Steven L. Orebaugh and Hillenn Cruz Eng
INTRODUCTION
The vertebral column forms part of the axis of the human body, extending in the midline from the base of the skull to the pelvis. Its four primary functions are protection of the spinal cord, support of the head, provision of an attachment point for the upper extremities, and transmission of weight from the trunk to the lower extremities. Pertinent to regional anesthesia, the vertebral column serves as the landmark for a wide variety of regional anesthesia techniques. It is important, therefore, that the anesthesiologist be able to develop a three-dimensional mental image of the structures comprising the vertebral column.
ANATOMIC CONSIDERATIONS
The vertebral column consists of 33 vertebrae (7 cervical, 12 thoracic, 5 lumbar, 5 sacral, and 4 coccygeal segments) (Figure 1). In the embryonic period, the spine curves into a C shape, forming two primary curvatures with their convex aspect directed posteriorly. These curvatures persist through adulthood as the thoracic and sacral curves. The cervical and lumbar lordoses are secondary curvatures that develop after birth as a result of extension of the head and lower limbs when standing erect. The secondary curvatures are convex anteriorly and augment the flexibility of the spine.
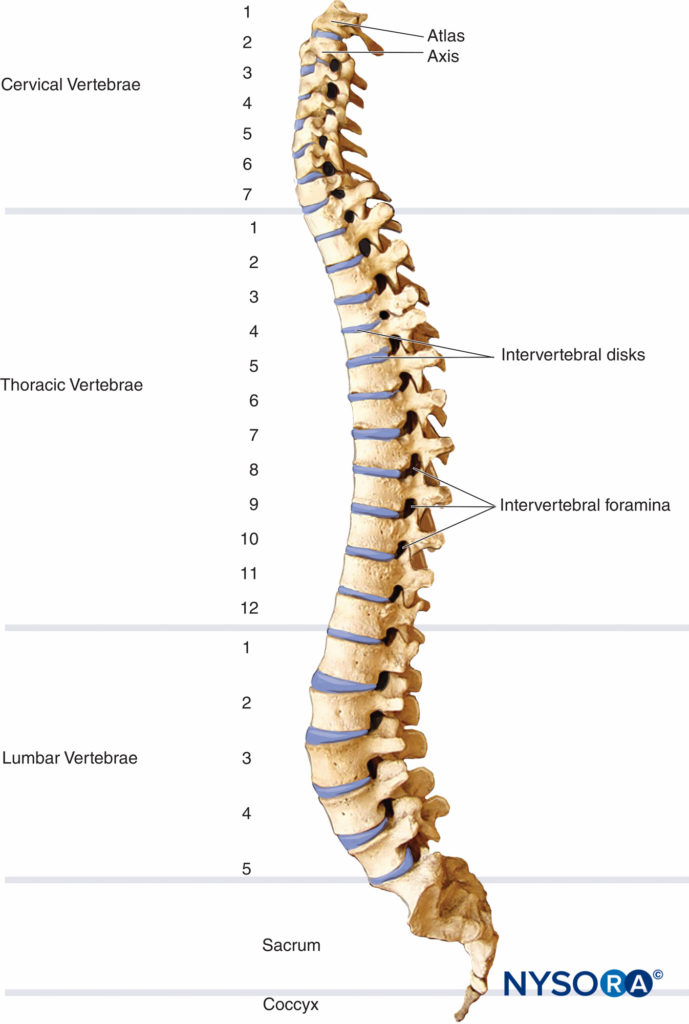
FIGURE 1. The vertebral column and the curvatures of the adult spine, lateral view.
Vertebrae
A typical vertebra consists of a vertebral arch posteriorly and a body anteriorly. This holds true for all vertebrae except C1. Two pedicles arise on the posterolateral aspects of each vertebra and fuse with the two laminae to encircle the vertebral foramen1 (Figure 2A, Figure 2B). These structures form the vertebral canal, which contains the spinal cord, spinal nerves, and epidural space. Fibrocartilaginous disks containing the nucleus pulposus, an avascular gelatinous body surrounded by the collagenous lamellae of the annular ligament, join the vertebral bodies. The transverse processes arise from the laminae and project laterally, whereas the spinous process projects posteriorly from the midline union of the laminae (Figure 2A, Figure 2B). The spinous process is frequently bifid at the cervical level and serves as an attachment for muscles and ligaments.
C1 (atlas), C2 (axis), and C7 (vertebra prominens) are described as atypical cervical vertebrae due to their unique features.
C1 is a ringlike bone that has no body or spinous process.
