Marina Gitman, Michael Fettiplace, and Guy Weinberg
INTRODUCTION
The introduction of cocaine as the first local anesthetic (LA) in the late nineteenth century was soon accompanied by reports of its systemic toxicity. The symptoms of toxicity were frequently described as seizures or respiratory failure, but some cases also included accounts of adverse cardiac effects. Often lethal, local anesthetic systemic toxicity (LAST) was treated with caffeine, ammonia, or even hypodermic ether. The development of procaine in 1904 did not solve the problem of systemic toxicity, and the Committee for the Study of Toxic Effects of Local Anesthetics published a report of 43 fatal cases linked to the use of LAs. Identification of contributing factors, emphasis on prevention and the almost-complete elimination of cocaine from clinical practice helped decrease the incidence of LAST for nearly 50 years.
However, the synthesis of long-acting, lipid-soluble LAs such as bupivacaine in the late 1950s with subsequent associated reports of LAST resulted in return of lethal LAST. These included multiple cases of fetal demise associated with paracervical nerve blocks, ventricular fibrillation after an interscalene nerve block, and what is considered to be the “sentinel” case of a young man who suffered a cardiac arrest after a caudal nerve block. The following several decades were plagued by isolated accounts describing a common problem: cardiovascular (CV) demise associated with LAST that was particularly resistant to available resuscitative measures, such as vasopressors (eg, epinephrine) and defibrillation.
MECHANISM OF LOCAL ANESTHETIC TOXICITY
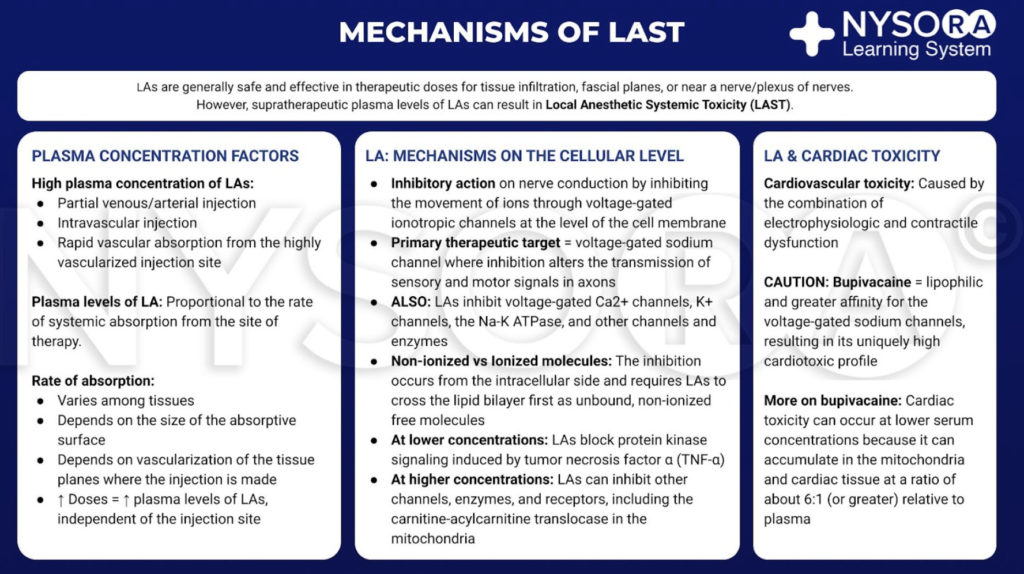
Local anesthetics are generally safe and effective when limited to the site of therapy, such as tissue infiltration, near a nerve or a plexus of nerves. However, if large amount of LA reaches the systemic circulation, supratherapeutic blood and tissue levels can cause toxicity. This transit into the blood may be due to inadvertent intravascular injection or vascular uptake from local spread. At the target site, LAs reduce the sodium ion flux through voltage-gated sodium channels by a combination of an increased energy barrier and steric hindrance. This nerve block occurs from the intracellular side and requires LAs to move across the lipid bilayer first. LAs also nerve block calcium channels and other channels at similar concentrations. At lower concentrations, LAs nerve block protein kinase signaling induced by tumor necrosis factor α. At higher concentrations, LAs can inhibit other channels, enzymes, and receptors, including the carnitine-acylcarnitine translocase in mitochondria.
