after which the Esmarch bandage is removed.
5. Following inflation of the proximal cuff and removal of the Esmarch bandage, 30–50 mL of 0.5% lidocaine HCl are injected via the indwelling plastic catheter, the volume depending on the size of the arm being anesthetized.
6. To the level of the procedure table, the intravenous cannula in the surgical extremity is withdrawn, and pressure is quickly applied over the site using sterile gauze.
7. About 25–30 minutes after the onset of anesthesia or when a patient complains of tourniquet pain, the distal cuff is inflated and the proximal cuff is deflated to minimize the development of tourniquet pain.
Lower Extremity IVRA
The only significant difference in IVRA for the upper and lower extremities is that the IVRA technique for the lower extremity requires relatively larger volumes of local anesthetic solutions by virtue of the obvious size disparity between upper and lower extremities. This is necessary to more completely fill the larger vascular compartment of the lower extremity from the distally placed intravenous cannula to the proximal tourniquet (100 mL vs. 50 mL).
PHARMACOLOGIC CONSIDERATIONS
Local Anesthetic Considerations
Lidocaine is the prototypical local anesthetic used for IVRA in the United States. In Europe, however, prilocaine may be more commonly used and in fact has been the subject of most clinical trials. Attempts have been made to maximize the efficacy of lidocaine while minimizing side effects or toxicity of the agent. Alkalinization of 0.5% lidocaine (using 1.4% sodium bicarbonate) for IVRA was studied in 31 patients. The authors found no clinical advantage to the practice of alkalinization of lidocaine with respect to sensory block, motor block, or the appearance of postoperative pain. When lidocaine was compared with alkalinized and nonalkalinized 2-chloroprocaine, both used as 0.5% concentrations and used exclusively for hand surgery, alkalinized chloroprocaine behaved similarly to lidocaine, but plain chloroprocaine offered no benefit and produced more side effects than seen with lidocaine.
Another study comparing IVRA with low-concentration/high-volume lidocaine (0.5% concentration of 30–50 mL lidocaine) and higher-concentration/low-volume lidocaine (2% concentration of 12–15 mL lidocaine) in patients undergoing upper extremity surgery showed a faster onset and delayed regression of sensory block in the higher-concentration/low-volume group. There were no significant differences in hemodynamic data such as systolic and diastolic blood pressure, mean blood pressure, and heart rate between the two groups.
Lidocaine has been compared with ropivacaine for upper extremity IVRA in two separate studies. Two doses of ropivacaine (1.2 and 1.8 mg/kg) were compared with one dose of lidocaine (3.0 mg/kg) in 15 volunteers. Recovery of sensory and motor block after tourniquet release was slowest with the high-dose ropivacaine group. More patients in the lidocaine group (5 of 5) experienced light-headedness following tourniquet release, versus only 1 in the high-dose ropivacaine group. In the second study, 51 patients were randomized to receive either ropivacaine 0.375% or lidocaine 0.5% in a volume of 0.4 mL/kg up to 25 mL. Postoperative analgesia as measured by first request for analgesics was superior in the ropivacaine group.
The progression of sensory block in the hand following IVRA with 20 mL of 0.3% ropivacaine and a double tourniquet placed in the forearm was studied in 10 healthy volunteers. The local anesthetic was injected through a 22-gauge intravenous catheter placed in a prominent vein on the dorsum of the hand after exsanguination of the hand using an Esmarch bandage and sequentially inflating the distal tourniquet to 150 mm Hg or 20 mm Hg above the systolic blood pressure (whichever was higher) and the proximal tourniquet to 250 mm Hg. The distal tourniquet was then deflated, and the Esmarch bandage was removed. Baseline values for cold and touch sensation were determined before the block, and updated values were obtained repeatedly and continuously, beginning 5 minutes after the local anesthetic injection and continuing until loss of sensation was obtained in all areas. There was an almost-immediate loss of cold perception followed by a delayed and uneven spread of loss of touch sensation. The initial spread of anesthesia was noted both proximally and distally in the dorsum of the hand and then progressed to the fingertips, with a delayed proximal spread over the palmar surface of the hand to the wrist.
Prilocaine has been compared with lidocaine, as well as with other local anesthetics used for IVRA. While evaluating the onset of sensory and motor block, 40 mL of 0.5% prilocaine (100 mg) was compared with the same volume and same concentration of chloroprocaine in 10 volunteers undergoing IVRA. Motor block onset did not differ between groups, and sensation was recovered almost equally well. However, recovery of motor function was shorter in the prilocaine group, and more chloroprocaine patients demonstrated signs of venous irritation or antecubital urticaria for 30–45 minutes after tourniquet deflation. Heart rate changes were also more notable in the chloroprocaine group. The same group of investigators expanded their study to include 60 patients, 30 in each of the respective groups detailed previously. Now, the investigators found that complete recovery of sensory block was faster in the prilocaine group (7.1 vs. 9.8 minutes). Otherwise, the incidence of side effects remained higher in the chloroprocaine group.
Next, these investigators compared 0.5% prilocaine with the same concentration of articaine (a newer amino amide-type local anesthetic that contains thiophene and is pharmacologically similar to mepivacaine) for upper extremity IVRA. Articaine, a potent local anesthetic with a low degree of toxicity by virtue of its rapid metabolism with esterases, was felt to be a suitable alternative to prilocaine. Ten volunteers participated in this double-blind, crossover comparison of the two agents.
They found no significant difference between the two with respect of onset of anesthesia or motor block or in recovery of sensory or motor function. However, 80% of the subjects experienced skin rashes after receiving articaine, versus 20% in the prilocaine group.
When 0.5% prilocaine was compared with the same concentrations of articaine or lidocaine in three groups of 10 patients each for IVRA, it was found that the onset of sensory block was significantly shorter in the articaine group, which also had the lowest peak plasma concentrations of local anesthetic following tourniquet release. Plain prilocaine 1% has been compared with the same local anesthetic combined with four different additives for IVRA: bupivacaine 0.25%, clonidine 150 μg, sufentanil 25 μg, or tenoxicam 20 mg. The sufentanil- added group demonstrated the most rapid onset of sensory block; postoperative pain scores were improved by adding either clonidine or tenoxicam. Otherwise, there were no significant differences among the five groups with respect to onset and duration of sensory block. Unlike the situation noted for lidocaine with addition of bicarbonate as an adjuvant, the addition of bicarbonate to prilocaine does seem to shorten onset time and prolong the duration of anesthesia during IVRA.
The use of mepivacaine for IVRA has been studied. Sixteen patients were evaluated using 1.4 mg/kg in 40 mL total for IVRA versus saline blocks performed in the same individuals on the contralateral arm. Arterial occlusion was maintained for 20 minutes. Reactive hyperemia was attenuated in the mepivacaine-treated arm for the 60-minute evaluation period, indicating that mepivacaine is a potent vasoconstrictor having a long duration of action. This finding has implications for the use of mepivacaine in individuals either with compromised upper extremity blood flow or with CRPS, for whom it probably should not be considered the local anesthetic of choice.
The same study group evaluated the effects of mepivacaine IVRA on intracutaneous capsaicin-induced burning pain and on microvascular skin blood flow as measured by Doppler perfusion imaging. The reactive hyperemia was less in the mepivacaine- treated arm 10 minutes after tourniquet release, and the area of the flare was smaller after capsaicin in the mepivacainetreated arms. The authors concluded that mepivacaine IVRA had no effects on post-IVRA sensory function of thin afferent fibers, but differentially decreased the spread of capsaicininduced flare.
NYSORA Tips
• Lidocaine is the prototypical drug used for IVRA in the United States; prilocaine is favored in Europe.
• Alkalinization of lidocaine confers minimal, if any, advantage to commercial lidocaine for IVRA.
• The potent vasoconstrictive properties of mepivacaine detract from its overall attractiveness as a primary agent for IVRA.
Adjuncts to Local Anesthetics for IVRA
A systematic review of the literature was undertaken to evaluate the use of adjuncts to local anesthetics for IVRA. Twenty-nine studies met the criteria of being randomized, double blind, and controlled. Data on 1217 study subjects were reviewed, and the agents studied included opioids (fentanyl, sufentanil, meperidine, and morphine); clonidine; muscle relaxants (atracurium, pancuronium, mivacurium); tramadol; nonsteroidal antiinflammatory agents (NSAIDs) (ketorolac, tenoxicam, acetylsalicylate); alkalinization using sodium bicarbonate; the addition of potassium; and temperature alterations. The authors found solid evidence supporting the use of NSAIDs in general and ketorolac in particular for improving postoperative analgesia and prolonging tourniquet tolerance during IVRA. Opioids fared poorly when used for IVRA, with only meperidine in doses of 30 mg or greater showing substantial postoperative benefits at the expense of postdeflation nausea, vomiting, and dizziness. Muscle relaxants improved postoperative motor block and were beneficial in fracture reduction in which muscular relaxation is imperative for good results.
