John D. Rae and Paul D. W. Fettes
INTRODUCTION
In busy clinical practice, it is not uncommon that intrathecal injection of local anesthetic in an attempt to accomplish spinal anesthesia, perfectly performed, fails. Indeed, despite the reliability of the technique, the possibility of failure can never be completely eliminated. Managing a patient with an ineffective or inadequate spinal anesthetic can be challenging, and prevention is better than cure. In this section, we discuss systematically the potential mechanisms by which spinal anesthesia may fail: detail strategies to decrease the failure rate and protocols for managing an incomplete spinal anesthetic.
NYSORA Tips
- Inability to reach the subarachnoid space, errors in drug preparation or injection, the unsatisfactory spread of the injectate within the cerebrospinal fluid (CSF), ineffective drug action on neural tissue, and difficulties relating to patient expectations and psychology rather than genuine block failure.
The dense neuraxial block obtained by the administration of a spinal (intrathecal) injection of local anesthetic is widely held to be among the most reliable regional techniques. The anatomy is usually straightforward to palpate and identify, the technique for needle insertion simple and easy to teach, and the presence of CSF acts as both a definite endpoint for needling and a medium for carriage of local anesthetic within the subarachnoid space. The simplicity of the procedure was succinctly described by Labat, one of the pioneers of regional anesthesia, almost 100 years ago.
‘Two conditions are, therefore, absolutely necessary to produce spinal anesthesia: Puncture of the dura mater and subarachnoid injection of an anesthetic agent.’ – Gaston Labat, 1922
Yet, despite this simplicity, failure is not uncommon. What constitutes failure? At the most basic level, a spinal anesthetic has been attempted but the satisfactory conditions for proceeding with surgery are not obtained. Failure encompasses a spectrum that includes the total absence of any neuraxial block or the development of a partial block that is of insufficient height, duration, or quality.
In experienced hands, most anesthesiologists would expect the failure rate of spinal anesthesia to be low, probably less than 1%. A retrospective analysis of almost 5000 spinal anesthetics by Horlocker and colleagues reported inadequate anesthesia in less than 2% of cases, and failure rates of under 1% have been described. Yet, the “failed spinal” demonstrates remarkable interinstitutional variation, and in some published reports, it may be much higher. One American teaching hospital quoted a surprising failure rate of 17%, with the majority of failures deemed “avoidable.” A second institution reported a 4% failure rate—more in keeping with expectations, but nonetheless significant. Analyzing their failures, “errors of judgment” were felt to be the main causative factor. The suggestion from these reports is that with meticulous attention to detail and appropriate management, most failures of spinal anesthesia could be prevented.
Patients undergoing an operation under spinal block expect reliable surgical anesthesia, and an inadequate block will generate anxiety for both patient and clinician. In addition, by conducting this invasive procedure, such as spinal anesthesia, we subject patients to small but well-established risks. For these reasons and to improve our own clinical practice, we must strive to minimize the incidence of failure, and to do this we must understand why failure occurs. Broadly, there are three areas where shortfalls may occur: faulty technique, lack of sufficient experience to troubleshoot “on the go” and the lack of attention to detail. It is helpful to distill the procedure into five distinct phases and analyze the keys to success at each stage. In sequence, these phases are lumbar puncture, injection of local anesthetic solution, spread of solution through the CSF, drug action on neural tissue, and patient management.
MECHANISMS OF FAILURE
From the Regional Anesthesia Manual e-Course: Mechanisms of failed spinal anesthesia infographic.
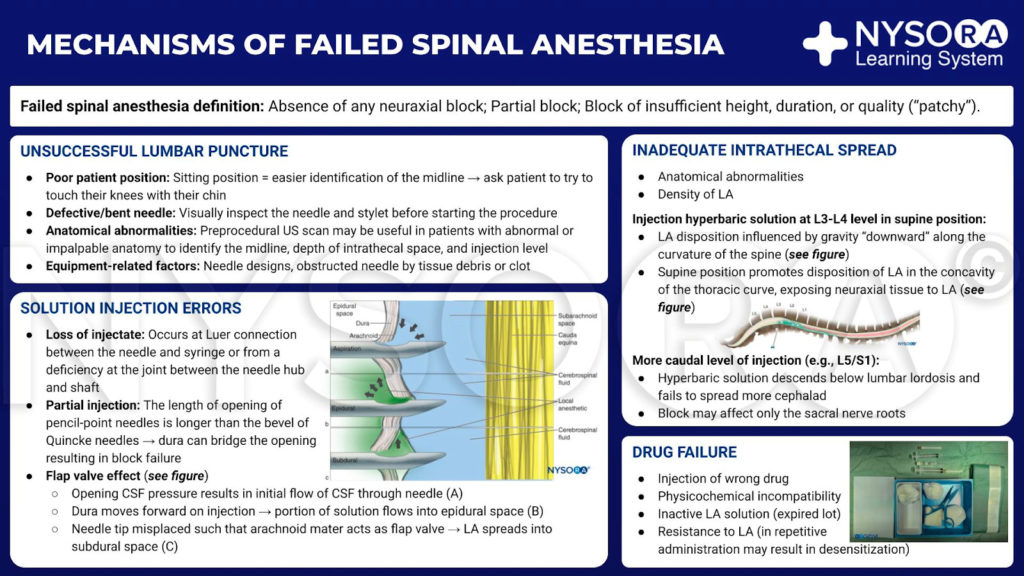
Unsuccessful Lumbar Puncture
The most evident cause of failure is an inability to successfully access the subarachnoid space. This may occur due to incorrect needling technique, poor patient positioning, anatomical abnormality, or equipment-related factors. The first two factors are operator and experience-dependent and therefore can be considered modifiable. Anatomical difficulties such as scoliosis, kyphosis, vertebral collapse, calcified ligaments, or obesity may increase the difficulty of lumbar puncture, particularly in the geriatric population, but can be overcome at least to some degree by good positioning and clinical experience. Issues with equipment may result in a lack of CSF flow despite the correct placement of the needle within the subarachnoid space. Manufacturing problems resulting in a needle with a blocked lumen are a theoretical possibility, but obstruction of the lumen by clot or tissue is more likely. For these reasons, the needle and stylet should be visually checked before starting the procedure, and to prevent blockage, the stylet should always be in place when the needle is advanced.
NYSORA Tips
- The needle and stylet should be visually checked before starting the procedure.
- Failure to obtain CSF flow despite an apparently successful needle placement(s) should raise the suspicion of needle blockage and prompt needle withdrawal and “flush test” to assure patency.