Learn more about the mechanism of action of local anesthetics
Although there is no clear consensus, cardiac toxicity is likely caused by the combination of electrophysiologic and contractile dysfunction. Compared with other LAs in common clinical use, bupivacaine is more lipophilic and has a greater affinity for the voltage-gated sodium channels. These qualities may contribute to its cardiotoxic profile. Of note, toxicity can occur at serum concentrations that are lower than expected because LAs accumulate in mitochondria and cardiac tissue at a ratio of about 6:1 (or greater) relative to plasma.
From the Compendium of Regional Anesthesia: Mechanisms of local anesthetic systemic toxicity infographic.
DIAGNOSIS AND CONTRIBUTING FACTORS
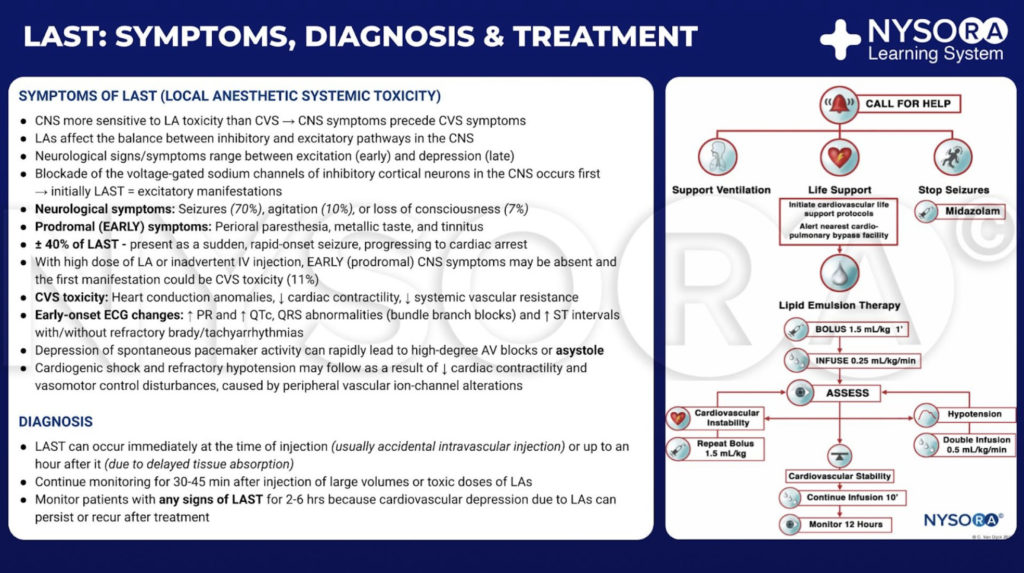
The typical presentation of LAST usually begins with prodromal symptoms and signs, such as perioral numbness, tinnitus, agitation, dysarthria, and confusion. These may be followed by more severe central nervous system (CNS) derangements such as seizures and coma. CV derangements can occur as well, initially presenting with hypertension and tachycardia, then bradycardia and hypotension, with progression to more serious complications, including ventricular arrhythmias and asystole. The majority of adverse events occur within 1 minute after injection of LA, but not all cases follow this pattern. Toxicity can have a delayed onset of greater than 1 hour after injection and can manifest as isolated CV dysfunction or as a combination of CNS and CV signs without the classical progression.
Variables that increase the risk of toxicity include the type of LA and dose, site of injection, the patient’s comorbidities, extremes of age, and small size or limited muscle mass. The lipophilicity of a LA is associated with toxicity. More lipophilic LAs like bupivacaine have an increased risk of toxicity relative to the less-lipophilic LAs like mepivacaine and lidocaine.
Higher total dose and the dose-to-weight ratio of the drug can potentially increase the possibility of LAST. In particular, skeletal muscle acts as a depot for systemically absorbed LA, which may account for the clinical risk of LAST in diminutive patients whose muscle mass is substantially less than normal. Accordingly, nerve blocks and epidural anesthetics that require larger doses carry an inherent risk for such patients. For example, bilateral transversus abdominus plane nerve blocks performed with as much as 40 mL of 0.5% ropivacaine can result in an increased incidence of local toxicity.
Last, but not least, the site of injection also contributes to the risk of vascular spread of the drug. The classical teaching that the vascular absorption of LAs is highest with intercostal nerve blocks followed by epidural and brachial plexus injections corresponds to the clinical data demonstrating that the highest incidence of LAST occurs with paravertebral nerve blocks, followed by upper extremity and trunk/lower extremity nerve blocks.