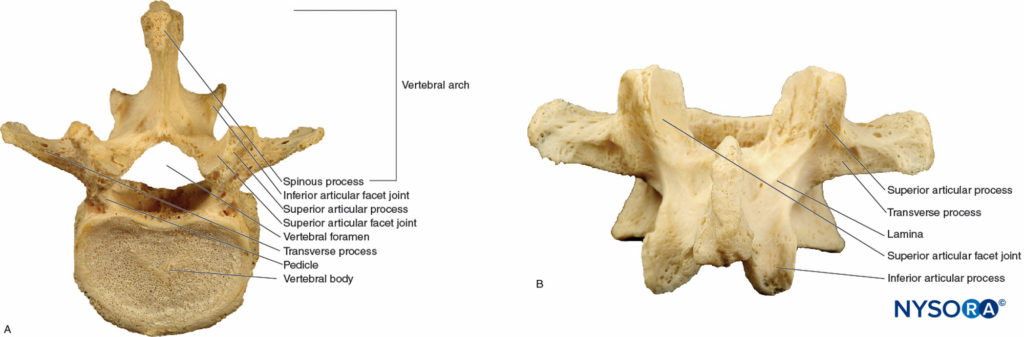
FIGURE 2. A typical vertebra. A: Superior view of the L5 vertebra. B: Posterior view of the L5 vertebra.
It is formed by two lateral masses with facets that connect anteriorly to a short arch and posteriorly to a longer, curved arch. The anterior arch articulates with the dens, and the posterior arch has a groove where the vertebral artery passes (Figures 3A). The odontoid process (dens) of C2 protrudes superiorly, hence the name axis (Figures 3B). Together, the atlas and axis form the axis of rotation for the atlantoaxial joint.
The C7 (vertebra prominens) has a long, nonbifid spinous process that serves as a useful landmark for a variety of regional anesthesia procedures (Figure 3C). The C7 transverse process is large and has only one posterior tubercle.
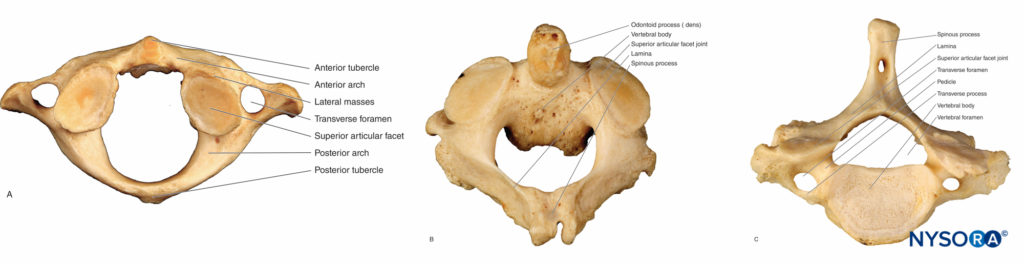
FIGURE 3. The atypical vertebrae. A: Superior view of the C1 vertebra (atlas). B: Superior view of the C2 vertebra (axis) with a bifid spinous process. C: Superior view of the C7 vertebra; the spinous process is nonbifid.
The interlaminar spaces in the thoracic spine are narrow and more challenging to access with a needle due to overlapping laminae. In contrast, the laminae of the five lumbar vertebrae do not overlap. The interlaminar space between adjacent lumbar vertebrae is rather large.
Vertebral facet (zygapophyseal) joints articulate posterior elements of adjacent vertebrae. The junction of the lamina and pedicles gives rise to inferior and superior articular processes (Figure 2A, Figure 2B). The inferior articular process protrudes caudally and overlaps the inferiorly adjacent vertebra’s superior articular process. This alignment is important to understand when performing interventional pain procedures such as facet joint injections, intra-articular steroid injections or radio-frequency denervation. Joint surfaces in the cervical spine are oriented halfway between the axial and coronal planes. This alignment allows an ample degree of rotation, flexion, and extension but little resistance to backward and forward shearing forces. Facet joints in the thoracic region are oriented in a more coronal plane, which provides greater protection against shearing forces but reduced rotation, flexion, and extension.
In the lumbar spine, joint surfaces are curved, with a coronal orientation of the anterior portion and a sagittally oriented posterior portion. Thoracic facets are located anterior to the transverse processes, whereas cervical and lumbar facets are located posterior to their transverse processes.
Five sacral vertebrae fuse to form the wedge-shaped sacrum, which connects the spine with the iliac wings of the pelvis4 (Figures 4A, 4B). In childhood, the sacral vertebrae are connected by cartilage, which progresses to osseous fusion after puberty, with only a narrow remnant of sacral disk remaining in adulthood.
Fusion is generally complete through the S5 level, although there can be complete lack of any posterior bony roof over the sacral vertebral canal. The sacral hiatus is an opening formed by the incomplete posterior fusion of the fifth sacral vertebra.