Alpha2 Agonists (Clonidine andDexmedetomidine)
Clonidine has been added to both prilocaine and lidocaine as an adjunct to IVRA for extremity surgery. When 2 μg/kg were added to prilocaine 0.5% in a randomized, double-blind fashion in 56 patients undergoing upper extremity surgery, there was no difference between groups regarding sensory or motor block onset or duration. The patients who had clonidine had a significant reduction in arterial blood pressure after tourniquet release (24%–48%), while heart rates remained unchanged.
The authors concluded that clonidine was of limited benefit as an adjunct to local anesthetics.35 The addition of clonidine to prilocaine dramatically suppressed tourniquet pain, but it did not alter postoperative pain following tourniquet deflation.
Clonidine was found to provide no measurable benefits when added to lidocaine for IVRA in patients undergoing carpal tunnel release.
Dexmedetomidine is appropriately eight times more selective toward the α-adrenoreceptors than clonidine. As such, it has been used in IVRA to determine if it might advance some of the beneficial findings noted with the latter agent. Thirty patients undergoing hand surgery under IVRA received 0.5% lidocaine alone or lidocaine plus dexmedetomidine 0.5 μg/kg.
The dexmedetomidine group showed a more rapid onset of sensory and motor block; prolonged sensory and motor block recovery; prolonged tolerance for the tourniquet; and improved quality of analgesia compared with the group that received local anesthetic only.
A shortened sensory block onset time; prolonged sensory and motor block recovery times; prolonged duration of analgesia for tourniquet; and prolonged postoperative analgesia were noted in patients undergoing IVRA with dexmedetomidine in another randomized, double-blind study comparing the effects of lornoxicam or dexmedetomidine in IVRA with prilocaine in patients undergoing hand or forearm surgery. In this study, IVRA was achieved with 2% prilocaine at 3 mg/kg in the control group (n = 25), 2% prilocaine at 3 mg/kg plus dexmedetomidine 0.5 μg/kg in the dexmedetomidine group (n = 25), and 2% prilocaine at 3 mg/kg plus lornoxicam 8 mg in the lornoxicam group (n = 25). In each group, the drugs were diluted with 0.9% normal saline to a total volume of 40 mL.
A more recent study comparing the effects of dexmedetomidine when added to lidocaine for IVRA and when administered parenterally as premedication before IVRA found that both these groups had similarly improved quality of anesthesia and perioperative analgesia. This study was done on patients undergoing carpal tunnel release randomized into three groups.
IVRA was done using 40 mL of 0.5% lidocaine. A single dose of dexmedetomidine 0.5 μg/kg and placebo (saline) solution in a total volume of 20 mL was administered to group P (n = 15) and group S (n = 15), respectively, before IVRA. Dexmedetomidine at 0.5 μg/kg of was added to lidocaine in group A (n = 15) during IVRA. The onset and recovery time of sensory and motor block, intraoperative-postoperative visual analog scale (VAS), Ramsay sedation score (RSS), analgesic requirements, hemodynamic variables, and side effects were noted. Significantly shortened sensory block onset and recovery time in groups P and A, shortened motor block onset time in group P, and decreased intraoperative VAS scores and analgesic requirement in groups P and A were found. Intraoperative RSS in group P and postoperative RSS in groups P and A were higher than in group S. Intraoperative and postoperative heart rate and postoperative mean arterial blood pressure (MAP) of group P were significantly lower than in groups A and S, respectively.
Opioids
Because opiate receptors were discovered to exist in the peripheral nervous system and with the demonstration that opioids may produce effective, long-lasting analgesia when injected in conjunction with local anesthetics for brachial plexus block, several investigators have attempted to decrease the potential for toxicity from local anesthetic–only IVRA by adding opioids to reduce the concentration of lidocaine. Although it has not been proven that the addition of fentanyl to lidocaine for IVRA results in improved analgesia while reducing the risks, the addition of fentanyl in 200-μg doses to prilocaine 0.5% did result in more complete analgesia than in patients who had 100 μg added, or when plain prilocaine was used for IVRA. Postoperative nausea and central nervous system side effects were higher in the fentanyl-added groups versus those who received local anesthetic alone. Two other studies, however, found that the addition of opioids to prilocaine did not improve success with the technique. More research on the effects of the addition of opioids to prilocaine for IVRA may ultimately resolve this discrepancy.
Some investigators have found that adding opioids and muscle relaxants to 0.25% lidocaine provides the same analgesia and muscular relaxation as that provided by 0.5% lidocaine alone, while reducing the likelihood of systemic toxicity. Adjuvants added to lidocaine have included fentanyl 50 μg plus pancuronium 0.5 mg, fentanyl plus rocuronium, fentanyl plus D-tubocurarine, and fentanyl plus vecuronium. In each case, the authors reported outstanding operating conditions, and because the lidocaine concentration was able to be reduced to 0.20% (ie, more than one-half the normal used), the potential for systemic toxicity was at least halved.
When meperidine 0.25%, 40 mL (100 mg), was used as a solitary agent for IVRA, complete motor block was produced, just as effective as that produced by lidocaine. Motor block onset was as rapid as or more rapid than sensory block onset in each of the 15 patients in this study group. However, when compared with plain lidocaine in this study, there was a higher incidence of dizziness, nausea, and pain at the injection site.
Tramadol
Tramadol has been evaluated for use in IVRA of the upper extremity. Sixty volunteers divided into four groups of 15 patients each received IVRA with 40 mL of tramadol 0.25% (100 mg), 0.9% normal saline, lidocaine 0.5%, or lidocaine plus tramadol 0.25%. The onset and recovery of sensory and motor block were similar between the tramadol and normal saline–only However, the addition of tramadol to lidocaine resulted in faster onset of sensory block at the expense of an increase in skin rash and painful burning sensations at the injection site. The conclusion of the authors was that tramadol alone does not possess local anesthetic effects but might modify the effects if added to a local anesthetic such as lidocaine.
In another study comparing 0.5% lidocaine with and without 50 mg of tramadol for upper extremity IVRA, the tramadol-added group experienced less tourniquet pain than the local-only group, but as in the study mentioned previously, there were several cases of skin urticaria in the tramadol group but not in the lidocaine-only group. Tramadol (100 mg) added to lidocaine for IVRA for upper extremity anesthesia acted similarly to sufentanil (25 μg) or clonidine (1 μg/kg) added to the local anesthesia with respect to intraoperative hemodynamic data, time to recovery of sensory block, onset and recovery of motor block, sedation scores, and postoperative pain.61 In summary, tramadol is ineffective as a solo agent for IVRA but may confer some advantage when added to lidocaine. This advantage, however, may be offset by the significant incidence of dermatologic side effects of tramadol given intravenously in an exsanguinated extremity.
Muscle Relaxants
A small dose of nondepolarizing muscle relaxant may be chosen as an adjunct to the local anesthetic administered for IVRA; however, because D-tubocurarine releases histamine even in judicious doses, it is probably best to avoid this agent altogether. Atracurium has been added to lidocaine in an effort to improve muscular relaxation during IVRA, particularly during the reduction of upper extremity fractures and dislocations. Adding 3 mg of atracurium to lidocaine for IVRA resulted in a decrease in the onset time of analgesia in the hand, but not at the tourniquet site. There was no added benefit to adding this agent or adding alfentanil to lidocaine in the same study. A study using 2 mg of atracurium added to 40 mL of 0.5% lidocaine for IVRA for hand surgery in 40 patients randomized to one of two groups found that the addition of the atracurium provided a greater degree of muscle relaxation, easier reduction of fractures, and better operating conditions, as well as less pain after surgery.
Neostigmine
Neostigmine has been suggested as a coanalgesic when used for epidural and intrathecal analgesia and anesthesia, but evidence of its benefit in the peripheral nervous system is lacking. In two studies, one using neostigmine added to lidocaine and the other using the adjuvant added to prilocaine, there have been conflicting findings. When neostigmine (1 mg) was added to 0.5% lidocaine for IVRA in a study of 54 volunteers randomized into one of three study groups, it was found that the addition of the adjuvant provided no benefit in terms of analgesia or anesthesia compared with controls. When one-half of the dose of neostigmine (0.5 mg) was added to prilocaine (3 mg/kg) for IVRA in 30 patients randomized to one of two treatment groups, it was found that the adjuvant group demonstrated shortened sensory and motor block onset times, prolonged sensory and motor block recovery times, improved quality of anesthesia, and prolonged time to first analgesic requirement versus the plain prilocaine group.