Positioning
Optimal positioning is vital to facilitate needle placement, particularly in more challenging cases. The choice of sitting or lateral position is of personal preference. Sitting may allow easier identification of the midline, particularly in the obese, and is often seen as the position of choice for “difficult” spinals; however, the reverse may also be true. In any event, the patient should be on a firm, level gurney or bed that can be adjusted in height for ergonomic ease. The patient should be asked to curl up, flexing the entire spine to maximize the space for needle insertion between spinous processes. Flexing of the hips, knees, and neck increases the effectiveness of this procedure. The presence of a skilled assistant to “coach” the patient and discourage any lateral or rotational movement is invaluable.
NYSORA Tips
- A useful position tip is to ask the patient to “try to touch their knees with their chin”.
- This typically leads to a satisfactory flexing of the spine and facilitates needle passage into epidural or subarachnoidal space.
Needle Insertion
The classically described site for lumbar puncture is in the midline between the spinous processes of the third and fourth lumbar vertebrae. This level can be estimated by drawing a line between the anterior superior iliac spines: Tuffier’s line. Evidence has shown that this landmark may be very accurate at estimating their level of needle insertion, and more detailed palpation and making sure that the presumptive L3/4 level makes sense (“reality check”). It must be emphasized that great care must be taken to insert needle below the conus medullaris, which in some individuals may be as low as the second lumbar interspace. The needle should be perpendicular to the skin in both planes and advanced with caution. Fine adjustments to the needle angle may be required if an obstruction is encountered, with a slight cephalad angulation most commonly required. Lateral alterations in needle angle may be required, especially in patients with significant scoliosis and when needle bone contact occurs at a greater depth (beyond spinal process), suggesting needle-contact with the laminae and the need to re-adjust the needle path lateral-medial. A clear knowledge of vertebral 3D-spacial anatomy and a mental image of where the needle tip is thought to lie will assist the operator in interpreting tactile feedback from the needle and guide alterations in needle angle.
In addition to the midline technique, lateral or paramedian approaches can be used. These have the advantage of avoiding ossified midline ligaments, particularly a problem in the elderly, but are more technically challenging procedures. If difficulty should be encountered, the same basic principles apply: Ensuring the patient is optimally positioned and a thorough understanding of the path of the needle and the likely obstacle may yield results.
Adjuncts
The ideal means of achieving the optimal spinal position is with a patient who is comfortable and calm, understands what is being asked of him or her, and has full trust in the anesthesia provider. Preprocedure counseling, the establishment of rapport, and a reassuring, professional manner can facilitate this during the spinal procedure. A small dose of anxiolytic medication may assist proceedings, but sedation should be titrated carefully on the basis that it is easier to give more drug than to mitigate the effects of an overdose. Care must be taken to infiltrate local anesthetic to provide effective analgesia without distorting the spinal anatomy; an initial intradermal injection will help to facilitate this. The purpose of these adjuncts is to attain the ideal position, allay patient concern, and minimize movement, thus providing the best possible conditions for lumbar puncture.
Ultrasound
The ubiquitous use of ultrasound in regional anesthesia has not been adopted as a routine neuraxial procedure but has several advantages to offer over a landmark technique. A pre-procedure scan can be useful in patients with abnormal or impalpable anatomy to identify the midline and level of injection and to assess the depth of dura from the skin. Its use in epidural techniques has been shown to increase success rates, reduce the need for multiple punctures, and improve patient comfort; it seems logical that this would translate to increased success with spinal anesthesia. Real-time scanning of needle placement for epidural insertion has been described but is not a technique in widespread use. The main obstacles to uptake of ultrasound in a neuraxial block are lack of awareness of the technique and limited training in this area, with the technique requiring knowledge of the sonoanatomy of the spine and a high degree of dexterity.
Pseudosuccessful Lumbar Puncture
Rarely, the flow of a clear fluid of non-cerebrospinal origin through the spinal needle may mimic successful lumbar puncture without this having occurred. There are two scenarios in which this may occur. “Topping up” a lumbar epidural in obstetric practice for a cesarean section may result in a reservoir of local anesthetic in the epidural space. An epidural spread of injectate has also been reported following lumbar plexus block. This may be mistaken for CSF at subsequent spinal injection.
Traditionally, bedside testing for glucose has been advocated to distinguish this fluid from CSF; however, a positive glucose test does not definitely confirm the presence of CSF as the fluid in the epidural space will rapidly equilibrate with extracellular fluid. Another, potential source of fluid mimicking CSF is the presence of a congenital arachnoid cyst. Tarlov cysts are meningeal dilatations of the posterior spinal nerve root, reportedly present in 4.5%–9% of the population. Such a cyst could result in CSF flow through the needle, but anesthetic injected may fail to result in anesthesia. The actual clinical relevance and occurrence of failed spinal anesthesia due to the “false CSF” flow from arachnoidal cysts is unknown.
Solution Injection Errors
Successful lumbar puncture is an absolute requirement for spinal anesthesia but does not preclude failure by a number of other mechanisms. To ensure a block suitable for surgery, a proper dose of local anesthetic must be calculated, prepared, and delivered to the site of action.
Dose Selection
Research into intrathecal drug spread has demonstrated that providing a dose within the therapeutic range is selected, alterations in drug dose have a relatively minor part to play in the height of spinal block achieved but are significant in governing the duration and quality of the result. The dose selected is dictated by a number of factors, including choice of local anesthetic, baricity of the solution, patient positioning, the nature of block desired, and the extent and length of planned surgery. To choose a suitable dose, the clinician must have knowledge of the clinical characteristics and pharmacokinetics of the intrathecally injected local anesthetics.
Trials of drug dosing during continuous intrathecal anesthesia have demonstrated that a satisfactory block can be achieved with relatively low anesthetic doses. Given that failure of a “single-shot” spinal is distressing for the patient and can be associated with increased morbidity (eg, the requirement for general anesthesia and airway management during cesarean section), doses used in practice are often deliberately in excess of the bare minimum required. The clinician must weigh the difficulties of managing hypotension or prolonged anesthesia versus the risk of block failure.
Studies have shown that in many circumstances, lower than commonly-used doses (ie, 5–10 mg rather than 15 mg of hyperbaric bupivacaine) can be used sufficiently to achieve effective block. This has the advantage of potentially lessening hypotension and, by increasing the speed of block regression, aiding postoperative mobility or decreasing the need for bladder catheterization. While these techniques can be successfully used in experienced hands and appropriately selected cases, the margin for error is significantly decreased. It becomes imperative that the entire volume of the syringe is successfully delivered into the subarachnoid space. Loss of even a small amount of injectate either via spillage (see the next section) or simply in the dead space of the needle and hub may result in an ineffective anesthetic.