It lies at the apex of the coccyx, which is formed by the union of the last four vertebrae (Figure 4C). This hiatus provides convenient access to the caudal ending of the epidural space, especially in children. The sacral cornu are bony prominences on each side of the hiatus that are easily palpated in small children and serve as landmarks for a caudal epidural block. For more information, see Caudal Anesthesia.
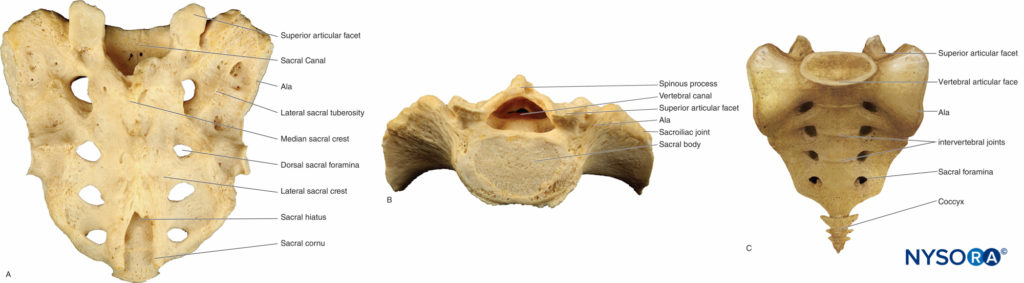
FIGURE 4. The sacrum and coccyx. A: Posterior view of the sacrum; the sacrum curves anteriorly proximal to its narrowing tip where it articulates with the coccyx. B: The base of the sacrum is directed upward and forward. C: Anterior view of the coccyx.
From the Compendium of Regional Anesthesia: Vertebral column animation.
Intervertebral Ligaments
The vertebral column is stabilized by a series of ligaments. The anterior and posterior longitudinal ligaments run along the anterior and posterior surfaces of the vertebral bodies, respectively, reinforcing the vertebral column. The supraspinous ligament, a heavy band that runs along the tips of the spinous processes, becomes thinner in the lumbar region (Figure 5).
This ligament continues as the ligamentum nuchae above T7 and attaches to the occipital external protuberance at the base of the skull. The interspinous ligament is a narrow web of tissue that attaches between spinous processes; anteriorly it fuses with the ligamentum flavum and posteriorly with the supraspinous ligament (Figure 5).
The ligamentum flavum is a dense, homogenous structure, composed mostly of elastin which connects the lamina of adjacent vertebrae, (Figure 5). The lateral edges of the ligamentum flavum surround facet joints anteriorly, reinforcing their joint capsule. When a needle is advanced toward the epidural space, there is an easily perceptible increase in resistance when the ligamentum flavum is encountered. More importantly for the practice of neuraxial anesthesia, a perceptible, sudden loss of resistance is encountered when the tip of the needle passes through the ligamentum and enters the epidural space.
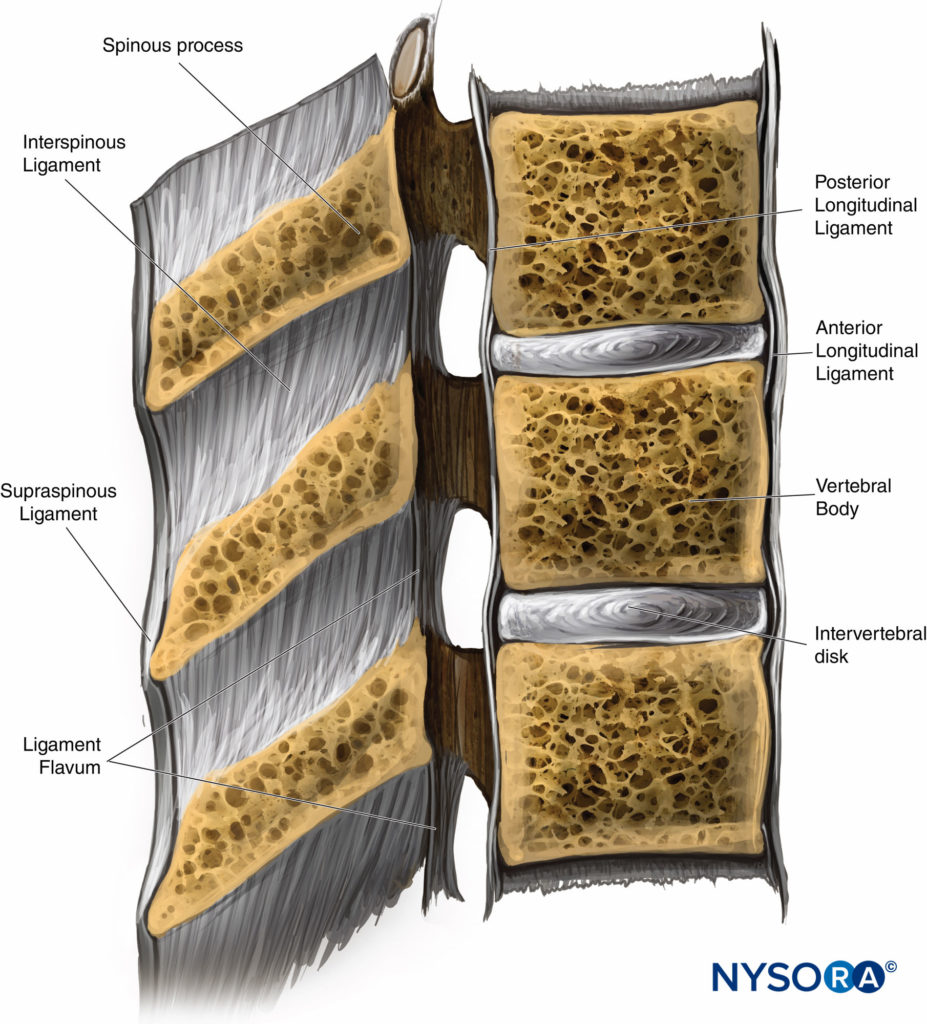
FIGURE 5. A cross-sectional view of the vertebral canal with the intervertebral ligaments, vertebral body, and spinous process.