A more recent study looking at the effect of adding 0.5 mg neostigmine to 40 mL of 0.5% lidocaine for IVRA in patients undergoing elective or emergency forearm and hand surgeries randomized into two groups, with 1 mL of isotonic saline added to 40 mL of 0.5% lidocaine in the control group, noted significantly shorter sensory and motor block onset times and longer recovery times in the neostigmine group when compared to the control group. The quality of intraoperative anesthesia and frequency of tourniquet pain were similar in both groups. It appears that the conflicting findings with two different doses of neostigmine added to lidocaine for IVRA will need to be confirmed by additional work incorporating larger patient sample sizes to resolve the discrepancy in the two small studies mentioned previously.
Nonsteroidal Anti-inflammatory Agents
Other attempts to improve IVRA with lidocaine have included using NSAIDs to suppress tourniquet pain while enhancing postoperative analgesia. Although ketorolac has shown some efficacy, other NSAIDs have not fared as favorably. Ketorolac was studied as an adjuvant to lidocaine using either a forearm or an upper arm tourniquet in patients undergoing hand or forearm surgery. In this study, the patients were randomized in to two groups: group UA, consisting of 20 patients undergoing IVRA with an upper arm tourniquet; and group FA, consisting of 20 patients undergoing IVRA with a forearm tourniquet. Patients in the upper arm tourniquet group received IVRA with 0.5% lidocaine at the dose of 3 mg/kg plus ketorolac 0.3 mg/kg. IVRA in the forearm tourniquet group was established with 0.5% lidocaine at 0.15 mg/kg plus ketorolac 0.15 mg/kg. There was no statistically significant difference in the onset and duration of sensory block and the need for analgesic supplementation between the two groups. Postoperative pain scores were also similar between the two groups. The authors concluded that forearm tourniquet IVRA with 0.5% lidocaine at a dose of 1.5 mg/kg plus ketorolac 0.15 mg/kg is a safe and clinically viable option that provides similar perioperative anesthesia and analgesia as that provided by upper arm tourniquet IVRA with 0.5% lidocaine at a dose of 3 mg/kg plus ketorolac 0.3 mg/kg, while reducing the dose of lidocaine and ketorolac by half.
Another NSAID, tenoxicam, was added to prilocaine in one study of 45 total patients. A 20-mg dose of the NSAID was used in patients undergoing IVRA for reduction of Colles fractures, with patients divided into three groups. One group received local anesthetic only; one received local plus tenoxicam; and one group had IVRA with local anesthetic only plus an intravenous NSAID. In this last group, the tenoxicam (20 mg) was injected in the contralateral arm, opposite the IVRA procedure arm. The group receiving the NSAID added to the local anesthetic had superior analgesia and lower pain scores than either of the other two groups of patients.
A more recent study comparing the intraoperative and postoperative analgesic effects of lornoxicam and fentanyl when added to lidocaine for IVRA in patients undergoing hand surgery showed increased sensory block recovery time and first analgesic requirement time in the lornoxicam group, with no increased incidence of side effects, when compared to the lidocaine-only group and the lidocaine with fentanyl group. In this study, a total of 45 patients were randomized into three groups. Patients in group 1 received 3 mg/kg of 2% lidocaine (40 mL); group 2 received 3 mg/kg lidocaine, 38 mL plus lornoxicam 2 mL (4 mg/mL); and group 3 received 3 mg/kg lidocaine, 38 mL plus 2 mL fentanyl (0.05 mg/mL). This study also concluded that addition of fentanyl to lidocaine IVRA (group 3) seemed to be superior to lidocaine IVRA (group 1) and lornoxicam added to lidocaine IVRA (group 2) in decreasing tourniquet pain; however, this was at the expense of increasing side effects like itching.
Dexketoprofen is another NSAID that has been studied as an adjunct to lidocaine for IVRA. In this prospective, randomized, placebo-controlled study, patients scheduled for elective hand or forearm soft tissue surgery were randomly divided into three groups. All 45 patients received 0.5% lidocaine as IVRA. Dexketoprofen 50 mg was given either intravenously or added into the IVRA solution, and the control group received an equal volume of saline both intravenously and as part of the IVRA. The times of sensory and motor block onset, recovery time, and postoperative analgesic consumption were recorded. Compared with controls, the addition of dexketoprofen to the IVRA solution resulted in more rapid onset of sensory and motor block, longer recovery time, decreased intra- and postoperative pain scores, and decreased postoperative analgesic requirements. The pharmacological formulation of dexketoprofen used in this study contained ethanol as an excipient. The authors stated that the neurolytic effect of ethanol may have contributed to the faster development of sensory and motor block and longer recovery times in the IVRA group in this study.
Other Specific Agents: Corticosteroids
The anti-inflammatory properties of glucocorticoid-type steroids have been evaluated when these agents have been added to local anesthetics for IVRA in patients with rheumatoid arthritis (RA). In a randomized, double-blind, crossover, placebo-controlled study, 20 patients with RA received either 50 mg methylprednisolone in mepivacaine 0.25% or mepivacaine plain for upper extremity IVRA. The other extremity received the opposite treatment. One week later, the same medications were injected into the contralateral extremities, respectively. Fifty percent of patients reported subjective improvement at 1 and 6 weeks; objective parameters like grip strength did not change until the 6-week evaluation, at which time a significant increase was noted, as was the reduction in grip diastasis and movement-invoked pain. This report suggested that corticosteroids administered by IVRA may provide sustained analgesia in certain RA sufferers.
Steroid IVRA has also been used as adjunctive treatment of CRPS type 1. Methylprednisolone (40 mg) was added to lidocaine for IVRA in a randomized, double-blind, placebo-controlled fashion in 22 patients. Treatments were applied once per week, for up to three sessions of blocks. The investigators found no benefit in adding the steroid to the local with regard to improvement in pain severity or shortening the course of the disease. Interestingly, a case series involving 168 patients with CRPS type 1 of the upper extremity treated with IVRA using 25 mL of 0.5% lidocaine and 125 mg of methylprednisolone diluted in 10 mL of normal saline and followed up over a 5-year period reported complete absence of pain in 92% of patients at the end of the follow-up period. IVRA was performed with the tourniquet kept inflated for 20 minutes, during which time the affected extremity was manipulated in an attempt to increase the range of motion. The tourniquet was then gradually deflated to avoid rapid entry of the injected agents to the circulation.
The same process was repeated once or twice a week depending on the intensity and persistence of the patient’s symptoms, and in between sessions patients were kept under mild physical therapy, which was not prolonged or stressful. An average of 4.8 sessions was needed to relieve the symptoms and to provide a functional extremity. The authors attributed the clinical results to the early stage of CRPS type 1 on initiating treatment and the increased dosing of methylprednisolone when compared to previous studies.
Acetaminophen
Because of its known analgesic effects, acetaminophen (APAP) (paracetamol) has been studied as an adjuvant to local anesthetics in patients undergoing hand surgery under IVRA. Sixty patients undergoing hand surgery were randomized into three groups. All groups received IVRA lidocaine (3 mg/kg) diluted with normal saline to a total volume of 40 mL. Group 1 received IVRA lidocaine plus intravenous saline. Group 2 received IVRA lidocaine and an APAP (300 mg) admixture plus intravenous saline; and group 3 received IVRA lidocaine plus intravenous APAP (300 mg). Sensory and motor block onset time, tourniquet pain, and analgesic use were assessed during surgery. After tourniquet deflation, VAS scores at 1, 2, 4, 6, 12, and 24 hours; the time to first analgesic requirement; total analgesic consumption in first 24 hours; and side effects were noted. There was no significant difference in the onset of sensory blocks between the three groups; however, the duration of sensory block was significantly longer in group 2. Motor block onset time was shorter and duration of motor block was longer in group 2. Tourniquet pain was reduced and the quality of anesthesia scores as reported by the anesthesiologist, who was blinded to the study drug, was also significantly higher in group 2. There was no demonstrable decrease in postoperative pain scores between the three groups. The authors did point out the arbitrary dosing of APAP (300 mg) as a deficiency of the study. Further dose-ranging studies are required to optimize the dosing of paracetamol when used as an adjuvant to lidocaine for IVRA.