Patient-dependent risk factors include organ dysfunction, the serum level of the binding proteins, and age. Preexisting cardiac disease can make patients more prone to the arrhythmogenic and myocardial depressant effects of LAs. Extreme caution is advised for those with decompensated heart failure, severe valvular pathology, or depressed ventricular function. Hepatic or renal dysfunction can result in decreased metabolism and clearance and a higher level of circulating drug. In addition, liver/kidney failure, malnutrition, or any other disease process that results in a decreased serum level of albumin can indirectly increase the level of the free drug for a given dose.
Patients at the extremes of age are more susceptible to toxicity, a finding that may be related to a number of factors. The elderly are more likely to have organ dysfunction, which will contribute to toxicity. Further, both elderly and pediatric patients may have diminished muscle mass and, as such, are more likely to receive a higher dose of drug for their weight. Most children are anesthetized when a nerve block is placed, so the early symptoms will be missed, and more serious CNS/cardiac derangement could be the first sign of toxicity.
From the Compendium of Regional Anesthesia: Symptoms, diagnosis, and treatment of local anesthetic systemic toxicity infographic.
OCCURRENCE OF TOXICITY
Neuraxial anesthesia and peripheral nerve blocks (PNBs) are the most commonly performed procedures requiring the use of LAs. The low volume of drug required for intrathecal dosing rarely poses a problem. However, the high volume required for epidural anesthesia and PNBs increases the risk of LAST. Currently available data indicate that the incidence of LAST associated with PNBs has decreased from 1.6–2/1000 in the 1990s to 0.08–0.98/1000 between 2003 and 2013. In fact, one recent study observed no cases of LAST with more than 9000 PNBs over a period of 6 years. Likewise, the incidence of LAST with epidural anesthesia decreased from 9.75/1000 in the early 1980s to 0.1–1.2/1000 in the 1990s and stayed at 0.1/1000 in 2003.
While large population studies are mostly limited to epidurals and PNBs, there are numerous reports describing LAST with other types of local anesthesia. For example, with the recent popularity of a transversus abdominus plane nerve block for abdominal procedures, there have been several cases of LAST after these nerve blocks were performed for cesarean sections.
Neurologic toxicity has also been described after the topical use of LAs, which anesthesia providers frequently use prior to airway instrumentation for awake intubation. This is likely underreported because the neurological symptoms may be mild (perioral numbness, tinnitus, agitation) and masked by preoperative sedation that precedes the induction of general anesthesia that immediately follows awake intubation.
Occasional causes of LAST outside the usual scope of an anesthesiologist include retrobulbar nerve blocks for ophthalmologic surgery and inferior alveolar nerve blocks for dental procedures. Toxicity from a retrobulbar nerve block is caused by subarachnoid spread of the anesthetic causing brainstem anesthesia; which can be manifest as altered mental status, apnea, and seizures. Specific reports of LAST after inferior alveolar nerve blocks are rare, but it is clearly a potential risk. The richly vascular area of the pterygomandibular space increases the risk for intravascular needle placement, which can be as high as 15.3% even among experienced oral surgeons. Finally, there is a recent increase in utilization of regional nerve blocks in emergency room settings and corresponding reports of LAST in the emergency room, but the scope of this problem is currently unknown.
TREATMENT
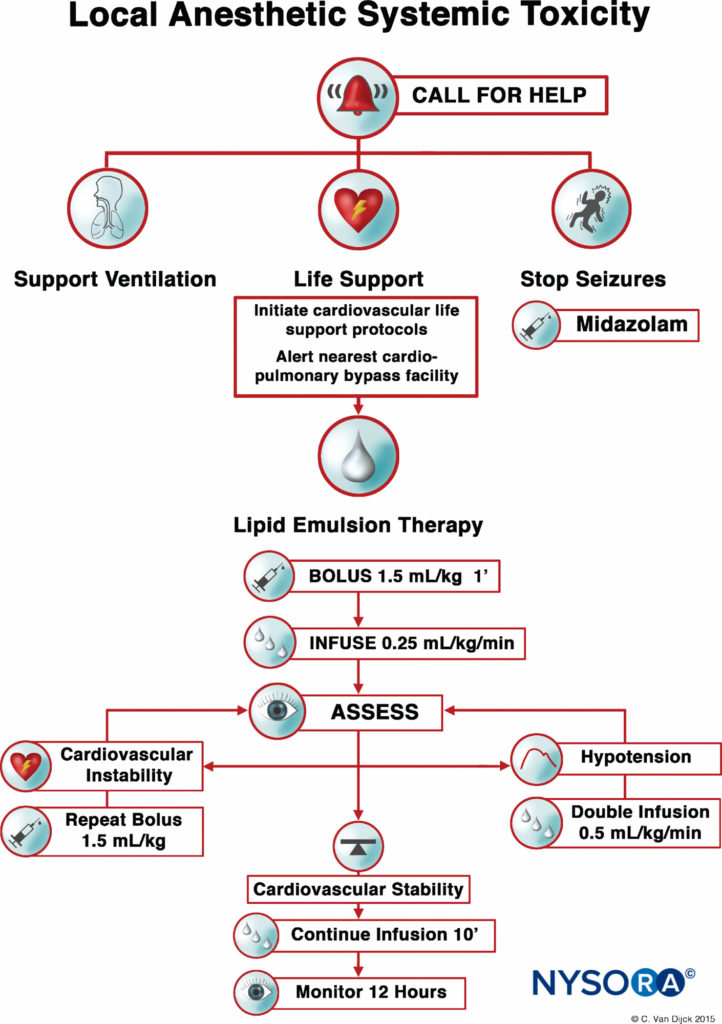