The ligamentum flavum consists of right and left halves that join at an angle of less than 90°. Importantly, this midline fusion may be absent to a variable degree depending on the vertebral level. These fusion gaps allow for veins to connect to vertebral venous plexuses. Of note, the fusion gaps are more prevalent at cervical and thoracic levels. Yoon et al reported that midline gaps between C3 and T2 occur in 87%–100% of individuals.
The incidence of the midline gap decreases at lower vertebral levels, with T4–T5 the lowest (8%). In theory, a midline gap poses a risk of failure to recognize a loss of resistance at the cervical and high thoracic levels when using the midline approach, resulting in an inadvertent dural puncture.
The ligamentum flavum is thinnest in the cervical and upper thoracic regions and thickest in the lower thoracic and lumbar regions. As a result, resistance to needle advancement is easier to appreciate when a needle is introduced at a lower level (eg, lumbar). At the L2–L3 interspace, the ligamentum flavum is 3- to 5-mm thick. At this level, the distance from the ligamentum to the spinal meninges is 4–6 mm. Consequently, a midline insertion of an epidural needle at this level is least likely to result in an inadvertent meningeal puncture with epidural anesthesia-analgesia.
The lateral wall of the vertebral canal has gaps between consecutive pedicles known as intervertebral foramina (Figure 1A). Because the pedicles attach more cephalad of the middle of the vertebral body, the intervertebral foramina are centered opposite the lower half of the vertebral body, with the vertebral disk at the caudal end of the foramen.
As a consequence, the borders of the intervertebral foramina are the pedicle at the cephalad and caudal ends, the vertebral body (cephalad) and the disk (caudally) on the anterior aspect, a portion of the next vertebral body most inferiorly, and posteriorly the lamina, facet joint, and ligamentum flavum.
Spinal Meninges
The spinal cord is an extension of the medulla oblongata. It has three covering membranes: the dura, arachnoid, and pia maters (Figure 6A). These membranes concentrically divide the vertebral canal into three distinct compartments: the epidural, subdural, and subarachnoid spaces. The epidural space contains fat, epidural veins, spinal nerve roots, and connective tissue (Figure 6B) The subdural space is a “potential” space between the dura and the arachnoid and contains a serous fluid.
The subdural compartment is formed by flat neuroepithelial cells that have long interlacing branches. These cells are in close contact with the inner dural layers. This space can be expanded by shearing the neuroepithelial cell layer connections with the collagen fibers of the dura mater.