Loss of Injectate
Leakage may occur at the Luer connection between needle and syringe or from a deficiency at the joint between needle hub and shaft. Considering the small volumes involved, even the smallest leak of the solution may result in a significant decrease in the dose of drug delivered. This pitfall can be avoided by ensuring a good connection between the syringe and needle hub and visually verifying that no leak is occurring.
Misplaced Injection
It is crucial that during the process of ensuring a leak-tight connection between needle and syringe, meticulous attention is paid to avoid accidental movement of the needle. Once the syringe is securely connected, aspiration of CSF can be used to confirm that the tip is still within the subarachnoid space. This maneuver in itself carries the potential for needle displacement, as does the injection of anesthetic solution. For this reason, it is imperative that the operator secure the needle position prior to any further manipulation. This can be achieved by stabilizing the dorsum of one hand against the patient’s back and anchoring the hub of the needle between thumb and forefinger while the other hand has control of the syringe. Many anesthesiologists would advocate aspirating CSF postinjection to ensure the needle position has not moved during the process. Although there is no evidence to suggest this reduces the failure rate, it may at least alert the anesthetist to the possibility that not all of the drug has reached its intended destination.
NYSORA Tips
- Gentle aspiration of 0.5-1 ml before injection to assure CSF retrieval from the subarachnoidal space.
- Gentle aspiration of 0.5-1ml at the end of the spinal injection can be done to assure that the needle tip stayed in the subarachnoidal space throughout the injection process.
- The aspirated 0.5ml-1ml is then re-injected and the needle is withdrawn.
Stabilization of the needle during injection is important with all types of spinal needles but particularly so with pencil-point” needles commonly in use. In these needles, the opening through which injectate emerges is some distance proximal to the tip; therefore, minimal posterior displacement of the needle can result in this opening being outside the subarachnoid space and subsequent block failure. As the length of the opening of pencil-point needles is significantly longer than the bevel of a Quincke needle, it is also possible for the dura to bridge this opening (Figure 1).
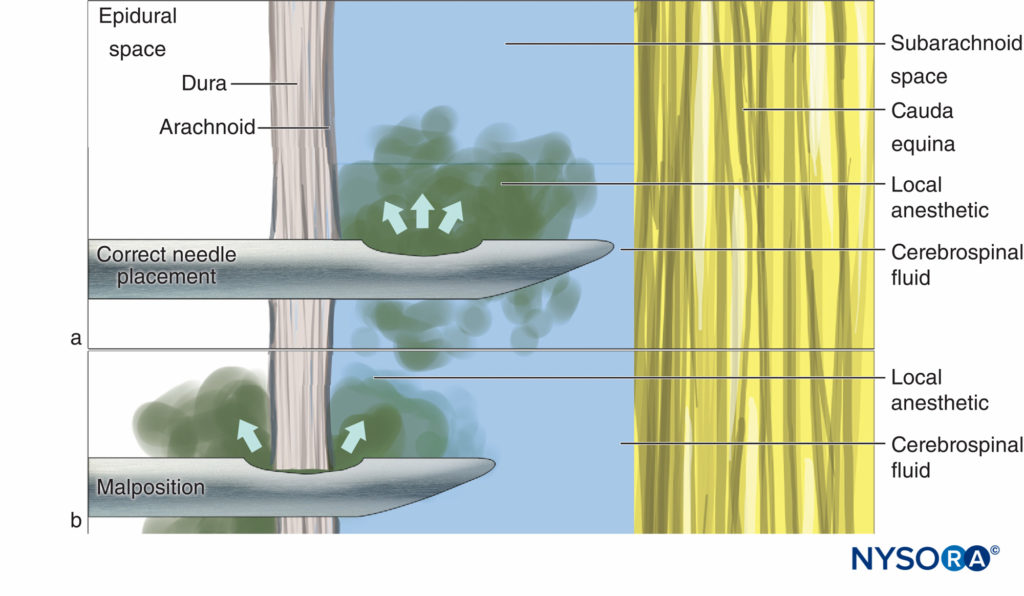
Figure 1. Correct needle placement with (A) all drug delivered to CSF and (B) malposition where some of the drug is lost into the epidural space.
This problem may be compounded by the dura mater working as a flap valve. The opening CSF pressure results in an initial successful flow of CSF through the needle (Figure 2a), but on injection, the dura moves forward and a portion of the solution flows into the epidural space (Figure 2b). As with leakage between the needle and syringe, given the small volumes involved, loss of even a small amount of injectate may substantially influence the quality of the block.
If the needle tip is misplaced such that the arachnoid mater acts as the flap valve, local anesthetic will spread into the subdural space (Figure 2c). Subdural block is well recognized as a potential side effect of epidural anesthesia (where it may result in a more extensive, prolonged, or unpredictable effect because of the larger volume of local anesthetic used for epidural anesthesia), but it has also been recorded as a consequence of attempted spinal anesthesia. Subdural injection is seen relatively frequently during myelography and its occurrence in daily clinical practice of anesthesiology is likely underestimated. Due to the initial flow of CSF and minute distances between the layers of the dura, these subtle misplacements are difficult to identify or eliminate. One suggested solution, once CSF has been successfully located, is to rotate the needle a full 360° before aspirating. Theoretically, this may lessen the chance of the dura layers catching on the opening of the needle.
Due to the initial flow of CSF and minute distances between the layers of the dura, these subtle misplacements are difficult to identify or eliminate. One suggested solution, once CSF has been successfully located, is to rotate the needle a full 360° before aspirating. Theoretically, this may lessen the chance of the dura layers catching on the opening of the needle.
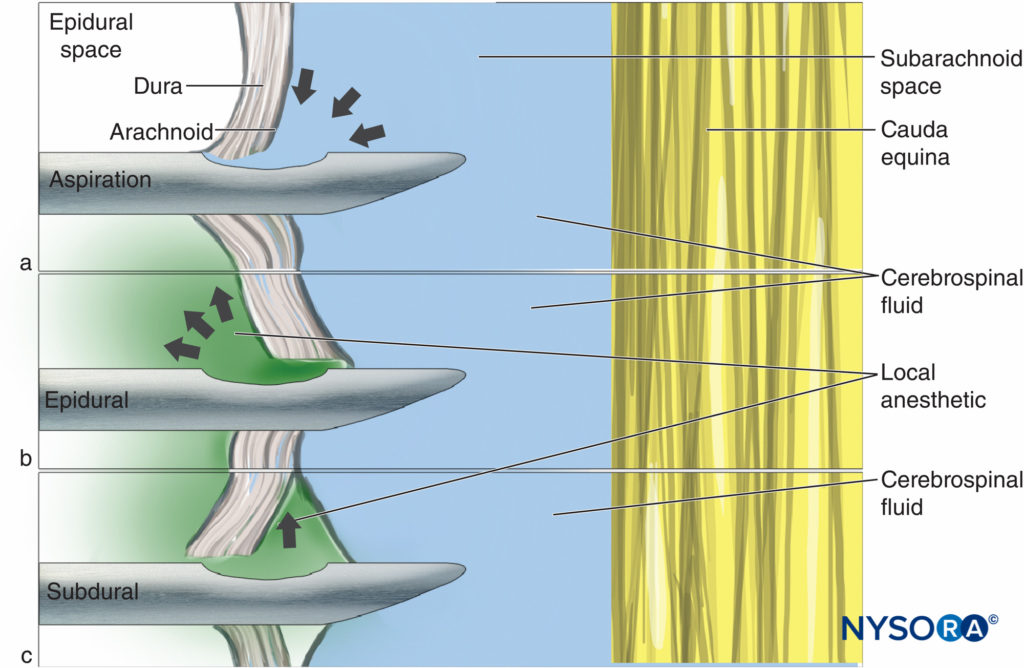
Figure 2. The flap valve effect: (A) CSF is aspirated but on injection the meningeal layers move, resulting in (B) epidural or (C) subdural injection of drug.