Another study evaluating the effect of APAP, when added to lidocaine in IVRA, on sensory and motor block onset time, tourniquet pain, and postoperative analgesia found a shorter sensory block onset time; delayed tourniquet pain onset time; and reduced postoperative pain scores and analgesic consumption. The dosing of acetaminophen was identical to the earlier study (0.5% lidocaine diluted with 300 mg of intravenous APAP to a total volume of 40 mL). The control group received 0.5% lidocaine diluted with 0.9% normal saline to a total volume of 40 mL. Time of onset and duration of motor block were not assessed in this study. In an attempt to explain the faster onset time of the sensory block in the lidocaine-acetaminophen group, the authors investigated the pH of the lidocaineacetaminophen mixture and found that the pH value of this mixture was 5.88, which was lower than the pH of the lidocaine–normal saline mixture, which was 6.16. This contradicts the fact that the higher the pH of the local anesthetic, the greater the nerve penetration and hence faster onset of neural block. In this study, the authors attributed the faster onset of sensory block to possible antinociceptive effects of APAP at peripheral sites.
Nitroglycerine
The effect of nitroglycerine (NTG), when added to lidocaine for IVRA, was studied in a prospective, randomized, double-blind study.76 Thirty patients undergoing hand surgery were randomly assigned to two groups. The control group (group C, n = 15) received a total dose of 40 mL with 3 mg/kg of lidocaine diluted with normal saline, and the NTG group (group NTG, n = 15) received an additional 200 μg NTG. Shortened sensory and motor block onset times; prolonged sensory and motor block recovery times; decreased tourniquet pain; and improved quality of anesthesia were noted in the NTG group. Postoperative analgesic requirements were also significantly decreased in the NTG group. The authors attributed the shorter onset time of sensory and motor block to the vasodilatory effects of NTG that promotes the distribution of local anesthetic to the nerves. Some of the other mechanisms that may contribute to the improved analgesia with NTG may include the metabolism of NTG to nitric oxide, which in turn causes an increase in the intracellular concentration of cyclic guanosine monophosphate, which produces
pain modulation in the central and peripheral nervous system. Nitric oxide generators have also been shown to induce anti-inflammatory effects and analgesia by blocking hyperalgesia and the neurogenic component of inflammatory edema by topical application.
Midazolam
Midazolam has been shown to hasten the onset of sensory and motor block and improve postoperative analgesia when added to bupivacaine for brachial plexus block. Midazolam was shown to have analgesic effects mediated through GABA (γ-aminobutyric acid) receptors in the spinal cord in animal studies. GABA receptors have also been found in peripheral nerves. Midazolam was also shown to reduce A-delta and C-fiber activity.
In a study designed to evaluate the effect of midazolam when added to lidocaine for IVRA, 40 patients undergoing hand surgery were randomly assigned to two groups. The control group received 3 mg/kg lidocaine 2% diluted with saline to a total volume of 40 mL, and the midazolam group received an additional 50 μg/kg of midazolam. There were no statistically significant differences in the sensory and motor block onset and recovery times between the two groups. However, a subjective pain assessment score using the numeric rating scale (NRS) for tourniquet pain was significantly decreased in the midazolam group. Anesthesia quality as evaluated by the patient and the surgeon was also significantly better in the midazolam group.
Postoperative NRS pain scores were also significantly lower in the midazolam group for the initial 2 hours postoperatively.
However, postoperative sedation scores were also higher in the midazolam group. The authors indicated that the enhanced postoperative analgesia after tourniquet deflation may be explained by the systemic effect of midazolam in addition to the peripheral analgesic effect.
Another study involved 60 patients undergoing hand surgery randomized into two groups, with the control group and the midazolam group receiving IVRA with exactly identical dosing of lidocaine and midazolam as in the previous study. This study showed shortened sensory and motor block onset times and prolonged sensory and motor block recovery times in addition to decreased tourniquet pain scores and postoperative pain scores. The conflicting findings with regard to the sensory and motor block onset times may warrant further studies with greater sample sizes to resolve the discrepancy. At present, all that can be stated is that midazolam appears to hold some promise as an adjunct to local anesthetic when used for upper extremity IVRA.
Ketamine
Ketamine is a potent analgesic agent whose principal mechanism of action is antagonism of N-methyl-D-aspartate (NMDA) glutamate receptors that play a crucial role in the pain-processing mechanism at the level of the spinal cord. Animal studies have indicated the presence of NMDA receptors in peripheral nerves. Ketamine has also been shown to produce transient block of peripheral nerve sodium and potassium channels. The possible presence of NMDA receptors in peripheral nerves as well as the ability of ketamine to block sodium channels locally points to a possible peripheral site of action for ketamine, in addition to its well-established central sites of action.
Ketamine was studied as an adjuvant in lidocaine IVRA in patients undergoing hand surgery and its efficacy in controlling intraoperative tourniquet pain, postoperative analgesia, and side effects was compared to the same dosage of ketamine administered systemically. In this randomized, double-blind, systemic control study, 40 patients undergoing outpatient hand surgery were randomized into two groups. In group “IVRA,” 0.1 mg/kg ketamine in 1 mL of normal saline was added to the IVRA lidocaine, and 1 mL of normal saline was administered via a peripheral intravenous line. In group “systemic,” 1 mL of normal saline was added to the IVRA syringe, and 0.1 mg/kg ketamine in 1 mL of normal saline was administered via a peripheral intravenous line. Both groups received 40 mL of 0.5% lidocaine for IVRA.
The study found no difference between the groups in the study parameters mentioned, and the authors concluded that IVRA ketamine and systemic intravenous ketamine were indistinguishable in terms of intraoperative tourniquet pain and postoperative analgesic consumption. The speed of onset and the duration of sensory and motor block were not measured in this study. Another study comparing the efficacy of ketamine and clonidine when they are added separately to 40 mL of 0.5% lidocaine for IVRA in patients undergoing hand or forearm surgery found delayed onset of tourniquet pain and decreased analgesic consumption in both these groups when compared to the control group receiving 40 mL of 0.5% lidocaine with saline added to it. Ketamine had a more potent effect on the study parameters when compared to clonidine. In this study involving 45 patients randomized into three groups, IVRA was performed using 40 mL of 0.5% lidocaine and saline, 1 μg/kg clonidine, or 0.1 mg/kg ketamine.
Specific IVRA Treatments for CRPS
Adrenergic blocking agents or antagonists, particularly those effective at the α receptor, have shown promise in the treatment of CRPS, particularly when these agents are used for IVRA. Other adrenergic adjuvants release and then subsequently prevent the reuptake of norepinephrine at the neurovascular junction. Their use in CRPS is intuitive because the pathophysiology of the disease is suspected to include the α receptor and to be mediated by norepinephrine. However, there is significant controversy regarding this topic, particularly when current research is compared with the findings of studies conducted almost 40 years ago. Guanethidine, reserpine, and bretylium have all been evaluated for IVRA for CRPS. When 15 mg guanethidine were added to 0.5% prilocaine in a group of 57 patients with CRPS of the upper extremity and hand, the guanethidine was not found more effective than normal saline in treating allodynia and burning pain of CRPS following distal radius fractures.
These findings corroborated work done in a double-blind, randomized, multicenter study 7 years earlier. Sixty patients with Reflex sympathetic dystrophy (RSD)/causalgia received four IVRA blocks at 4-day intervals with either guanethidine or placebo in 0.5% lidocaine. Long term, there was no difference noted between the placebo group and the guanethidine group, and only 35% of patients overall in all groups had a resolution of their problem.
Bretylium has been used as well in CRPS. In a randomized, controlled trial, 0.5% lidocaine was compared with the same local anesthetic to which bretylium 1.5 mg/kg were added. A decrease in pain of 30% or more was considered significant. The bretylium-local group had pain relief for 20 ± 17.5 days, as opposed to the lidocaine-only group, wherein analgesia persisted for only 2.7 ± 3.7 days. The bretylium was far superior to the local anesthetic alone in treating CRPS in this study.
Intravenous regional anesthesia with bretylium was utilized to demonstrate that a reduction in sympathetic tone of exercising forearm muscles would increase blood flow, reduce muscle acidosis, and attenuate reflex responses. IVRA with bretylium increased blood flow as well as oxygen consumption in the exercising forearm, although both venous potassium and hydrogen ion content were elevated during the exercise phase, implying that reflex effects were unaffected by bretylium block.
COMPLICATIONS
Complications due to IVRA may be classified either as drug or equipment (ie, tourniquet) related. Drug-related complications depend on the agents, including local anesthetics and adjuvants, being administered directly into the vascular system. Equipment-related complications include all devices and techniques used to isolate the vascular space from the systemic circulation. Inadvertent or unintentional deflation of the cuff, cuff failure, a sudden increase in venous pressure within the occluded tissue to a level higher than cuff pressure, and an intact interosseous circulation may all contribute to complications of IVRA.