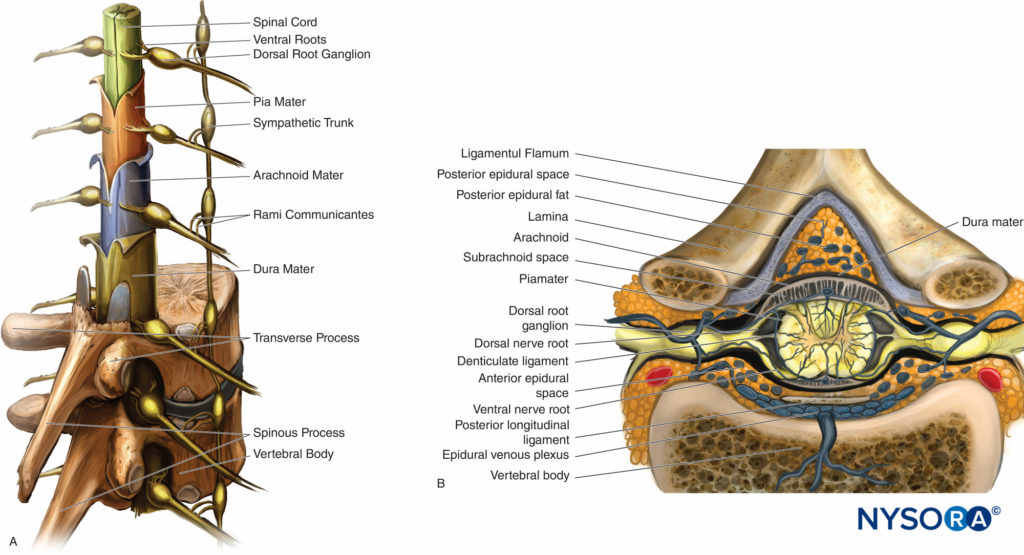
FIGURE 6. A. Sagittal view of the spinal cord with meningeal layers, dorsal root ganglia, spinal nerves, and sympathetic trunk. B. Crosssectional view of the spinal cord depicting the ligamentum flavum in respect to the posterior epidural space. Notice the close proximity of the posterior epidural space to the subarachnoid space.
This expansion of the subdural space can be caused mechanically by injecting air or a liquid such as contrast media or local anesthetics, which, by applying pressure in the space, separates the cell layers. The subarachnoid space is traversed by threads of connective tissue extending from the arachnoid mater to the pia mater. It contains the spinal cord, dorsal and ventral nerve roots, and cerebrospinal fluid (CSF). The subarachnoid space ends at the S2 vertebral level.
Spinal Cord
There are eight cervical neural segments. The eighth segmental nerve emerges between the seventh cervical and first thoracic vertebrae, whereas the remaining cervical nerves emerge above their same-numbered vertebrae. Thoracic, lumbar, and sacral nerves emerge from the vertebral column below the same-numbered bony segment1 (Figure 6A). Anterior and posterior spinal nerve roots arise from rootlets along the spinal cord. The roots of the upper and lower extremity plexuses (brachial and lumbosacral) are significantly larger compared to other levels.
The dural sac is continuous from the foramen magnum to the sacral region, where it spreads distally to cover the filum terminale. In children, the dural sac terminates lower, and in some adults, the sac termination can be as high as L5. The vertebral canal contains the dural sac, which adheres superiorly to the foramen magnum, to the posterior longitudinal ligament anteriorly, the ligamentum flavum and laminae posteriorly, and the pedicles laterally.
The spinal cord tapers and ends as the conus medullaris at the level of the L1–L2 intervertebral disk (Figure 7A). The filum terminale, a fibrous extension of the spinal cord, extends caudally to the coccyx. The cauda equina is a bundle of nerve roots in the subarachnoid space distal to the conus medullaris (Figure 7A).
The spinal cord receives blood primarily from one anterior and two posterior spinal arteries that derive from the vertebral arteries (Figure 7B). Other major arteries that supplement blood supply to the spinal cord include the vertebral, ascending cervical, posterior intercostal, lumbar, and lateral sacral arteries. The single anterior spinal artery and two posterior spinal arteries run longitudinally along the length of the cord and combine with segmental arteries in each region. The major segmental artery (Adamkiewicz) is the largest segmental artery and is found between the T8 and L1 vertebral segments. The Adamkiewicz artery is the major blood supplier to two-thirds of the spinal cord. Injury of this artery may result in anterior spinal artery syndrome, characterized by loss of urinary and fecal continence as well as impaired motor function of the legs. The radicular arteries are branches of the spinal arteries and run within the vertebral canal and supply the vertebral column. Radicular veins drain blood from the vertebral venous plexus and eventually drain into the major venous system: the superior and inferior vena cava and the azygos venous system of the thorax.