Presently, the three pillars of LAST treatment consist of seizure management, advanced cardiac life support (ACLS), and prompt administration of a 20% lipid emulsion. For hemodynamically stable patients with isolated seizure activity, intravenous benzodiazepines may be used. Small doses of propofol are considered by some an acceptable alternative for seizure control but can worsen cardiac dysfunction that may develop with LAST. Supplemental oxygen is appropriate for any patient exhibiting signs of LAST, but for patients with apnea, hemodynamically unstable arrhythmias, or cardiac arrest, immediate, more aggressive airway management or circulatory support is required. The goals are to maintain pulmonary ventilation and adequate organ perfusion with well-oxygenated blood and to avoid further acidosis until initiation of lipid emulsion therapy.
Before the introduction of lipid emulsion resuscitation, the treatment of severe cardiac toxicity was limited to ACLS and cardiopulmonary bypass. The use of vasopressors during resuscitation potentially worsened acidosis and arrhythmias. The cardiopulmonary bypass had been used in some cases; unfortunately, not all hospitals have that capability. The idea that a lipid-rich substance has the potential to reverse the effects of certain drugs began in the 1960s, when several animal experiments demonstrated that intravenous administration of an oil emulsion decreased the duration of action of thiopental or decreased the free fraction of chlorpromazine in blood. Serendipitously, in 1997, the case of LAST in a young woman with isovaleric acidemia and carnitine deficiency inspired a series of animal experiments. Carnitine is required for the transport of fatty acids into the mitochondria for β-oxidation, and accumulation of cytoplasmic acylcarnitines (eg, during myocardial ischemia) is associated with arrhythmias. So, Weinberg et al hypothesized that overloading cells with exogenous fatty acids by infusing a lipid emulsion would exacerbate LA toxicity. Surprisingly, the opposite was seen. Infusing a fat emulsion decreased and even reversed LA toxicity.
In 2006, the first successful resuscitation of a human patient with a lipid emulsion was reported. Since then, there have been many clinical reports describing effective reversal of LAST in adults and children. Treatment of toxicity with an intravenous lipid emulsion has been termed lipid resuscitation therapy (LRT). The mechanism of LRT is multimodel in action, with lipid exerting both a scavenging effect (previously known as the “lipid sink”) and a direct cardiotonic effect.
The scavenging effect is moderated by the lipid emulsion’s ability to take up lipophilic moieties and transfer them around the blood to sites of storage and detoxification. This provides a “lipid shuttle” effect. However, the scavenging effect is not sufficient to explain the rapid recovery. A second effect occurs whereby in laboratory models infusing the lipid emulsion increases cardiac output through a combination of volume and direct cardiotonic effects to improve cardiac output once the cardiac concentration of drug drops below ion channel–blocking thresholds. A 20% lipid emulsion is efficacious in the treatment of LAST caused by bupivacaine as well as other less-soluble LAs, such as ropivacaine, mepivacaine, and lidocaine.
The lipid emulsion LD50 (median lethal dose) tested in a rat model was found to be much higher than the doses used for lipid rescue in humans. Potential side effects include interference with clinical laboratory measurements (hemoglobin, methemoglobin, electrolytes, base excess); allergic reactions; nausea/emesis; dyspnea; and chest pain. Nonetheless, actual reported side effects are limited to bronchospasm, hyperamylasemia, and laboratory measurement interference.