Lidocaine is the most commonly utilized local anesthetic for IVRA and is therefore the agent about which most complications have been reported. Fortunately, lidocaine does not accumulate to any great extent at sodium channels at therapeutic plasma concentrations, and because it both rapidly binds to and dissociates from the channel, toxic accumulations of the drug at the channel are atypical.97,98 Excessive plasma concentrations of lidocaine, as are associated with intravenous boluses of large doses with a faulty tourniquet system, result in peripheral vasodilation and diminished cardiac contractility, usually seen clinically as hypotension. The usual onset of IVRA using lidocaine in 0.5% concentrations is rapid (about 4.5 ± 0.3 minutes), and the termination of anesthesia once the tourniquet has been deflated is also rapid (5.8 ± 0.5 minutes).99 Usually, there are no signs or symptoms of cardiovascular or central nervous system toxicity if the tourniquet is deflated at least 30 minutes after the drug is injected into the venous system, although tinnitus has been noted at the 20- and 27-second postdeflation periods following standard inflation times.
However, a literature search in the American National Library of Medicine’s PubMed®, in Embase®, and in MEDLINE®, spanning the period from 1950 to 2007 revealed 24 cases of seizures, with seizures reportedly occurring in 12 cases while the cuff was still inflated and in 9 cases after the cuff was deflated. Information was not available in three cases. Seizures occurring during tourniquet inflation were reported with tourniquet pressure exceeding the initial systolic arterial blood pressure by 150 mm Hg. Seizures occurring after tourniquet deflation were reported with tourniquet times as long as 60 minutes and with a delay of up to 10 minutes after tourniquet deflation. The lowest dose of local anesthetic associated with a seizure was 1.4 mg/kg for lidocaine, 4 mg/kg for prilocaine, and 1.3 mg/kg for bupivacaine.
Although about 70% of the administered lidocaine dose remains within the tissues of the isolated limb after tourniquet deflation, the remaining 30% enters the systemic circulation during the ensuing 45 minutes. Much more drug is released from the tissues of the isolated limb into the circulation after tourniquet deflation if the limb is inadvertently exercised, emphasizing the importance of maintaining the previously anesthetized extremity quiescent for some time immediately following tourniquet deflation.
The other commonly utilized local anesthetic used for IVRA, prilocaine, is associated with the formation of methemoglobin (MetHb), which occurs about 4 to 8 hours after its administration. Fortunately, significant methemoglobinemia has not been reported when prilocaine has been used for IVRA. Prilocaine (0.5%) administered for IVRA has an onset of analgesia of about 11 minutes (±6.8 minutes), and termination of analgesia following tourniquet deflation averages 7.2 minutes (±4.6 minutes).25 The use of this agent for IVRA appears to be extraordinarily safe. Indeed, in one survey of 45,000 prilocaine IVRA blocks, there were no serious side effects and no deaths using this drug via this technique.102 In terms of effectiveness, prilocaine seems to be equivalent to lidocaine when used for IVRA.
When opioids are administered in combination with local anesthetics for IVRA in an attempt to prolong analgesia following cuff deflation, occasional side effects typically attributed to opioids administered systemically may be noted following cuff deflation. These include nausea, vomiting, and mild sedation.
When neurovascular blocking drugs are administered in conjunction with local anesthetics to improve surgical conditions in patients undergoing fracture reduction, there have been no reports of complications from these adjuvants.
NYSORA Tips
• An intact tourniquet system is essential for the successful and safe performance of IVRA.
• Unintentional deflation of the tourniquet or the presence of a vascular communication even with an intact, functioning tourniquet may result in severe systemic toxicity.
• When the surgical procedures is shorter than 30 minutes, intermittent cuff deflation and inflation may effectively prolong the time to achieve peak arterial concentrations of the local anesthetic but may not be entirely reliable in minimizing toxicity due to release of local anesthetic into the circulation.
• The tourniquet should not be deflated until at least 30 minutes has elapsed from the time local anesthetic (and adjuvants, if used) is injected into the isolated venous system.
In addition, the tourniquet itself may be a source of complications because it may cause ischemic pain and discomfort.
Systemic hypertension may result from tourniquet inflation that is sustained or prolonged. Equipment misuse or malfunction is an important, and avoidable, source of complications due to this technique. Even an intact, fully functional tourniquet may be associated with leakage of administered drugs from a supposedly isolated extremity into the systemic circulation.
Lower limb IVRA has an almost 100% incidence of local anesthetic leakage from beneath the tourniquet, versus about a 25% incidence for upper extremity block. As a corollary to this leakage phenomenon, the use of IVRA for lower extremity analgesia has an associated high incidence of poor-quality block (almost 40% in one prospective study). Drug may leak past an apparently fully functioning cuff and gain access to the systemic circulation via the interosseous circulation, which is not affected by the occlusion of muscles, soft tissues, and the accompanying vascular channels included therein. This factor has been recognized for almost 50 years, yet it does not appear to be significant in the production of complications due to IVRA.
Tourniquet deflation after IVRA is associated with signs and symptoms of systemic local anesthetic toxicity, ranging from mild events related to the central nervous system, such as tinnitus and perioral numbness, to seizures, and finally to devastating cardiovascular collapse. These correlate with local anesthetic concentrations in arterial blood and not to venous concentrations.
Another complication due to IVRA is tourniquet pain, which not uncommonly occurs if a double pneumatic device is not utilized (Figure 1 and Figure 9). We recommend the use of such a tourniquet for any procedure performed using IVRA that is expected to last longer than 30 minutes. Even when such guidelines are followed, however, untoward events occur following tourniquet deflation after a “safe” time interval. Very rare, isolated reports of neurologic complications, including damage to the median, ulnar, and musculocutaneous nerves, are associated with IVRA. The cause of such complications appears to be direct pressure of the tourniquet applied to these nerves, which subsequently exhibit histologic changes resembling crush injuries. It is recommended that tourniquet time not exceed 2 hours to reduce the likelihood of capillary and muscle damage secondary to tissue acidosis.
Compartment syndrome may occur rarely following IVRA, especially when IVRA is used for reduction of long-bone lower extremity fractures, and may be due both to the large volume of local anesthetic injected to effect analgesia and to inadequate or incomplete exsanguination of the limb prior to performing the block. There is a case report of this complication following inadvertent injection of hypertonic saline solution when local anesthetic was intended to be injected.
A 33-year-old pregnant patient undergoing IVRA for endoscopic carpal tunnel release experienced a severe episode of phantom limb sensation after the injection of the local anesthetic.
The symptoms resolved on dissipation of the IVRA. There is one report of the devastating necessity for amputation of the arm in a 28-year-old patient whose radial and ulnar arteries thrombosed following IVRA after a brief tourniquet occlusion time.Whether this resulted from unsuspected intraarterial injection of drug, a drug administration error, or perhaps an idiosyncratic drug reaction is purely speculative. Three cases including either death or permanent brain damage associated with IVRA were reported in the ASA Closed Claims Project for the years 1980 to 1999. Specifics of these cases are not known.
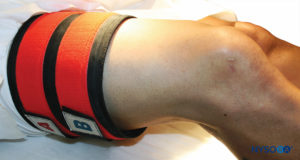
FIGURE 9.The double tourniquet system is placed on the proximal right thigh, in preparation for IVRA of the right lower extremity.
Local Anesthetic Toxicity
Although lidocaine is the most commonly utilized local anesthetic agent for IVRA in the United States, in Europe prilocaine 0.5% is more routinely chosen. Prilocaine, however, is metabolized to orthotoluidine, an oxidizing compound capable of converting hemoglobin to MetHb. This is usually only of concern when the dose of prilocaine exceeds 600 mg, which, even for lower extremity IVRA in which volumes as large as 100 mL are utilized, should not be attained (ie, 100 mL × 0.5% prilocaine = 500 mg).
NYSORA Tips
• Severe methemoglobinemia is a medical emergency requiring prompt recognition and appropriate treatment.
• A good history and high level of suspicion are required to make the diagnosis.
• For methemoglobinemia due to drug exposure, traditional first-line therapy consists of the infusion of methylene blue (MB).
• Dextrose should be given because the major source of NADH (reduced [hydrogenated] nicotinamide adenine dinucleotide) in the red blood cells is the catabolism of sugar through glycolysis. Dextrose is also necessary to form NADPH (reduced [hydrogenated] NAD phosphate) through the hexose monophosphate shunt, which is necessary for MB to be effective.
• The dose of MB is 1 to 2 mg/kg IV over 5 minutes (total dose should not exceed 7–8 mg/kg).
• MB can cause dyspnea, chest pain, or hemolysis.