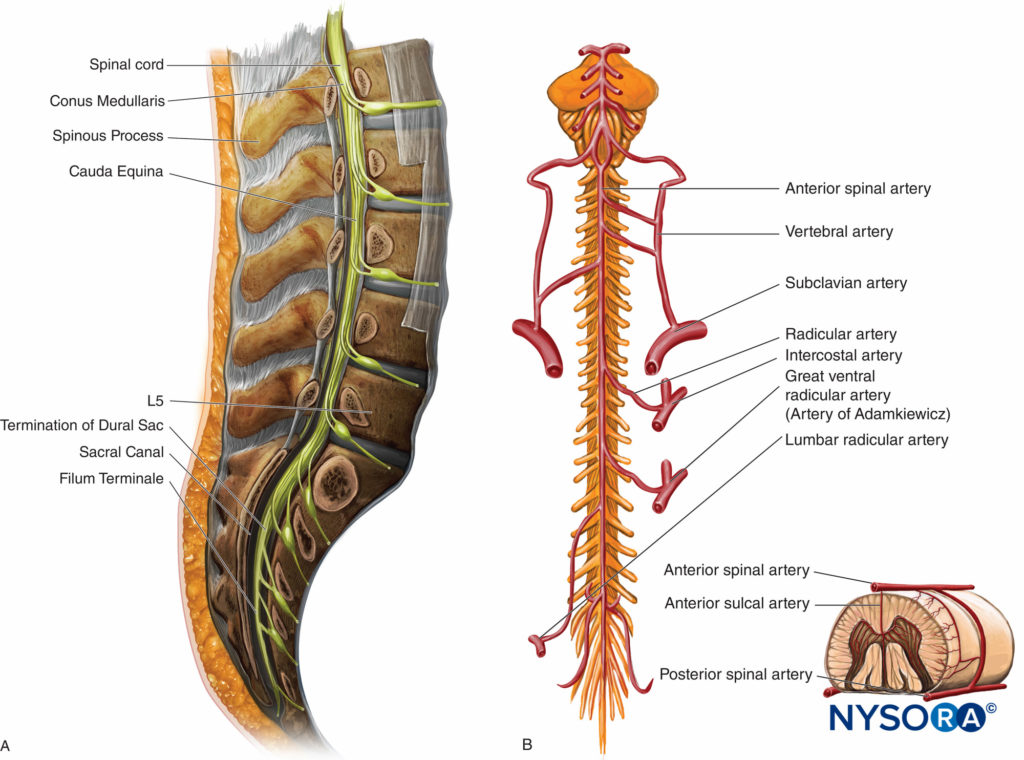
FIGURE 7. A. Sagittal view of the lumbar vertebrae. The spinal cord terminates at the L1-L2 interspace. B. Arterial supply to the anterior spinal cord. The Artery of Adamkiewicz emerges from T8-L1 vertebral segments. The small insert demonstrates the blood supply to the spinal cord (one anterior and two posterior arteries).
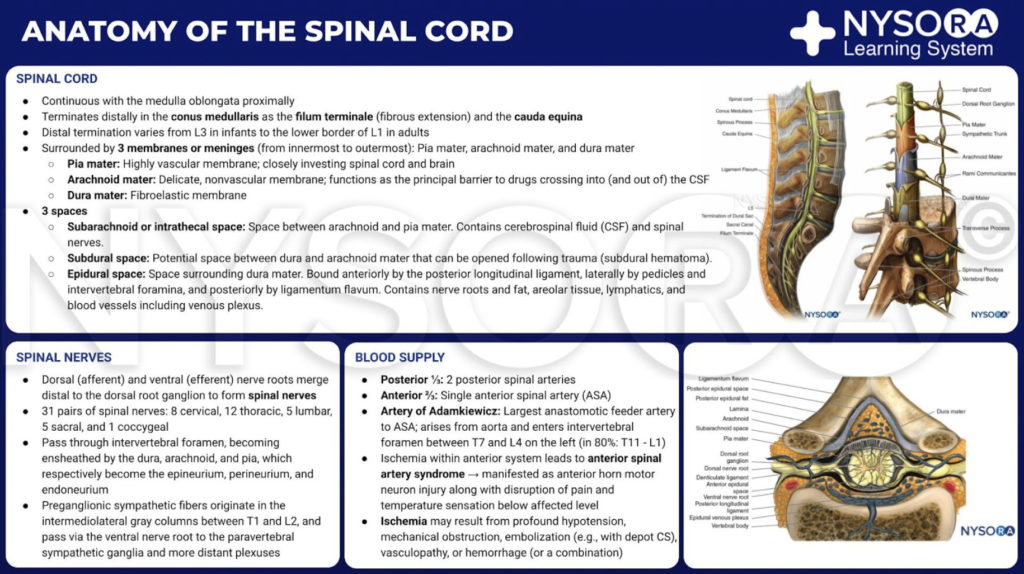
From the Compendium of Regional Anesthesia: Anatomy of the spinal cord infographic.
MOVEMENTS OF THE SPINE
The fundamental movements through the vertebral column are flexion, extension, rotation, and lateral flexion in the cervical and lumbar spine. Movement between individual vertebrae is relatively limited, although the effect is compounded along the entire spine. Thoracic vertebrae, in particular, have limited mobility due to the rib cage. Flexion is greatest in the cervical spine, whereas extension is greatest in the lumbar region. The thoracic and sacral regions are the most stable.