Inadequate Intrathecal Spread
Even when the entire volume of injectate is successfully delivered to the intrathecal space, the spread of solution within the CSF can be somewhat unpredictable. The practitioner must have an understanding of the common factors affecting intrathecal spread and the degree to which they may be manipulated.
Anatomical Abnormality
Dispersion of injectate within the CSF is dictated by the complex interaction between the anatomy of the spinal canal, the physical properties of the solution, and gravity.
The normal kyphotic and lordotic curvatures of the vertebral column are important anatomical factors affecting the spread of solution, and the presence of anatomical abnormality, including scoliosis, will alter this. Preoperative examination of the patient may allow identification of such anatomical abnormalities. The actual effect of anatomical deviations on the block quality is unpredictable; variability in the block height is probably more common than block failure.
To achieve a uniform symmetrical block, the local anesthetic should diffuse freely within CSF, without anatomical barriers. For instance, it is also possible for the ligaments that support the spinal cord to form a barrier to the spread of anesthetic within the subarachnoid space. By acting as septae, these anomalies, although uncommon, may cause a unilateral block, or limited cephalad spread. Other examples of spinal pathologies that may impede the spread or effect of injectate include spinal stenosis and adhesions from spinal surgery or from previous administration of intrathecal chemotherapy.
In one case report, two occurrences of failed spinal anesthesia in the same patient were investigated with magnetic resonance imaging (MRI) and revealed larger-than-normal CSF volume in the dural sac below the termination of the cord. The volume of CSF within the subarachnoid space has since been shown to be an important cause of the interindividual variation in the degree of cephalad spread of anesthetic. MRI studies found a negative correlation between lumbosacral CSF volume and peak sensory block height. A similar picture may be encountered in patients with connective tissue diseases, including Marfan syndrome, who may develop dural ectasia, a pathological enlargement of the dura.
Density of the Local Anesthetic Solution
Density of the injected solution relative to the CSF is another important determinant of intrathecal spread. “Plain” bupivacaine is commonly regarded as isobaric, although it is in fact slightly hypobaric compared to CSF at 37°C. Its spread through CSF is by local turbulent currents and diffusion, which results in a block of somewhat unpredictable spread (in some cases no higher than the second lumbar dermatome) with a relatively slow onset to maximal block height. However, it tends to give reliable anesthesia to the lower extremities with limited spread to the thoracic level. The combination of the slow onset and lower block height results in less risk of cardiovascular instability.
The use of hyperbaric solutions to influence the spread within CSF was described more than 100 years ago by Barker, an early proponent of neuraxial block in the United Kingdom. This is typically achieved by the addition of dextrose to accomplish a density higher than that of the CSF. Commercial preparations of hyperbaric local anesthetic contain up to 8% glucose, although even preparations containing 1% glucose will result in a predictable block.
Following the injection of a hyperbaric solution at the level of L3/L4 in a supine subject, this solution travels predominantly by bulk flow under the influence of gravity “downward” along the curvature of the spine. It naturally moves to the concavity of the thoracic curve (Figure 3), exposing the neuraxial tissue to local anesthetic. If, however, the level of injection is more caudal, the hyperbaric solution may descend below the lumbar lordosis and fail to spread more cephalad (Figure 4), particularly if the injection is performed while sitting and the patient is not quickly placed supine.
This manifests clinically as a block of only the sacral nerve roots, as reported with a caudally placed spinal catheters. In some circumstances, a “saddle” block is intentionally sought.
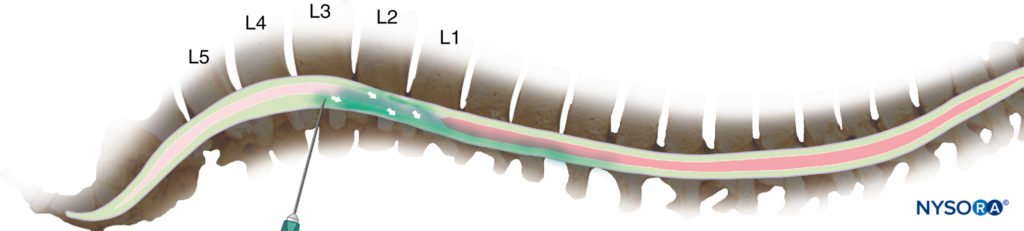
Figure 3. Injection at the second or third lumbar interspace will normally result in a significant fraction of the drug spreading cranially from the point of injection (but too high an injection risks inadvertent damage to the spinal cord).
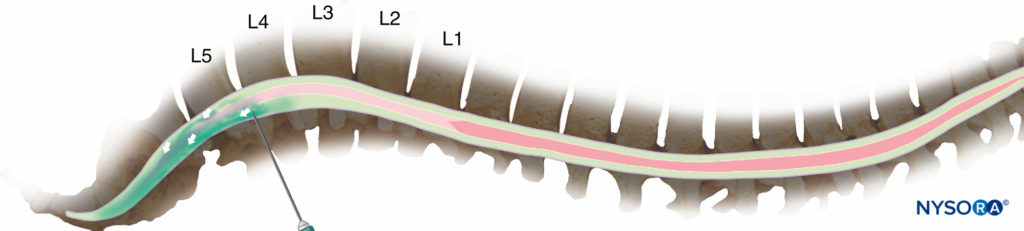
Figure 4. Injection at the fourth interspace or lower reduces the risk of cord damage, but it may result in predominantly caudal spread of the drug and an inadequate block for surgery.
Drug Failure
Assuming successful lumbar puncture, adequate drug delivery, and normal anatomy, the final possible cause of an ineffective spinal anesthetic is a failure of drug to exhibit block on the neural tissue.