Transaminitis, hepatosplenomegaly, and bacterial contamination are typically associated with prolonged use of a lipid emulsion and do not play a role in the short-term administration for LAST. Although the use of high volumes of lipid emulsion (especially 30%) in premature and low birth weight neonates has been associated with death from fat accumulation in the lungs, there are case reports in neonates, toddlers, and older children of successful reversal of drug overdose (bupivacaine and non-LAs) using standard recommended regimes of 20% lipid. Last, as mentioned, one should exercise caution with the use of propofol in this setting: It is not a substitute for a lipid emulsion. There is insufficient lipid content in standard sedating or antiseizure doses of propofol to exert a benefit in the overdose setting; however, propofol can compromise CV stability.
After numerous case reports validated the role of LRT as an effective treatment of LAST, the American Society of Regional Anesthesia and Pain Medicine (ASRA) issued a practice advisory in 2010 followed in 2012 by a checklist for managing LAST (Figure 1). The guidelines stress the importance of immediate cardiopulmonary resuscitation and provide a detailed algorithm for the dosing and administration of the lipid emulsion.
Timely use of the LRT, at the earliest signs of toxicity, can improve resuscitative efforts and decrease the amount of vasopressors used. As with any life-threatening emergency, securing intravenous access is essential; however, intraosseous administration of lipid emulsion is a possible alternative if intravenous access proves problematic.
PREVENTION
As always, the best treatment is prevention. This is especially true for LAST. The efficacy and availability of LRT does not decrease the potential morbidity even in cases of successful treatment. The presence of a “silver bullet” does not remove the need for caution. For that reason, the use of ultrasound, intravascular markers, incremental injection with aspiration, less toxic drugs, and the lowest effective dose is recommended.
NYSORA Tips
- There is a greater likelihood for LA systemic toxicity in petite patients (small muscle mass), those at the extremes of age, and patients with preexisting heart disease or carnitine deficiency.
- Roughly half the cases of LAST are atypical, with no seizures (other CNS symptoms), only CV toxicity or delayed onset.
- The incidence of toxicity increases with injections near richly vascular areas. It is highest with paravertebral injections, followed by upper and lower extremity PNBs.
- Prevention of LAST-related morbidity requires optimizing a complete system for regional anesthesia: patient selection, nerve block choice, drug and dose, complete monitoring and use of USGRA when possible, and preparing for LAST by having a kit available and practicing with simulation.
- Prevention also includes raising awareness and educating our non-anesthesiology colleagues about proper use of LAs and risks, including management of LAST.
Ultrasound offers several potential advantages. It allows for direct visualization of injectable spread of drug, detection of unintended intravascular injection, and the use of smaller volumes of LAs. Moreover, there is evidence that the use of ultrasound for PNBs can reduce the incidence of LAST.
An intravascular marker such as 10–15 μg of epinephrine has reasonable (albeit imperfect) sensitivity and positive predictive value and can be administered with a test dose. An increase in heart rate of 10 beats/minute or greater or an increase in systolic blood pressure of 15 mm Hg or greater suggests an intravascular injection. Incremental injection of LAs (usually 3–5 mL) and frequent aspiration have been regularly recommended and, together with the use of the test dose, may have contributed to the decrease in the incidence of LAST seen with epidurals.
Finally, the use of the lowest effective dose provides an additional margin of safety. It is also reasonable to adjust a dose downward for patients recognized to have a condition that could increase their susceptibility to LAST. This seems somewhat redundant if one always uses the least dose necessary for any nerve block. Prudence is the point. None of these measures is in itself precise or perfect; hence, it is vital not to rely on a single prevention step, but to incorporate several plus common sense to put patient safety first.
AWARENESS AND EDUCATION
Anesthesiologists use LAs every day in various practice locations and for a wide variety of procedures. Thus, every site where LAs are used in potentially toxic doses should be equipped with basic resuscitation equipment and a 20% lipid emulsion. In addition, the ASRA checklist for the treatment of LAST can help guide the treatment process. An electronic decision support tool was shown to improve adherence to guidelines during simulation of management of LAST and may be of benefit during actual cases. Finally, education of non-anesthesia providers is crucial to raise their awareness of both the risk of LAST and its treatment. Patients could be saved if such very rare events are properly diagnosed and managed by non-anesthesiologists or others among the uninitiated who will otherwise remain ignorant of the risk. Education differs among institutions and departments, but there is suboptimal knowledge of LA dosing, safety precautions, and treatment of LAST among other specialties. For that reason, the ASRA checklist and the electronic decision support tool can be invaluable for physicians in the event of LA toxicity.