SPECIAL CONSIDERATIONS
In the United States and most developed countries, there is an increase in the aging population. This trend carries with it an increased prevalence of spinal deformities, such as spinal stenosis, scoliosis, hyperkyphosis, and hyperlordosis. Elderly patients present anesthetic challenges when neuraxial techniques are required. With advancing age, a diminishing thickness of intervertebral disks results in decreased height of the vertebral column. Thickened ligaments and osteophytes also contribute to difficulty in accessing both the subarachnoid and epidural spaces. The frequency of spinal deformities in older adults can be as high as 70%.
Adult scoliosis, in particular, is frequently encountered in older adults. In fact, Schwab et al demonstrated that scoliosis was present in 68% of an asymptomatic volunteer population older than 60 years of age. A thorough understanding of the scoliotic spine will aid in successfully performing central neuraxial block in this patient population. In the scoliotic spine, vertebral bodies are rotated toward the convexity of the curve, and their spinous processes face into the concavity of the curve (Figure 8).
The diagnosis of scoliosis is made when there is a Cobb angle of greater than 10° in the coronal plane of the spine in a skeletally mature patient. The Cobb angle, which is used to measure the magnitude of scoliosis, is formed between a line drawn parallel to the superior endplate of one vertebra above the curve deformity and a line drawn parallel to the inferior endplate of the vertebra one level below the curve deformity (Figure 8). In untreated patients, there is a strong linear relationship between the Cobb angle and the degree of vertebral rotation in both thoracic and lumbar curves, with maximum rotation occurring at the apex of the scoliotic curve. A compensatory curvature of the spine always occurs in the opposite direction of the scoliotic curve.
Scoliosis usually presents in childhood or adolescence and is diagnosed during routine physical examination. Untreated, it may become progressive and result in respiratory impairment and gait disturbances. Scoliosis may also go undiagnosed and present later in life as back pain.
Treatment depends on the severity of the scoliosis. Mild scoliosis (11°–25°) is usually observed. Moderate scoliosis (25°–50°) in the skeletally immature patient frequently progresses and therefore is most often braced. Patients with severe scoliosis (>50°) are usually treated surgically.
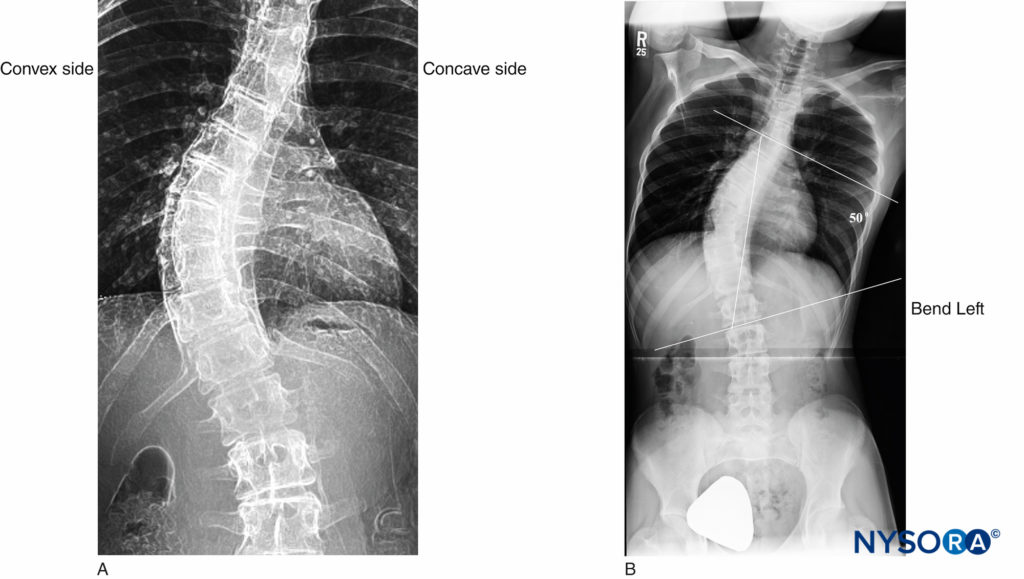
FIGURE 8. Adolescent scoliotic spine. A: S-shaped scoliosis of the thoracolumbar spine. B: Cobb angle of 50°.
The degree of vertebral body rotation along the long axis of the spine influences the orientation of a needle during insertion for neuraxial anesthesia. In the patients with scoliosis, the vertebral body rotates toward the convex side of the curve. As a result of this rotation, the spinous processes point toward the midline (the concave side). This results in a larger interlaminar space on the convex side of the spine. A direct path to the neuraxial space is created by this vertebral body rotation, allowing the use of a paramedian approach from the convex side of the curve (Figure 9). Surface landmarks, particularly the spinous process, may be difficult to identify in the severe scoliotic spine. X-rays, and most recently preprocedural ultrasound scanning, may be useful to determine the longitudinal angulation of the spine, the location and orientation of the spinous process, as well as the depth of the lamina.