Injection of Incorrect Drug
Anesthetics for intrathecal use are commonly supplied in ampoules of aqueous solution, ready for use. Preparations of local anesthetics specifically made for use in spinal anesthesia minimize the opportunity for errors during drug preparation. Nevertheless, the presence of other clear solutions on the spinal tray gives the potential for confusion and inadvertent injection of the wrong drug, with consequent block failure or neurotoxicity. Local anesthetic used for skin preparation is the common culprit; chlorhexidine solution may also be present, although recent guidelines advise separating this from the procedural area due to the risk of contamination and possible adhesive arachnoiditis. The relatively high incidence of so-called syringe swaps in general anesthetic practice has led to almost-universal use of syringe labels. The potential for syringe swaps can be further decreased by meticulous preparation, reducing the number of unnecessary drug ampoules on the tray, and adopting a consistent system for drawing up solutions—for example, always using a certain size of syringe for each particular drug.
Physicochemical Incompatibility
The common practice of utilizing adjuvants to local anesthetic in spinal injections necessitates the mixing of solutions, introducing the possibility of a chemical reaction, potentially reducing efficacy. Clinical experience has shown that the commonly used opioids appear compatible with local anesthetics, but there are few hard data to support this and even fewer for mixing with other adjuncts, such as midazolam, clonidine, or ketamine.
The mixing of three substances for intrathecal injection, not uncommon in today’s practice, must further raise the opportunity for chemical interaction. This reaction could result in the formation of a precipitate, which would be obvious within the syringe, but less evident would be a reduction in pH of the solution. This could decrease the fraction of un-ionized drug within the injectate, thereby reducing the mass of local anesthetic capable of diffusing into neural tissue and available for neural block. One example of this effect may be illustrated by a case report of a higher failure rate following addition of vasoconstrictor to the local anesthetic solution.
Inactive Local Anesthetic Solution
Amide local anesthetics such as bupivacaine, ropivacaine, and lidocaine are stable compounds, which are heat sterilized in solution and may be stored for years without significant impact on their efficacy. Regardless, several cases of spinal anesthetic failure thought to be related to local anesthetic inactivity have been published. Local anesthetic inactivity may be more common with ester-type anesthetic agents, which are less chemically stable and over time may undergo hydrolysis, degrading their effectiveness.
Local Anesthetic Resistance
Several cases of failed spinal anesthesia have been attributed to local anesthetic resistance. These authors postulated that the causes were altered activity of local anesthetic at the sodium channel as a result of mutation of the sodium channel. This altered activity, however, has not been demonstrated at cellular level, nor mutations found in the patients described. Mutations of sodium channels (channelopathies) do occur but they are rare and are associated with significant neurological disease.
Specifically, Nav1.1 mutations are associated with intractable epilepsy and Nav1.7 mutations are associated with chronic pain. To our knowledge mutations of the sodium channel, however, do not exist in asymptomatic individuals.
Failure of Subsequent Management
A well-executed spinal anesthetic typically results in reliable anesthesia. However, perioperative management of a patient under spinal anesthesia is just as important for success. For instance, the patient may perceive unblocked sensations of movement, pressure, or traction experienced intraoperatively to be painful or uncomfortable experiences. This likelihood is heightened by awareness of the clinical environment and the patient’s underlying views, fears, and expectations of the hospital setting, potentiated by the stress of undergoing a surgical procedure. Failure to address these psychological aspects of spinal anesthesia can lead to anxiety, distress, and the need to convert an adequate spinal anesthetic to general anesthesia.
Even for the most composed patients, lying supine in operating theater completely awake while undergoing an operative procedure may be unnatural and anxiety-provoking experience. The surgery is performed may require the patient to lie in an awkward position for a considerable time (eg, during hip arthroplasty). Operating tables are primarily designed to provide good surgical conditions and are often narrow and uncomfortable. Manipulation of the intra-abdominal viscera may result in activation of unblocked parasympathetic nerves and the experience of unpleasant sensations. Patient selection and expectation management are also important to success. Adequate pre-procedure patient counseling, positive suggestion, and a supportive, reassuring manner intraoperatively are all essential ingredients to success. A judicious use of sedative adjunctive medications such as benzodiazepines and intraoperative infusions of propofol and remifentanil can further contribute to the patient acceptance of the spinal anesthesia, satisfaction and improve overall perioperative experience. With appropriate monitoring and cautious dosing, there are few situations outside obstetric anesthesia for which sedation would be contraindicated. Some patients can also benefit from or prefer alternative distraction techniques, such as listening to music.
Testing the Block
There are wide variations in practice regarding the assessment of the adequacy of a spinal anesthetic, but some form of test is commonly carried out, particularly in obstetric anesthesia. Common techniques include testing for motor effect by asking the patient to move his or her legs and then testing the different sensory modalities, such as light touch, cold, or pinprick sensation. Carried out well, this can be a confidence-building procedure; however, it also may instill doubt in the patient about the quality of the block or the anesthetist. If testing is commenced prematurely, without allowing adequate time for the spinal anesthesia to “set in”, the patient may assume that the anesthetic failed and become anxious. For similar reasons, it is recommended that testing should start in the lower dermatomes, where the onset of the block will be the most rapid. By moving cephalad from this point, the development of anesthesia can be demonstrated and anxiety prevented.
It should be noted that achievement of a block height adequate for surgery does not guarantee that the quality of the block is sufficient for surgery, particularly when pinprick or perception to cold are used as testing modalities. Provided the patient is not deeply sedated, block quality can be assessed by asking the operator to covertly apply a painful stimulus prior to incision without warning the patient. This can be achieved by pinching the skin with surgical forceps out of the patient’s line of sight.
Combined Spinal-epidural and Catheter Techniques
Most commonly, intrathecal anesthetic techniques utilize a one-off injection that, as discussed, may not always provide satisfactory surgical anesthesia. The placement of an intrathecal catheter or a combined spinal-epidural (CSE) technique can be useful to extend the height of the block or prolong its duration, which adds versatility. The presence of an accurately placed catheter will allow an inadequate block to be supplemented or infusion of local anesthetic can be used to provide continuous analgesia. Placement and maintenance of these catheters, however, requires a higher level of knowledge and technical expertise on the part of the operator. The subarachnoidal injection during CSE requires a small volume of local anesthetic thus, the discussed issues whereby a proportion of the injectate is lost via leakage or dead-space remain pertinent. Use of intrathecal catheters has declined as of late because of the increased potential for infection with the introduction of a catheter into the CSF and because of case reports of arachnoiditis resulting from the concentrated effect of local anesthetic on nerve roots.