REFERENCES
- Drasner K: Local anesthetic systemic toxicity. A historical perspective. Reg Anesth Pain Med 2010;35:162–166.
- Mayer E: The toxic effects following the use of local anesthetics: an analysis of the reports of forty-three deaths submitted to the Committee for the Study of Toxic Effects of Local Anesthetics of the American Medical Association, and the recommendations of the committee. JAMA 1924;82:875–876.
- Edde RR, Deutsch S: Cardiac arrest after interscalene brachial-plexus block. Anesth Analg 1977;56:446–447.
- Prentiss JE: Cardiac arrest following caudal anesthesia. Anesthesiology 1979;50:51–53.
- Clarkson CW, Hondeghem LM: Mechanism for bupivacaine depression of cardiac conduction: fast block of sodium channels during the action potential with slow recovery from block during diastole. Anesthesiology 1985;62:396–405.
- Coyle DE, Sperelakis N: Bupivacaine and lidocaine block of calciummediated slow action potentials in guinea pig ventricular muscle. J Pharmacol Exp Ther 1987;242:1001–1005.
- Piegeler T, Votta-Velis, Bakhshi FR, et al: Endothelial barrier protection by local anesthetics: ropivacaine and lidocaine block tumor necrosis factor-a-induced endothelial cell Src activation. Anesthesiology 2014;120: 1414–1428.
- Weinberg GL, Palmer JW, VadeBoncouer, et al: Bupivacaine inhibits acylcarnitine exchange in cardiac mitochondria. Anesthesiology 2000;92: 523–528.
- Wolfe JW, Butterworth JF: Local anesthetic systemic toxicity: update on mechanisms and treatment. Curr Opin Anesthesiol 2011;24:561–566.
- Albright EA: Cardiac arrest following regional anesthesia with etidocaine or bupivacaine. Anesthesiology 1979;51:285–287.
- Heavner JE: Cardiac toxicity of local anesthetics in the intact isolated heart model: a review. Reg Anesth Pain Med 2002;27:545–555.
- Hiller N, Mirtschink P, Merkel C, et al: Myocardial accumulation of bupivacaine and ropivacaine is associated with reversible effects on mitochondria and reduced myocardial function. Anesth Analg 2013; 116:83–92.
- Fettiplace MR, Pichurko A, Ripper R, et al: Cardiac depression induced by cocaine or cocaethylene is alleviated by lipid emulsion more effectively than by sulfobutylether-B-cyclodextrin. Acad Emerg Med 2015;22:508–517.
- Di Gregorio, Neal JM, Rosenquist RW, et al: Clinical presentation of local anesthetic systemic toxicity. A review of published cases, 1979–2009. Reg Anesth Pain Med 2010;35:181–187.
- Pertrar S: Total local anesthetic administered is integral to the syndrome of local anesthetic systemic toxicity. Anesthesiology 2014;121: 1130–1131.
- Barrington MJ, Kluger R: Ultrasound guidance reduces the risk of local anesthetic systemic toxicity following peripheral nerve lockade. Reg Anesth Pain Med 2013;38:289–299.
- Eng HC, Ghosh SM, Chin KJ: Practical use of local anesthetics in regional anesthesia. Curr Opin Anesthesiol 2015;27:382–387.
- Calenda E, Baste JM, Hajjej R, et al: Toxic plasma concentration of ropivacaine after a paravertebral block in a patient suffering from severe hypoalbuminemia. J Clin Anesth 2014;26:149–151.
- Fagenholz PJ, Bowler GM, Carnochan FM, et al: Systemic local anaesthetic toxicity from continuous thoracic paravertebral block. Br J Anaesth 2012;109:260–262.
- Lonnqvist PA: Toxicity of local anesthetic drugs: a pediatric perspective. Paediatr Anaesth 2012;22:39–43.
- Auroy Y, Narchi P, Messiah A, et al: Serious complications related to regional anesthesia: results of a prospective survey in France. Anesthesiology 1997;87:447–486.
- Auroy Y, Benhamou D, Barques L, et al: Major complications of regional anesthesia in France: the SOS Regional Anesthesia Hotline Service. Anesthesiology 2002;97:1274–1280.
- Brown, Ransom DM, Hall JA, et al: Regional anesthesia and local anesthetic-induced systemic toxicity: seizure frequency and accompanying cardiovascular changes. Anesth Analg 1995;81:321–328.