FIGURE 9. Paramedian approach in a scoliotic spine; arrow B represents the needle realignment towards the convex side of the scoliotic spine compared to arrow A, which depicts the usual paramedian approach in a normal spine.
NYSORA Tips
- The spinal cord ends at the L1-to-L2 level; performing spinal anesthesia at or above this level is not recommended.
- Failure of the ligamentum flavum to fuse in the cervical and upper thoracic levels may reduce the sense of loss of resistance with a midline approach to epidural anesthesia. A paramedian approach may be more suitable at these levels because the needle is advanced to a point where the presence of a ligamentum flavum is most reliable, enabling successful access to the epidural space.
- In patients with scoliosis, a paramedian approach from the convex side may be more successful.
REFERENCES
- Standring S (ed): Gray’s Anatomy: The Anatomical Basis of Clinical Practice, 40th ed. Churchill Livingston, Elsevier Health, 2008.
- Hogan QH: Lumbar epidural anatomy. A new look by cryomicrotome section. Anesthesiology 1991;75:767-775.
- Scapinelli R: Morphological and functional changes of the lumbar spinous processes in the elderly. Surg Radiol Anat 1989;11:129.
- Aggarwal A, Kaur H, Batra YK, et al: Anatomic consideration of caudal epidural space: A cadaver study. Clin Anat 2009;22:730.
- Hogan QH: Epidural anatomy: New observations. Can J Anaesth 1998; 45:40.
- Zarzur E: Anatomic studies of the human lumbar ligamentum flavum. Anesth Analg 1984;63:499.
- Yoon SP, Kim HJ, Choi YS: Anatomic variations of cervical and high thoracic ligamentum flavum. Korean J Pain 2014;27:321.
- Lirk P, Colvin J, Steger B, et al: Incidence of lower thoracic ligamentum flavum midline gaps. Br J Anaesth 2005;94:852.
- Lirk P, Kolbitsch C, Putz G, et al. Cervical and high thoracic ligamentum flavum frequently fails to fuse in the midline. Anesthesiology 2003; 99:1387.
- Reina MA, Lopez Garcia A, de Andres JA, Villanueva MC, Cortes L: Does the subdural space exist? Rev Esp Anestesiol Reanim 1998;45:367.
- Kostelic JK, Haughton VM, Sether LA: Lumbar spinal nerves in the neural foramen: MR appearance. Radiology 1991;178:837.
- MacDonald A, Chatrath P, Spector T, et al: Level of termination of the spinal cord and the dural sac: A magnetic resonance study. Clin Anat 1999;12:149.
- Schwab F, Dubey A, Gamez L, et al: Adult scoliosis: prevalence, SF-36, and nutritional parameters in an elderly volunteer population. Spine 2005;30:1082.
- McLeod A, Roche A, Fennelly M: Case series: Ultrasonography may assist epidural insertion in scoliosis patients. Can J Anaesth 2005;52:717.
- Aebi M: The adult scoliosis. Eur Spine J 2005;14:925.
- Smith JS, Shaffrey CI, Fu KM, et al: Clinical and radiographic evaluation of the adult spinal deformity patient. Neurosurg Clin N Am 2013;24:143.
- White AA, Panjab MM: Clinical Biomechanics of the Spine, 2nd ed. Lippincott, 1990.
- Suzuki S, Yamamuro T, Shikata J, et al: Ultrasound measurement of vertebral rotation in idiopathic scoliosis. J Bone Joint Surg Br 1989;71:252.
- Glassman SD, Berven S, Bridwell K, et al: Correlation of radiographic parameters and clinical symptoms in adult scoliosis. Spine 2005;30:682.
- Bowens C, Dobie KH, Devin CJ, et al: An approach to neuraxial anaesthesia for the severely scoliotic spine. Br J Anaesth 2013;111:807.
- Huang J: Paramedian approach for neuraxial anesthesia in parturients with scoliosis. Anesth Anal 2010;111:821.
- Ko JY, Leffert LR: Clinical implications of neuraxial anesthesia in the parturient with scoliosis. Anesth Analg 2009;09:1930.
- Chin KJ, Perlas A, Chan V, et al: Ultrasound Imaging facilitates spinal anesthesia in adults with difficult surface anatomic landmarks. Anesthesiology 2001;115:94.
- Chin KJ, Karmakar MK, Peng P: Ultrasonography of the adult thoracic and lumbar spine for cetral neuraxial block. Anesthesiology 2011; 114:1459.
- Chin KJ, MacFarlane AJR, Chan V, Brull R: The use of ultrasound to facilitate spinal anesthesia in a patient with previous lumbar laminectomy and fusion: A case report. J Clin Ultrasound 2009;37:482.