• MB provides an artificial electron transporter for the reduction of MetHb via the NADPH-dependent pathway. The response is rapid; the dose may be repeated in
1 hour if the level of MetHb is still high 1 hour after the initial infusion.
• Rebound methemoglobinemia may occur up to 18 hours after MB administration due to prolonged absorption of lipophilic agents (benzocaine) from adipose tissue. It is reasonable to perform serial measurements of MetHb levels following treatment with MB. MB should not be administered to patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency because the reduction of MetHb by MB is dependent on NADPH generated by G6PD (hemolysis). An alternative treatment for these patients is ascorbic acid (2 mg/kg).
• Blood transfusion or exchange transfusion may be helpful in patients who are in shock. Hyperbaric oxygen has been used with anecdotal success in severe cases.
Deflation of the tourniquet after surgery is a critical step in minimizing the possibility of toxicity associated with IVRA. First, it is absolutely mandatory that the tourniquet not be deflated unless at least 30 minutes have elapsed since the injection of the local anesthetic, even if the duration of surgery or of the manipulation has been brief. If the surgery is brief, and the patient needs to recover in the PACU, it is acceptable to clamp off the distal tourniquet while it is inflated, remove the patient from the operating area (with the tourniquet inflated), and transfer the patient to a monitored care setting. At no time should anyone remove the clamped tourniquet, however, until the 30-minute period commencing with the injection of local anesthetic solution has elapsed. At such time, the patient should be continually monitored for at least 15 minutes following tourniquet unclamping in the PACU. At least one case of cardiac arrest has been reported when the tourniquet was released soon after the injection of local anesthetic, where the duration of surgery was extremely short.
Second, it is absolutely essential that the tourniquet deflation be accomplished in a “cyclical” fashion as follows: The cuff is deflated (after a minimum of 30 minutes) and is immediately reinflated. The patient is observed or questioned carefully for the occurrence of symptoms associated with local anesthetic toxicity, such as tinnitus, light-headedness, metallic taste in the mouth, and so on. Obviously, signs of stimulation of the central nervous system may also represent local anesthetic toxicity and must also be sought. If there are no such signs or symptoms after about 1 minute, the cuff is once again deflated and again immediately reinflated for a period of about 1 to 2 minutes, with the patient being observed and queried for systemic local anesthetic toxicity. If none appears by this time, the tourniquet may be safely deflated and removed from the extremity. The safety of such cycled deflating/reinflating is that, with each deflation, only a small fraction of the administered (and unbound) local anesthetic is allowed to enter the systemic circulation, minimizing the possibility of a sudden, sustained increase in the blood level of the local aneshtetic.
SUMMARY
Intravenous regional anesthesia is a valuable adjunct to the armamentarium of clinicians in any specialty dealing with the acutely injured patient. The simplicity of the technique and the relative safety (if strict adherence to the previously listed protocol is maintained) make it an attractive alternative to brachial plexus block (for upper extremity surgery or manipulation) and spinal or epidural block (for lower extremity surgery or manipulation). Simply being able to identify and access a peripheral vein and apply a pneumatic tourniquet make this one of the most “user-friendly” regional block modalities in clinical practice. There is no requirement for being facile with a peripheral nerve stimulator or interpreting images obtained from an ultrasound machine. One of the only potential disadvantages associated with IVRA is the finite duration of anesthesia/analgesia associated with its use. A relative inability to prolong analgesia long into the postprocedure period detracts from its utility. For those occasions, continuous catheter insertion and maintenance by way of plexus anesthesia ensures offering an attractive alternative.
REFERENCES
- Bier A: Uber einen neun weg local anaesthesia an den gliedmassen zuerzeugen. Arch Klin Chir 1908;86:1007–1016.
- Bier A: On a new method of local anesthesia. Muench Med Wschir 1909; 56:589.
- Bier A: Concerning venous anesthesia. Berl Klin Wschr 1909;46: 477–489.
- Bier A: On local anesthesia with special reference to vein anesthesia. Edinburgh Med J 19105:103–123.
- Morrison J: Intravenous local anesthesia. Br J Surg 1930–1931;18:641–647.
- Herreros L: Regional anesthesia by the intravenous route. Anesthesiology 1946;7:558–560.
- Holmes CMcK: Intravenous regional analgesia. Lancet 1963;1:245–247.
- Kalman S, Svenson H, Lisander B, et al: Quantitative sensory changes in humans after intravenous regional block with mepivacaine. Reg Anesth Pain Med 1999;24:236–241.
- Wahren L, Torebjork E, Nystorm B: Quantitative sensory testing before and after regional guanethidine block in patients with neuralgia in the hand. Pain 1991;46:23–30.
- Smith M, Sprung J, Zura A, et al: A survey of exposure to regional anesthesia techniques in American anesthesia residency training programs. Reg Anesth Pain Med 1999;24:11–16.
- Blaheta H, Vollert B, Zuder D, et al: Intravenous regional anesthesia (Bier’s block) for botulimum toxin therapy of palmar hyperhidrosis is safe and effective. Dermatol Surg 2002;28:666–671.
- Bosdotter Enroth S, Rystedt A, Covaciu L, et al: Bilateral forearm intravenous regional anesthesia with prilocaine for botulinum toxin treatment of palmar hyperhidrosis.. J Am Acad Dermatol 2010;63(3): 466–474.
- Nascimento MSA, Klamt JG, and Prado WA: Intravenous regional block is similar to sympathetic ganglion block for pain management in patients with complex regional pain syndrome type 1. Braz J Med Biol Res 2010; 43(12):1239–1244.
- Glickman L, Mackinnon S, Rao T, et al: Continuous intravenous regional anesthesia. J Hand Surg 1992;17:82–86.
- Mabee J, Shean C, Orlinsky M, et al: The effects of simulated Bier block IVRA on intracompartmental tissue pressure. Acta Anaesthesiol Scand 1997;41:208–213.
- Aarons CE, Fernandez MD, Willsey M, Peterson B, Key C, Fabregas J: Bier block regional anesthesia and casting for forearm fractures: Safety in the pediatric emergency department setting. J Pediatr Orthop 2014;34:45–49.
- Singh R, Bhagawat A, Bhadoria P, Kohli A: Forearm IVRA, using 0.5% lidocaine in a dose of 1.5 mg/kg with ketorolac 0.15 mg/kg for hand and wrist surgeries. Minerva Anestesiol 2010;76:109–114.
- Chiao FB, Chen J, Lesser JB, Resta-Flarer F, Bennett H: Single-cuff forearm tourniquet in intravenous regional anesthesia results in less pain and fewer sedation requirements than upper arm tourniquet. Br J Anaesth 2013;111(2):271–275.
- Benlabed M, Jullien P, Guelmi K, et al: Alkanization of 0.5% lidocaine for intravenous regional anesthesia. Reg Anesth 1990;15:59–60.
- Lavin P, Henderson C, Vaghadia H: Non-alkalinized and alkalinized 2-chlorprocaine versus lidocaine for intravenous regional anesthesia during outpatient hand surgery. Can J Anaesth 1999;46:939–945.
- Ulus A, Gurses E, Oztrurk I, Serin S: Comparative evaluation of two different volumes of lidocaine in intravenous regional anesthesia. Med Sci Monit 2013;19:978–983.
- Chan V, Weisbrod M, Kaszas Z, et al: Comparison of ropivacaine and lidocaine for intravenous regional anesthesia in volunteers: A preliminary study on anesthetic efficacy and blood level. Anesthesiology 1999;90: 1602–1608.
- Peng P, Coleman M, McCartney C, et al: Comparison of anesthetic effect between 0.375% ropivacaine versus 0.5% lidocaine in forearm intravenous regional anesthesia. Reg Anesth Pain Med 2002;27;595–599.
- Horn JL, Cordo P, Kunster D, et al: Progression of forearm intravenous regional anesthesia with ropivacaine. Reg Anesth Pain Med 2011;36(2): 177–180.
- Pitkanen M, Suzuki N, Rosenberg P: Intravenous regional anesthesia with 0.5% prilocaine or 0.5% chlorprocaine. A double blind comparison in volunteers. Anaesthesia 1992;47:618–619.
- Pitkanen M, Kytta J, Rosenberg P: Comparison of 2-chloroprocaine and prilocaine for intravenous regional anesthesia of the arm: A clinical study. Anaesthesia 1993;48:1091–1093.
- Pitkanen M, Xu M, Haasio J, et al: Comparison of 0.5% articaine with 0.5% prilocaine in intravenous regional anesthesia of the arm: A cross over study in volunteers. Reg Anesth Pain Med 1999;24:131–135.