Insertion can be technically challenging, and leaving an excessive length of catheter in situ may result in local anesthetic pooling in the caudal portion of the dural sac. Finally, the relatively uncommon use of the spinal catheters may be related to the potential risk of error whereby intrathecal catheter could be confused with an epidural catheter, which is much more commonly used in clinical practice. This may lead to an error in “topping up” and overdose with consequent development of high spinal anesthesia.
FAILED SPINAL ANESTHESIA
Despite meticulous technique and local anesthetic and dose selection, subarachnoidal injection carries a small risk of failed spinal anesthesia. Moreover, even when the level of spinal block appears to be adequate during testing, spinal anesthesia can fail to provide adequate operating conditions intraoperatively. To the patient, this may be a source of pain, anxiety, and psychological trauma and to the anesthesia provider one of stress, complaints, and potential medicolegal sequelae. For that reason, the possibility of block failure should be discussed with all patients as part of the consent process to ensure that both parties are cognizant of the possibility of this occurring and the steps to be taken if it does. If the duration or extent of the planned procedure is unclear, an alternative technique should be considered. In patients with severe comorbidities, respiratory compromise or a difficult airway, the traditional conversion to general anesthesia may be hazardous. For these reasons, prevention is better than cure, and meticulous attention to detail is crucial.
Management of the Failed Spinal Anesthesia
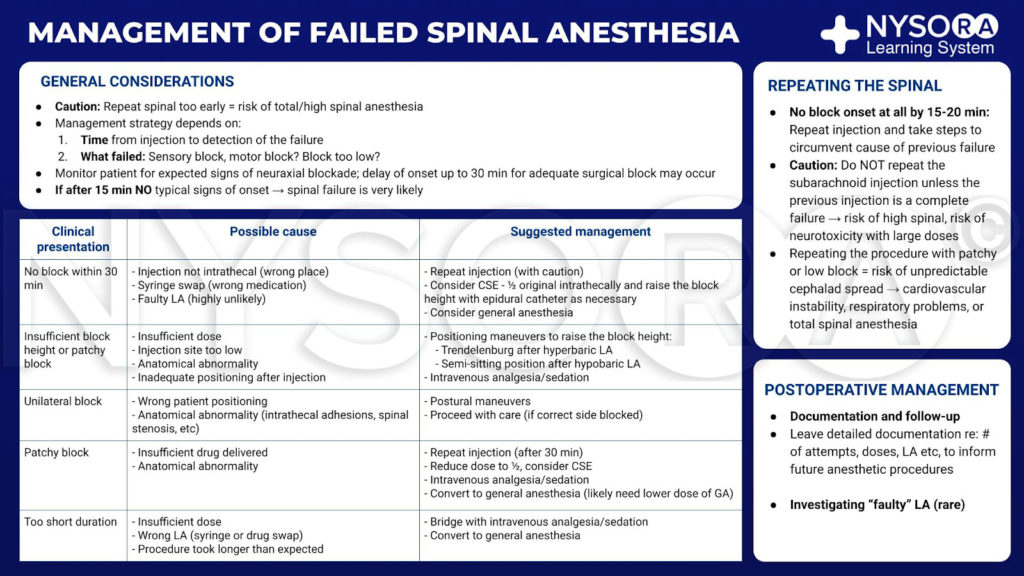
From the Regional Anesthesia Manual e-Course: Management of failed spinal anesthesia infographic.
The strategy for managing an inadequate spinal anesthetic is dictated by two factors: the time at which failure is detected and the nature of the failure. After the subarachnoidal injection, the anesthesia provider should closely monitor the patient for the expected signs of neuraxial block. The consequences of autonomic nervous system block, such as decrease of blood pressure with or without the presence of compensatory tachycardia provide an early clue of onset of spinal anesthesia even without any formal testing. Lack of autonomic response or slower-than-expected development of motor or sensory block should alert the clinician to potential of inadequate or failed spinal anesthesia. Although usually swift, the development of anesthesia can be more gradual in some patients, and additional observation time should be contemplated before starting surgery or assuming failure. If 15 minutes have lapsed since intrathecal injection and the spinal block does not follow a typical onset pattern, anticipated, it is highly likely that the spinal anesthetic will be inadequate for surgery and additional anesthetic interventions will be required. The possible flaws in the block, their likely origins, and suggested solutions are outlined (Table 1):
1. No block: An incorrect solution was injected, the solution was injected into an incorrect anatomical location, or the local anesthetic is defective. The options are to repeat the process or administer a general anesthetic. If repeating the spinal injection, sufficient time (20 minutes) must be allowed to pass to ensure that there is truly no block developing. If a second injection is performed after a successful but slowly developing first procedure, a “total spinal” may result.
2. Spinal block of insufficient height: Potential causes are that the local anesthetic has been lost during injection (eg, leakage at needle-syringe connection), lumbar puncture was in too low a lumbar interspace, or an anatomical barrier is preventing diffusion of anesthetic. Manipulating posture and utilizing gravity may overcome these difficulties. If hyperbaric formulation was used, the patient should be placed in Trendelenburg position with the hips and knees flexed. This will flatten the lumbar lordosis, allowing injectate to travel cephalad. Change in position after injection of isobaric bupivacaine is unlikely to be successful.
3. Unilateral block: The most common problem is patient position, although an anatomical barrier to spread formed by the longitudinal ligaments could lead to unilateral spinal anesthesia. Bilateral spread of the block can be encouraged by moving the patient so that the unblocked side is downward (although again positional change is less likely to be helpful when plain solutions have been used). A unilateral block should be sufficient for ipsilateral lower limb surgery, but the surgeon must be warned that the other limb is not anesthetized.
4. Patchy block: This describes a block that appears to have spread adequately but is of inconsistent quality with variable sensory and motor block. There are multiple possible explanations but most common is the administration of an insufficient dose of anesthetic drug, either due to underdosing or solution not reaching the target. Additional sedation and opiate analgesia may prove successful particularly if anxiety is a prominent factor. Alternatively, conversion to general anesthesia may be required.
5. Inadequate duration: The most likely culprit is the delivery of an insufficient dose of local anesthetic. Another possibility is a “syringe swap” by which a short-acting agent such as lidocaine is injected instead of the intended bupivacaine. Last, the procedure may have lasted longer than anticipated. As previously stated, the only realistic solutions are additional intravenous analgesia, sedation, or general anesthesia.
