Application of ultrasound in pain medicine (USPM) is a rapidly growing medical field in interventional pain management [1]. In general, the application of USPM can be divided into three areas: peripheral, axial, and musculoskeletal structures. In this chapter, we will review the relevant anatomy, sonoanatomy, and the injection techniques for three peripheral structures: lateral femoral cutaneous nerve (LFCN), intercostal nerve (ICN), and suprascapular nerve (SSN).
1. LATERAL FEMORAL CUTANEOUS NERVE BLOCK
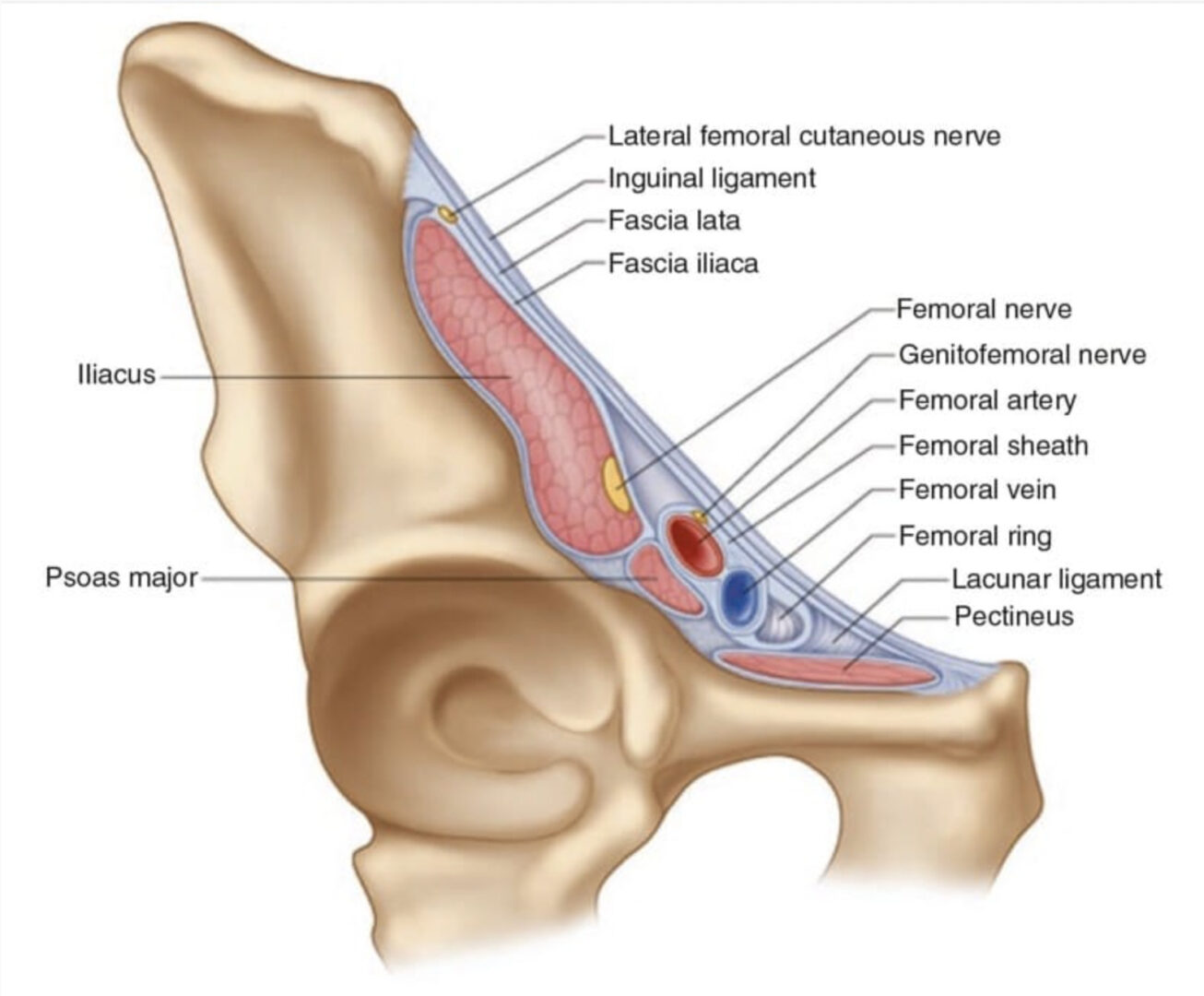
The LFCN provides sensory innervation to the skin of the anterior and lateral parts of the thigh as far as the knee (Fig. 1). Regional block of the LFCN is performed for acute pain relief following surgical procedures and for the diagnosis and treatment of meralgia paresthetica [2, 3]. Meralgia paresthetica refers to a symptom complex of pain, numbness, tingling, and paresthesia in the anterolateral thigh that is secondary to nerve entrapment or compression, trauma, or stretch. The incidence in a primary care setting is estimated at 4.3 per 10,000 person years [4].