- Simon M, Gielen M, Albernik N, et al: Intravenous regional anesthesia with 0.5% articaine, 0.5% lidocaine, or 0.5% prilocaine. A double-blind randomized clinical study. Reg Anesth 1997;22:29–34.
- Hoffman V, Vercauteren M, Van Steenberge A, et al: Intravenous regional anesthesia. Evaluation of four different additives to prilocaine. Acta Anaesthesiol Belg 1997;48:71–76.
- Armstrong P, Brockway M, Wildsmith J: Alkalinization of prilocaine for intravenous regional anesthesia. Anaesthesia 1990;45:11–13.
- Solak M, Akturk G, Erciyes N, et al: The addition of sodium bicarbonate to prilocaine solution during IV regional anesthesia. Acta Anaesthesiol Scand 1991;35:572–574.
- Kalman S, Bjorn K, Tholen E, et al: Mepivacaine as an intravenous regional block interferes with reactive hyperemia and decreases steady state blood flow. Reg Anesth 1997;22:552–556.
- Kalman S, Liderfalk C, Wardell K, et al: Differential effect on vasodilation and pain after intradermal capsaicin in humans during decay of intravenous regional anesthesia with mepivacaine. Reg Anesth Pain Med 1998;23:402–408.
- Choyce A, Peng P: A systematic review of adjuncts for intravenous regional anesthesia for surgical procedures. Can J Anaesth 2002;49: 32–45.
- Kleinschmidt S, Stockl W, Wilhelm W, et al: The addition of clonidine to prilocaine for intravenous regional anesthesia. Eur J Anaesthesiol 1997;14:40–46.
- Cucchia G, Chasot-Di Dio V, et al: Effect of addition of clonidine to local anesthetic during the Bier block on the pre- and postoperative analgesia. Br J Anaesth 1997;78(Suppl 1):78–79.
- Ivie CS, Viscomi CM, Adams DC, Friend AF, Murphy TR, Parker CJ: Clonidine as an adjunct to intravenous regional anesthesia: A randomized, double-blind, placebo controlled dose ranging study. Anaesthesiol Clin Pharmacol 2011;27(3):323–327.
- Memis D, Turan A, Karamanlioglu B, et al: Adding dexmedetomidine to lidocaine for intravenous regional anesthesia. Anesth Analg 2004;98: 835–840.
- Kol IO, Ozturk H, Kaygusuz K, Gursoy S, Comert B, Mimaroglu C: Addition of dexmedetomidine or lornoxicam to prilocaine in intravenous regional anesthesia for hand or forearm surgery: A randomized controlled study. Clin Drug Investig 2009;29(2):121–129.
- Mirzak A, Gul R, Erkutlu I, Alptekin M, Oner U: Premedication with dexmedetomidine alone or together with 0.5% lidocaine for IVRA. J Surg Res 2010;164(2):242–247.
- Fields H, Emson P, Leigh B, et al: Multiple opiate receptor sites on primary afferent fibers. Nature 1980;284:351–353.
- Young W, Wamsley J, Zarbin M, et al: Opioid receptors undergo axonal flow. Science 1980;210:76–78.
- Boogaerts J, Balatoni E, Lafont N, et al: Utilisation des morphiniques dans les blocs nerveux peripheriques. Congres Ser Ars Medicina 1985;3:143–150.
- Gobeaux D, Landais A: Utilisation de deux morphiniques dans les blocs du plexus brachial. J Can Anesth 1988;36:437–440.
- Gobeaux D, Landais A, Bexon G, et al: Adjonction de fentanyl la lidocaine adrenaline pour le blocage du plexus brachial. J Can Anesth 1987;35:195–199.
- Viel E, Eledjam J, de la Coussaye J, et al: Brachial plexus block with opioids for postoperative pain relief: Comparison between buprenorphine and morphine. Reg Anesth 1989;14:274–278.
- Candido K, Khan M, Raja D, et al: Brachial plexus block for postoperative pain relief. Reg Anesth 2000;25:23.
- Arhtur J, Mian T, Heavner J, et al: Fentanyl and lidocaine versus lidocaine for Bier block. Reg Anesth 1992;17:223–227.
- Bobart V, Hartmannsgruber M, Atanassoff P, et al: Analgesia/anesthesia after fentanyl plus lidocaine vs. plain lidocaine for intravenous regional anesthesia. Anesth Analg 1998:86:S-3.
- Pitkanen M, Rosenberg P, Pere P, et al: Fentanyl- prilocaine mixture for intravenous regional anesthesia in patients undergoing surgery. Anaesthesia 1992;47:395–398.
- Armstrong P, Power I, Wildsmith J: Addition of fentanyl to prilocaine for intravenous regional anesthesia. Anaesthesia 1991;46:278–280.
- Gupta A, Begntsson M, Bjornsson A, et al: Lack of peripheral analgesic effect of low-dose morphine during intravenous regional anesthesia. Reg Anesth 1993;18:250–253.
- Abdulla W, Fadhil N: A new approach to intravenous regional anesthesia. Anesth Analg 1992;75:597–601.
- Sztark F, Thicoipe M, Favarel-Garriques J, et al: The use of 0.25% lidocaine with fentnayl and pancuronium for intravenous regional anesthesia. Anesth Analg 1997;84:777–779.
- Subxedar D, Gevirtz C, Malik V, et al: Intravenous regional anesthesia: Prospective evaluation of 0.25% lidocaine with fentanyl and rocuronium. Reg Anesth 1997;22:41.
- Thapar P, Skerman J: Evaluation of 0.2% lidocaine with fentanyl and D-tubocurarine for intravenous regional anesthesia. Reg Anesth 1997; 84: S342.
- Santhosh MC, Rohini BP, Roopa S, Raghavendra PR: Study of 0.5% lidocaine alone and combination of 0.25% lidocaine with fentanyl and vecuronium in intravenous regional anesthesia for upper limb surgeries. Rev Bras Anestesiol 2013;63(3):254–257.
- Acalovschi I, Cristea T: Intravenous regional anesthesia with meperidine. Anesth Analg 1995;81:539–543.
- Acalovschi I, Cristea T, Margarit S, et al: Tramadol added to lidocaine for intravenous regional anesthesia. Anesth Analg 2001;92:209–214.
- Tan S, Pay L, Chan S: Intravenous regional anesthesia using lignocaine and tramadol. Ann Acad Med Singapore 2001;30:516–519.
- Alayurt S, Memis D, Pamucku Z: The addition of sufentanil, tramadol or clonidine to lignocaine for intravenous regional anaesthesia. Anaesth Intensive Care 2004;32:22–27.
- Kurt N, Kurt I, Aygunes B, et al: Effects of adding alfentanil or atracurium to lidocaine solution for intravenous regional anesthesia. Eur J Anaesthesiol 2002;19:522–525.
- Elhakim M, Sadek R: Addition of atracurium to lidocaine for intravenous regional anesthesia. Acta Anaesthesiol Scand 1994;38:542–544.
- McCartney C, Brill S, Rawson R, et al: No anesthetic or analgesic benefit of neostigmine 1 mg added to intravenous regional anesthesia with lidocaine 0.5% for hand surgery. Reg Anesth Pain Med 2003;28:414–417.
- Turan A, Karamanlyoglu B, Memis D, et al: Intravenous regional anesthesia using prilocaine and neostigmine. Anesth Analg 2002;95: 1419–1422.
- Sethi D, Wason R: Intravenous regional anesthesia using lidocaine and neostigmine for upper limb surgery. J Clin Anesth 2010;22(5): 324–328.
- Singh R, Bhagwat A, Bhadoria P, Kohli A: Forearm IVRA, using 0.5% lidocaine in a dose of 1.5 mg/kg with ketorolac 0.15 mg/kg for hand and wrist surgeries. Minerva Anestesiol 2010;76(2):109–114.
- Jones N, Pugh S: The addition of tenoxicam to prilocaine for intravenous regional anaesthesia. Anaesthesia 1996;51:446–448.
- Sertoz N, Kocaoglu N, Ayanoglu HO: Comparison of lornoxicam and fentanyl when added to lidocaine in intravenous regional anesthesia. Rev Bras Anestesiol 2013;63(4):311–316.
- Yurtlu S, Hanci V, Kargi E, Erdogan G, et al: The analgesic effect of dexketoprofen when added to lidocaine for intravenous regional anesthesia: A prospective, randomized, placebo-controlled study. J Int Med Res 2011;39(5):1923–1931.
- Bengtsson A, Bengtsson M, Nilsson I, et al: Effects of intravenous regional administration of methylprednisolone plus mepivacaine in rheumatoid arthritis. Scand J Rheumatol 1998;27:277–280.