TABLE 1. Mechanisms of failure and suggested management.
| Clinical Presentation | Possible Cause | Suggested Management |
|---|---|---|
| No block | Injection not into CSF Syringe swap Faulty local anesthetic | Repeat injection (with caution) General anesthesia |
| Insufficient block height or density | Insufficient drug delivered Injection site too low Anatomical abnormality | Postural maneuvers Intravenous analgesia/sedation |
| Unilateral block | Patient positioning Anatomical abnormality | Postural maneuvers Proceed with care (if correct side blocked) |
| Patchy block | Insufficient drug delivered Anatomical abnormality | Repeat injection (with caution) Intravenous analgesia/sedation General anesthesia |
| Inadequate duration | Insufficient drug delivered Syringe swap Lengthy procedure | Intravenous analgesia/sedation General anesthesiaallowed |
In all these scenarios, judicious use of analgesia and sedation will prove invaluable in the management of unsatisfactory block. Intravenous infusions of propofol and remifentanil can be used at low concentrations to good effect. Postoperative documentation of events and patient follow-up are important
Repeating the Block
If no appreciable block is seen at 15–20 minutes, then the most logical step is to repeat the injection, taking steps to eliminate the proposed cause of previous failure. Unless the previous injection is a complete failure, repeating subarachnoidal injection should not be done routinely. This is because high concentrations of local anesthetic intrathecally can be neurotoxic, and repeating the procedure may lead to such a concentration, particularly if there is an anatomical barrier preventing spread. Lesions of the cauda equina have been reported following multiple injections via an indwelling an intrathecal catheter.
Repeating the procedure, particularly in the context of a patchy or low block, can lead to unpredictable extensive cephalad spread with the potential for cardiovascular instability, respiratory embarrassment, or total spinal anesthesia.
Moreover, if block failure is secondary to anatomical factors, then repeat injection is unlikely to produce a more favorable result. A unilateral block thought secondary to a longitudinal anatomical barrier may tempt the anesthetist into performing a second injection on the opposite side, but there is no guarantee that this will not follow the path of the first attempt. An obstruction to intrathecal spread may also distort the epidural space, so epidural anesthesia may prove no more successful.
Postoperative Management
Documentation and Follow-up
At the postoperative visit, the patient should be given a full explanation of events. A detailed account of proceedings should be documented in the medical record to inform future anesthetic procedures. Rarely, unusual patterns of failure may signal the presence of serious neurological pathology, and if there are other signs or symptoms, then neurology consultation is advised. If a patient has experienced failure of spinal anesthesia on more than one occasion, MRI of the spine may be used to exclude or delineate abnormal anatomy.
Investigating “Faulty” Local Anesthetic
Although spinal anesthetic failure is an uncommon occurrence, certain circumstances may lead the anesthetist to closely scrutinize the local anesthetic agent. Lack of effect following a technically undemanding procedure or multiple failures within the same theater or department raises the possibility of a faulty batch of local anesthetic. Hyperbaric bupivacaine is the most commonly reported culprit, most likely due to its prevalence in current practice. Amide local anesthetics are chemically stable compounds that undergo heat sterilization as part of normal preparation. In addition to this, modern quality control procedures mean that drug failure is a rare occurrence, but if all other factors are eliminated, it must be considered. If the anesthetic used in the procedure has been retained, some authorities advocate infiltrating the skin to test its efficacy. Corroboration with reports from colleagues, pharmacy, and other hospitals will help to establish if others have had similar problems, although concerns of anesthetic failure have rarely been borne out by case reports.
SUMMARY
With proper technique, training, and meticulous attention to detail, failure rate of spinal anesthesia should be less than 1%. Good communication and appropriate management can mitigate against many of the common difficulties. Even best practice cannot completely eliminate the possibility of failure; thus, the careful assessment of the adequacy of the spinal block and management strategy should the failure occur intraoperatively should always be contemplated.
Read more about Spinal Anesthesia at this link on NYSORA: Spinal Anesthesia
NEW: For in-depth lessons, the newest illustrations, animations, infographics and clinical videos on everything regional anesthesia from A to Z, visit the Regional Anesthesia Manual e-course on NYSORA’s Learning System (LMS). Use the Compendium to take notes of additional important information using the built-in note-taking tool to make your own scripts and never lose them. You also get access to the NYSORA’s activity feed, a community where you can exchange information with your colleagues worldwide. Here’s a sample of the information in the Compendium, which is an always-updated time-saver for special clinical cases, and great for exams.
REGISTER FOR YOUR FREE 1 WEEK TRIAL to NYSORA REGIONAL ANESTHESIA MANUAL E-COURSE and get:
– Access to Step-by-step techniques for spinal, epidural, and nerve block procedures and 60 nerve blocks
NYSORA’s new illustrations, animations, and clinical videos
Access on any device via the desktop platform and mobile app
Real-time updates
– Latest management protocols
– Access to the community with real-time practice tips and knowledge sharing
– Infographics for exam preparation (e.g. EDRA)
REFERENCES
- Fettes PDW, Jansson JR, Wildsmith JAW: Failed spinal anaesthesia: Mechanisms, management, and prevention. Br J Anaesth 2009; 102:739–48.
- Labat G: Regional Anesthesia: Its Technic and Clinical Application. Saunders, 1922.
- Horlocker TT, McGregor DG, Matsushige DK, Schroeder DR, Besse JA: A retrospective review of 4767 consecutive spinal anesthetics: Central nervous system complications. Anesth Analg 1997;84:578–584.
- Harten JM, Boyne I, Hannah P, Varveris D, Brown A: Effects of height and weight adjusted dose of local anaesthetic for spinal anaesthesia for elective caesarean section. Anaesthesia 2005;60:348–353.
- Levy JH, Islas JA, Ghia JN, Turnbull C: A retrospective study of the incidence and causes of failed spinal anesthetics in a university hospital. Anesth Analg 1985;64:705–710.
- Munhall RJ, Sukhani R, Winnie AP: Incidence and etiology of failed spinal anesthetics in a university hospital. Anesth Analg 1988;67:843–848.
- Charlton JE. Managing the block. In Wildsmith JAW, Armitage EN, McClure JH (eds): Principles and Practice of Regional Anaesthesia, 3rd ed. Churchill Livingstone, 2003; 91-–109.
- Rubin AP. Spinal anaesthesia. In Wildsmith JAW, Armitage EN, McClure JH, eds: Principles and Practice of Regional Anaesthesia, 3rd ed: Churchill Livingstone, 2003: 125–138.
- Broadbent CR, Maxwell WB, Ferrie R, Wilson DJ, Gawne-Cain M, Russell R: Ability of anaesthetists to identify a marked lumbar interspace. Anaesthesia 2000;55:1106–1126.
- Kwok WH, Karmakar M: Spinal and Epidural Block. New York School of Regional Anesthesia. September 20, 2013. https://www.nysora.com/techniques/ neuraxial-and-perineuraxial-techniques/ultrasound-guided/3276-spinal-andepidural-block.html.
- Lang SA, Prusinkiewicz C, Tsui BCH: Failed spinal anesthesia after a psoas compartment block. Can J Anesth 2005;52:74–78.