- Barrington MJ, Watts SA, Gledhill SR, et al: Preliminary results of the Australian Regional Anaesthesia Collaboration. A prospective audit of more than 7000 peripheral nerve and plexus blocks for neurologic and other complications. Reg Anesth Pain Med 2009;34:534–541.
- Sites BD, Taenzer AH, Herrick MD, et al: Incidence of local anesthetic systemic toxicity and postoperative neurologic symptoms associated with 12,668 ultrasound-guided nerve blocks. An analysis from a prospective clinical registry. Reg Anesth Pain Med 2012;37:478–482.
- Orebaugh SL, Kentor ML, Williams BA: Adverse outcomes associated with nerve stimulator-guided and ultrasound-guided peripheral nerve blocks by supervised trainees: update of a single-site database. Reg Anesth Pain Med 2012;37:577–582.
- Tanaka K, Watanabe R, Harada T, et al: Extensive application of epidural anesthesia and analgesia in a university hospital: incidence of complications related to technique. Reg Anesth 1993;18:34–38.
- Kenepp NB, Gutsche BB: Inadvertent intravascular injections during lumbar epidural anesthesia. Anesthesiology 1981;54:172–173.
- Griffiths JD, Le NV, Grant S, et al: Symptomatic local anaesthetic toxicity and plasma ropivacaine concentrations after transversus abdominis plane block for caesarean section. Br J Anaesth 2013;110:996–1000.
- Weiss E, Jolly C, Dumoulin JL, et al: Convulsions in 2 patients after bilateral ultrasound-guided transversus abdominis plane blocks for cesarean section. Reg Anesth Pain Med 2014;39:248–251.
- Giordano D, Panini A, Pernice C, et al: Neurologic toxicity of lidocaine during awake intubation in a patient with tongue base abscess. Case report. Am J Otoralyngol 2014;35:62–65.
- Gunja N, Varshney K: Brainstem anaesthesia after retrobulbar block: a rare cause of coma presenting to the emergency department. Emerg Med Australas 2006;18:83–85.
- Dahle JM, Iserson KV: ED treatment of brainstem anesthesia after
retrobulbar block. Am J Emerg Med 2007;25:105–106. - Tatum PL, Defalque RJ: Subarachnoid injection during retrobulbar block: a case report. Am Assoc Nurse Anesth J 1994;62:49–52.
- Zenous AT, Ebrahimi H, Mahdipour M, et al: The incidence of intravascular needle entrance during inferior alveolar nerve block injection.J Dent Res Dent Clin Prospects 2008;2:38–41.
- Hahn C, Nagdev A: Color Doppler ultrasound-guided supraclavicular brachial plexus block to prevent vascular injection. West J Emerg Med 2014;15:703–705.
- Monti M, Monti A, Borgognoni F, et al: Treatment with lipid therapy to resuscitate a patient suffering from toxicity due to local anesthetics. Emerg Care J 2014;10:41–44.
- Harvey M, Cave G, Chanwai G, et al: Successful resuscitation from bupivacaine-induced cardiovascular collapse with intravenous lipid emulsion following femoral nerve block in an emergency department. Emerg Med Australas 2014;23:209–214.
- Weinberg GL: Treatment of local anesthetic systemic toxicity. Reg Anesth Pain Med 2010;35:188–193.
- Soltesz EG, van Pelt F, Byrne JG, et al: Emergent ardiopulmonary bypass for bupivacaine cardiotoxicity. J Cardiothorc Vasc Anesth 2003;17: 357–358.
- Russell RL, Westfall BA: Alleviation of barbiturate depression. Anesth Analg 1962;41:582–585.
- Krieglstein J, Meffert A, Niemeyer DH: Influence of emulsified fat on chlorpromazine availability in rabbit blood. Experientia 1974;30: 924–926.
- Corr PB, Yamada KA: Selected metabolic alterations in the ischemic heart and their contributions to arrhythmogenesis. Herz 1995;20:156–168.
- Weinberg GL, Laurito CE, Geldner P, et al: Malignant ventricular dysrhythmias in a patient with isovaleric academia receiving general and local anesthesia for suction lipectomy. J Clin Anesth 1997;9:668–670.
- Weinberg GL, Ripper R, Murphy P, et al: Lipid infusion accelerates removal of bupivacaine and recovery from bupivacaine toxicity in the isolated rat heart. Reg Anesth Pain Med 2006;31:296–303.
- Rosenblatt MA, Abel M, Fischer GW, et al: Successful use of a 20% lipid emulsion to resuscitate a patient after a presumed bupivacaine-related cardiac arrest. Anesthesiology 2006;105:217–218.