- Taskaynatan M, Ozgul A, Tan A, et al: Bier block with methylprednisolone and lidocaine in CRPS Type 1: A randomized, double-blinded placebo controlled study. Reg Anesth Pain Med 2004;29:408–412.
- Varitimidis SE, Papatheodorou LK, Dailiana ZH, Poultsides L, Malizos KN: Complex regional pain syndrome type I as a consequence of trauma or surgery to upper extremity: Management with intravenous regional anesthesia, using lidocaine and methylprednisolone. J Hand Surg Eur 2011;36(9):771–777.
- Sen H, Kulachi Y, Bicerer E, Ozkan S, Dagli G, Turan A: The analgesic effect of paracetamol when added to lidocaine for intravenous regional anesthesia. Anesth Analg 2009;109(4):1327–1330.
- Ko MJ, Lee JH, Cheong SH, et al: Comparison of the effects of acetaminophen to ketorolac when added to lidocaine for intravenous regional anesthesia. Korean J Anesthesiol 2010;58(4):357–361.
- Sen S, Ugur B, Aydin ON, et al: The analgesic effect of nitroglycerin added to lidocaine on intravenous regional anesthesia. Anesth Analg 2006;102(3):916–920.
- Lauretti GR, Perez MV, Reis MP, Pereira NL: Double-blind evaluation of transdermal nitroglycerine as an adjuvant to oral morphine for cancer pain management. J Clin Anesth 2002:14:83–86).
- Lauretti GR, de Olivera R, Reis MP, et al: Transdermal nitroglycerine enhances spinal sufentanil postoperative analgesia following orthopedic surgery. Anesthesiology 1999;90:734–739.
- Ferreira SH, Lorenzette BB, Faccioli LH: block of hyperalgesia and neurogenic edema by topical application of NTG. Eur J Pharmacol 1992;217:207–209.
- Jaro K, Batra YK, Panda NB: Brachial plexus block with midazolam and bupivacaine improves analgesia. Can J Anaesth 2005;52:822–826.
- Laig N, Khan MN, Arif M, Khan S: Midazolam with bupivacaine for improving analgesia quality in brachial plexus block for upper limb surgeries. J Coll Physicians Surg Pak 2008;18:674–678.
- Nishiyama T, Tamai H, Hanaoka K: Serum and cerebrospinal fluid concentrations of midazolam after epidural administration in dogs. Anesth Analg 2003;96:159–162.
- Bhisitkul RB, Villa JE, Kocsis JD: Axonal GABA receptors are selectively present in normal and regenerated sensory fibers in rat peripheral nerves. Exp Brain Res 1987:66:659–663.
- Brown DA, Marsh S: Axonal GABA receptors in mammalian peripheral nerve trunks. Brain Res 1978;156:187–191.
- Cairns BE, Sessle BJ, Hu JW: Activation of peripheral GABA-A receptors inhibits temporomandibular joint-evoked jaw muscle activity. J Neurophysiol 1999;81:1966–1969.
- Kontinen VK, Dickenson AH: Effects of midazolam in the spinal nerve ligation model of neuropathic pain in rats. Pain 2000;85:425–431.
- Farouk S, Aly AJ: Quality of lidocaine analgesia with and without midazolam for intravenous regional anesthesia. J Anesth 2010;24(6): 864–868.
- Kashefi P, Montazeri K, Honarmand A, Safavi M, Hosseini HM: The analgesic effect of midazolam when added to lidocaine for intravenous regional anesthesia. J Res Med Sci 2011;16(9):1139–1148.
- Carlton SM, Hargett GL, Coggeshall RE: Localization and activation of glutamate receptors in ummyelinated axons of rat glabrous skin. Neurosci Lett 1995;197:25–28.
- Brau ME, Sander F, Vogel W, Hempelmann G: Blocking mechanisms of ketamine and its enantiomers in enzymatically demyelinated peripheral nerve as revealed by single-channel experiments. Anesthesiology 1997;86:394–404.
- Viscomi CM, Friend C, Parker C, Murphy T, Yarnell M: Ketamine as an adjuvant in lidocaine intravenous regional anesthesia: A randomized, double-blind, systemic control trial. Reg Anesth Pain Med 2009;34(2): 130–133.
- Georgias NK, Maidatsi PG, Kyriakidis AM, et al: Clonidine versus ketamine to prevent tourniquet pain during intravenous regional anesthesia with lidocaine. Reg Anesth Pain Med 2001;26(6):512–517.
- Livingstone J, Atkins R: Intravenous regional guanethidine block in the treatment of posttraumatic complex regional pain syndrome 1(algodystrophy) of the hand. J Bone Joint Surg Br 2002;84:380–386.
- Ramamurthy S, Hoffman J: Intravenous regional guanethidine in the treatment of reflex sympathetic dystrophy/causalgia. A randomized, double-blind study. Guanethidine Study Group. Anesth Analg 1995;81: 718–723.
- Hord A, Rooks M, Stephens B, et al: Intravenous regional bretylium and lidocaine for treatment of reflex sympathetic dystrophy: A randomized, double-blind study. Anesth Analg 1992;74:818–821.
- Lee F, Shoemaker J, McQuillan P, et al: Effects of forearm Bier block with bretylium on the hemodynamic and metabolic responses to handgrip. Am J Physiol Heart Circ 2000;279:H586–H593.
- Bader A, Concepcion M, Hurley R, et al: Comparison of lidocaine and prilocaine for intravenous regional anesthesia. Anesthesiology 1988;69: 409–412.
- Tucker G, Boas R: Pharmacokinetic aspects of intravenous anesthesia. Anesthesiology 1971;34:538–549.
- Ware R: Intravenous regional anesthesia using bupivacaine. A double blind comparison with lignocaine. Anaesthesia 1979;34:231–235.
- Smith C, Steinhaus J, Haynes C: The safety and effectiveness of intravenous regional anesthesia. South Med J 1968;61:1057–1060.
- Guay J: Adverse events associated with intravenous regional anesthesia (Bier Block): A systematic review of complications. J Clin Anesth 2009;21: 585–594.
- Bartholomew K, Sloan J: Prilocaine for Bier’s block: How safe is safe? Arch Emerg Med 1990;7:189–195.
- Sukhani R, Garcia C, Munhall R, et al: Lidocaine disposition following intravenous regional anesthesia with different deflation techniques Anesth Analg 1989;68:633–637.
- Mazze R, Dunbar R: Plasma lidocaine concentrations after caudal, lumbar epidural, axillary block and intravenous regional anesthesia. Anesthesiology 1966;27:574–579.
- Dunbar R, Mazze R: Intravenous regional anesthesia: Experience with 779 cases. Anesth Analg 1967;46:806–813.
- Davies J, Walford A: Intravenous regional anesthesia for foot surgery. Acta Anaesthesiol Scand 1986;30:145–147.
- Kim D, Shuman C, Sadr B: Intravenous regional anesthesia for outpatient foot and ankle surgery. A prospective study. Orthopedics 1993:16; 1109–1113.
- Cotev S, Robin G: Experimental studies on intravenous regional anesthesia using radioactive lignocaine. Br J Anaesth 1966;38:936–940.
- Hargrove R, Hoyle J, Parker J: Blood lidocaine levels following intravenous regional analgesia. Anaesthesia 1996;21:37–41.
- Larsen U, Hommelgaard P: Pneumatic tourniquet paralysis following intravenous regional anesthesia. Anaesthesia 1987;42:526–528.
- Shaw-Wilgis E: Observations on the effects of tourniquet ischemia. J Bone Joint Surg 1971;1104:190.
- Mabee J, Bostwick T, Burke M: Iatrogenic compartment syndrome from hypertonic saline injection in Bier block. J Emerg Med 1994;12: 473–476.
- Quigley J, Popich G, Lanz U: Compartment syndrome of the forearm and hand: A case report. Clin Orthop 1981;161:247–251.
- Hastings H 2nd, Misamore G: Compartment syndrome resulting from intravenous regional anesthesia. J Hand Surg 1987;12:559–562.
- Dominguez E: Distressing upper extremity phantom limb sensation during intravenous regional ansthesia. Reg Anesth Pain Med 2001;26:72–74.
- Luce E, Mangubat E: Loss of hand and forearm following Bier block: A case report. J Hand Surg 1983;8:280–283.
- Lee LA, Posner K, Domino KB, et al: Injuries associated with regional anesthesia in the 1980’s and 1990’s. A closed claims analysis. Anesthesiology 2004;101:143–152.
- Kennedy B, Duthie A, Parbrook G, Carr T: Intravenous regional anesthesia: An appraisal. Br Med J 1965;5440:954–957.