- Stace JD, Gaylard DG: Failed spinal anaesthesia. Anaesthesia 1996;51: 892–893.
- Acosta L, Quinones-Hinojosa A, Schmidt MH, Weinstein PR: Diagnosis and management of sacral Tarlov cysts. Case report and review of the literature. Neurosurg Focus 2003;15:E15.
- Lee JA, Atkinson RS: Sir Robert Macintosh’s Lumbar Puncture and Spinal Analgesia. Churchill Livingstone, 1978.
- Hocking G, Wildsmith JAW: Intrathecal drug spread. Br J Anaesth 2004; 93:568–578.
- Hurley RJ, Lambert DH: Continuous spinal anesthesia with a microcatheter technique: Preliminary experience. Anesth Analg 1990;70: 97–102.
- Atallah MM, Shorrab AA, Abdel Mageed YM, Demian AD: Low-dose bupivacaine spinal anaesthesia for percutaneous nephrolithotomy: The suitability and impact of adding intrathecal fentanyl. Acta Anaesthesiol Scand 2006;50:798–803.
- Ben-David B, Levin H, Tarhi D: An unusual explanation for a failed spinal. Can J Anaesth 1995;45:448–449.
- Tarkkila PJ: Incidence and causes of failed spinal anesthetics in a university hospital: A prospective study. Reg Anesth 1991;16:48–51.
- Crone W: Failed spinal anesthesia with the Sprotte needle. Anesthesiology 1991;75:717–718.
- Thomson GE, McMahon D: Spinal needle manufacture, design and use. Bailliere’s Clin Anaesthesiol 1993;7:817–830.
- Collier CB: Accidental subdural injection during attempted lumbar epidural block may present as a failed or inadequate block: Radiological evidence. Reg Anesth Pain Med 2004;29:45–51.
- Singh B, Sharma P: Subdural block complicating spinal anesthesia. Anesth Analg 2002;94:1007–1009.
- Gershon RY: Local anesthesia for caesarean section with a subdural catheter. Can J Anaesth 1996;43:1068–1071.
- Jones MD, Newton TH: Inadvertent extra-arachnoid injection in myelography. Radiology 1963;80:818–822.
- Bier A: Versuche ueber Cocainiserung des Rueckenmarkes. Dtsche Z Chir 1899;51:361–369.
- Armstrong PJ: Unilateral subarachnoid anaesthesia. Anaesthesia 1989;44: 918–919.
- Adler R, Lenz G: Neurological complaints after unsuccessful spinal anaesthesia as a manifestation of incipient syringomyelia. Eur J Anaesthesiol 1998;15:105–105.
- Westphal M, Gotz T, Booke M: Failed spinal anaesthesia after intrathecal chemotherapy. Eur J Anaesthesiol 2005;22:235–236.
- Hirabayashi Y, Fukuda H, Saitoh K, Inoue S, Mitsuhata H, Shimizu R: Failed spinal anaesthesia: Cause identified by MRI. Can J Anaesth 1996;43:1072–1075.
- Carpenter RL, Hogan QH, Liu SS, Crane B, Moore J: Lumbosacral cerebrospinal fluid volume is the primary determinant of sensory block extent and duration during spinal anesthesia. Anesthesiology 1998;89: 24–29.
- Lacassie HJ, Millar S, Leithe LG, et al: Dural ectasia: A likely cause of inadequate spinal anaesthesia in two parturients with Marfan’s syndrome. Br J Anaesth 2005;94:500–504.
- Logan ML, McClure JH, Wildsmith JAW: Plain bupivacaine—An unpredictable spinal anaesthetic agent. Br J Anaesth 1986;58:292–296.
- Wildsmith JAW, McClure JH, Brown DT, Scott DB: Effects of posture on the spread of isobaric and hyperbaric amethocaine. Br J Anaesth 1981;53: 273–278.
- Barker AE: A report on clinical experiences with spinal analgesia in 100 cases. BMJ 1907;i:665–674.
- Morrison LMM, McClure JH, Wildsmith JAW: Clinical evaluation of a spinal catheter technique in femoro-popliteal graft surgery. Anaesthesia 1991;46:576–578.
- Manchikanti L, Hadley C, Markwell SJ, Colliver JA: A retrospective analysis of failed spinal anesthetic attempts in a community hospital. Anesth Analg 1987;66:363–366.
- Bouchard P, Caillet JB, Monnet F, Banssillon V: [Spinal anesthesia and multiple sclerosis]. Ann Fr Anesth Reanim 1984;3(3):194–198.
- Levesque P, Marsepoil T, Ho P, Venutolo F, Lesouef JM: [Multiple sclerosis disclosed by spinal anesthesia]. Ann Fr Anesth Reanim 1988; 7(1):68–70.
- Vadalouca A, Moka E, Sykiotis C: Combined spinal-epidural technique for total hysterectomy in a patient with advanced, progressive multiple sclerosis. Reg Anesth Pain Med 2002;27(5):540–541.
- Horlocker TT, Wedel DJ: Regional anesthesia in the immunocompromised patient. Reg Anesth Pain Med 2006;31(4):334–345.
- Chin KJ, Macfarlane AJ, Chan V, Brull R: The use of ultrasound to facilitate spinal anesthesia in a patient with previous lumbar laminectomy and fusion: A case report. J Clin Ultrasound 2009;37(8):482–485.
- Prasad GA, Tumber PS, Lupu CM: Ultrasound-guided spinal anesthesia. Can J Anaesth 2008;55(10):716–717.
- Costello JF, Balki M: Cesarean delivery under ultrasound-guided spinal anesthesia [corrected] in a parturient with poliomyelitis and Harrington instrumentation. Can J Anaesth 2008;55(9):606–611.
- Douglas MJ, Swenerton JE: Epidural anesthesia in three parturients with lumbar tattoos: A review of possible implications. Can J Anaesth 2002; 49(10):1057–1060.
- Mavropoulos A, Camann W: Use of a lumbar tattoo to aid spinal anesthesia for cesarean delivery. Int J Obstet Anesth 2009;18(1):98–99.
- Balki M, Carvalho JC: Intraoperative nausea and vomiting during cesarean section under regional anesthesia. Int J Obstet Anesth 2005; 14(3):230–241.
- Bier A: On a new method of local anesthesia. Muench Med Wschir 1909; 56:589.Mishriky BM, Habib AS: Metoclopramide for nausea and vomiting prophylaxis during and after cesarean delivery: A systematic review and meta-analysis. Br J Anaesth 2012;108(3):374–383.
- Carpenter RL, Caplan RA, Brown DL, Stephenson C, Wu R: Incidence and risk factors for side effects of spinal anesthesia. Anesthesiology 1992;76(6):906–916.