- Cave G, Harvey M, Willers J, et al: LIPAEMIC report: results of clinical use of intravenous lipid emulsion in drug toxicity reported to an online lipid registry. J Med Toxicol 2014;10:133–142.
- Presley JD, Chyka PA: Intravenous lipid emulsion to reverse acute drug toxicity in pediatric patients. Ann Pharmacother 2013;47:735–743.
- Fettiplace MR, Weinberg G: Past, present, and future of lipid resuscitation therapy. JPEN J Parent Enteral Nutr 2015;39(1 Suppl):72S–83S, 2015.
- Wagner M, Zausiq YA, Ruf S, et al: Lipid rescue reverses the bupivacaineinduced block of the fast Na+ current (INa) in cardiomyocytes of the rat left ventricle. Anesthesiology 2014;120:724–736.
- Mazoit JX, Le Guen R, Beloeil H, et al: Binding of long-lasting local anesthetics to lipid emulsions. Anesthesiology 2009;110:380–386.
- Kuo IK, Akpa BS: Validity of the lipid sink as a mechanism for the reversal of local anesthetic systemic toxicity. Anesthesiology 2013;118:1350–1361.
- Fettiplace MR, Ripper R, Lis K, et al: Rapid cardiotonic effects of lipid emulsion infusion. Crit Care Med 2013;41:e156–e162.
- Fettiplace MR, Akpa BS, Ripper R, et al: Resuscitation with lipid emulsion: dose-dependent recovery from cardiac pharmacotoxicity requires a cardiotonic effect. Anesthesiology 2014;120:915–925.
- Ozcan MS, Weinberg G: Update on the use of lipid emulsions in local anesthetic systemic toxicity: a focus on differential efficacy and lipid emulsion as part of advanced cardiac life support. Int Anesthesiol Clin 2011;49:91–103.
- Hiller DB, DiGrigorio G, Kelly K, et al: Safety of high volume lipid emulsion infusion. A first approximation of LD50 in rats. Reg Anesth Pain Med 2010;35:140–144.
- Ozcan MS, Weinberg G: Intravenous lipid emulsion for the treatment of drug toxicity. J Intensive Care Med 2014;29:59–70.
- Brull SJ: Lipid emulsion for the treatment of local anesthetic toxicity: patient safety implications. Anesth Analg 2008;106:1337–1339.
- Neal JM, Bernards CM, Butterworth JF 4th, et al: ASRA practice advisory on local anesthetic systemic toxicity. Reg Anesth Pain Med 2010;35:152–161.
- Neal JM, Mulroy MF, Weinberg GL: American Society of Regional
Anesthesia and Pain Medicine checklist for managing local anesthetic
systemic toxicity: 2012 version. Reg Anesth Pain Med 2012;37:16–18. - Fettiplace MR, Ripper R, Lis K, et al: Intraosseous lipid emulsion: an effective alternative to IV delivery in emergency situation. Crit Care Med 2014;42:157–160.
- Neal JM: Ultrasound-guided regional anesthesia and patient safety. Reg Anesth Pain Med 2010;35:S59–S67.
- Orebaugh SL, Williams BA, Vallejo M, et al: Adverse outcomes associated with stimulator-based peripheral nerve blocks with versus without ultrasound visualization. Reg Anesth Pain Med 2009;34:251–255.
- Salinas FV, Hanson NA: Evidence-based medicine for ultrasound-guided regional anesthesia. Anesthesiol Clin 2014;32:771–787.
- Mulroy MF, Hejtmanek MR: Prevention of local anesthetic systemic
toxicity. Reg Anesth Pain Med 2010;35:177–180. - Neal JM: Local anesthetic systemic toxicity. Improving patient safety one step at a time. Reg Anesth Pain Med 2013;38:259–261.
- Neal JM, Hsiung Rl, Mulroy MF, et al: ASRA checklist improves trainee performance during a simulated episode of local anesthetic systemic toxicity. Reg Anesth Pain Med 212;37:8–15.
- McEvoy MD, Hand WR, Stoll WD, et al: Adherence to guidelines for the management of local anesthetic systemic toxicity is improved by an electronic decision support tool and designated “reader.” Reg Anesth Pain Med 2014;392:299–305.
- Sagir A, Goyal R: An assessment of awareness of local anesthetic systemic toxicity among multi-specialty postgraduate residents. J Anesth 2015; 29:299–302.
Marina Gitman, Michael Fettiplace, and Guy Weinberg