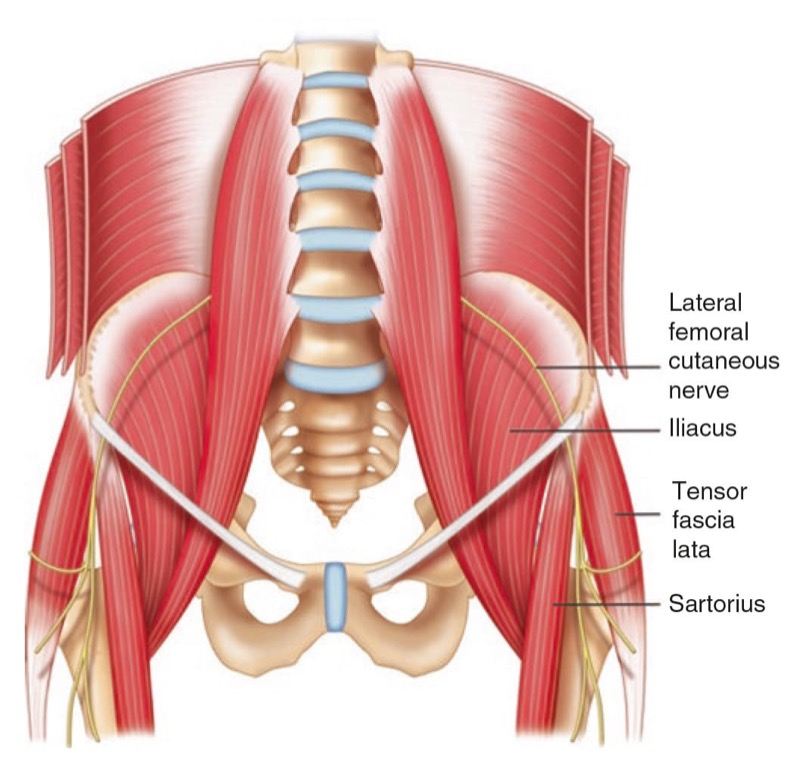
Fig. 1 The pathway of a typical course of lateral femoral cutaneous nerve is shown. Note that the nerve courses beneath the inguinal ligament and runs superficially to the sartorius muscle and then in between this muscle and tensor fascia lata muscle. (Reproduced with permission from Philip Peng Educational Series)
2. ANATOMY
The LFCN is a purely sensory nerve that arises from branches of dorsal divisions of the second and third lumbar nerves. It emerges from the lateral border of the psoas major and crosses the iliacus muscle obliquely, toward the anterior superior iliac spine (ASIS) [5]. The nerve then passes under the inguinal ligament at a distance 36 ± 20 mm medial to the ASIS, and after entering the thigh, the LFCN turns laterally and downward, where it typically divides into the anterior and posterior branches (Fig.1) [6]. The course and location of the LFCN as it crosses the inguinal ligament have been found to be quite variable. While the nerve courses medial to the ASIS most of the time, it can pass over or even posterior to the ASIS in up to 25% of patients [5–7]. Though in the vast majority of cases, the LFCN enters the thigh superficial to the sartorius muscle beneath the fascia lata, in 22% of cases, the LFCN passes through the muscle itself [8]. The LFCN has been shown to cross under the inguinal ligament as far as 4.6–7.3 cm medial to the ASIS [6, 9, 10]. The LFCN divides into an anterior and a posterior branch in the thigh. The anterior branch becomes superficial at a variable distance below the inguinal ligament and divides into branches that are distributed to the skin of the anterior and lateral parts of the thigh, as far as the knee. The posterior branch pierces the fascia lata and subdivides into filaments that pass backward across the lateral and posterior surfaces of the thigh, supplying the skin from the level of the greater trochanter to the middle of the thigh [11].
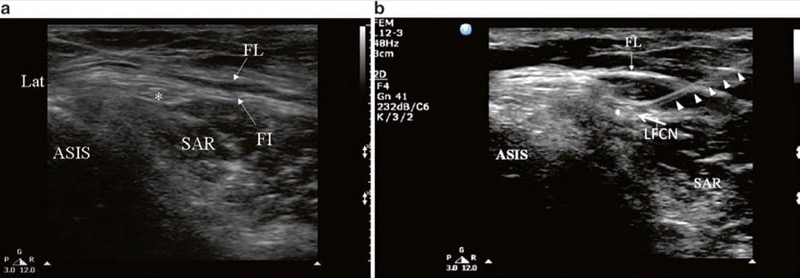
Fig. 2 Ultrasonographic image of lateral femoral cutaneous nerve (LFCN) (a) before and (b) after injection. FL fascia lata, FI fascia iliaca, SAR sartorius muscle, ASIS anterior superior iliac spine. Solid arrow head indicates the path of the needle; LFCN is indicated by asterisk. (Reproduced with permission from Lippincott Williams & Wilkins)
3. LITERATURE REVIEW OF INJECTION TECHNIQUES
The traditional approach to blocking the LFCN is a blind, landmark-assisted technique. The success of this method is variable, quoted success rates being as low as 38% [12]. The low success rate of the block can be attributed to the wide anatomic variability in the course of the LFCN, as well as to the lack of any predictable relationship of the LFCN to palpable vascular structures or bony landmarks [3].
There are a few published reports of the use of ultrasound to identify and block the LFCN [3, 13–16]. One of these was a study that demonstrated greater accuracy in identifying the LFCN with ultrasound in both cadavers and volunteers [13]. In the cadavers, 16 out of 19 needles (84.2%) inserted with ultrasound guidance were in contact with the LFCNs compared with 1 out of 19 (5.3%) when needles were inserted according to landmarks. In the same study, 16 out of 20 (80%) marked positions identified using ultrasound imaging corresponded to the LFCN position in human volunteers identified by percutaneous nerve stimulator compared to 0 out of 20 positions marked by anatomic landmarks.
In a case series of 10 patients with a mean BMI of 31, the author reported that the LFCN could be visualized by ultrasound in all patients and that sensory block was successful in all cases [3]. The technique was not complicated by coincidental block of any nearby nerves, nor did any patients complain of paresthesia from the needle coming into direct contact with the LFCN. In a prospective case series of 20 patients, US-guided injections of perineural steroids were performed around the LFCN at three different levels (at the level of the anterior superior iliac spine, just distal to the inguinal ligament, and lower thigh). The level of injection was that closest to “swelling” (increased cross-sectional area) of the nerve as perceived on sonographic examination. All patients displayed complete or partial resolution of symptoms of meralgia paresthetica at 12 months after the injections [14].
4. ULTRASOUND-GUIDED BLOCK TECHNIQUE
Locating this nerve with ultrasound can be a challenge as the LFCN is a small nerve and its course is highly variable.
However, a few important principals may assist the beginners to locate the nerve:
1. A sound knowledge of anatomy of the course and direction of the LFCN as well as the structures around LFCN [16].
2. The nerve is better appreciated with dynamic scanning or sweeping view because of the size of the nerve and its proximity with fascia layer [3, 16].
3. The LFCN may appear as hyperechoic, hypoechoic, or mixed structure, depending on the course of the nerve itself (under or through the inguinal ligament or over the iliac crest), the special tissue architecture in the corresponding area, and the frequency of the transducer used (the higher frequency probe is likely to produce artifacts) [3, 13, 16].
4. In patients with severe or advanced symptoms of meralgia paresthetica, the LFCN is likely to be swollen or enlarged (pseudoneuroma) and likely to be picked up (ultrasonography) [8].
5. The LFCN can be usually found in the infra-inguinal region, either superficial to the sartorius muscle or between sartorius and tensor of fascia lata muscles.
With the patient in the supine position, the ASIS and the inguinal ligament are marked on the skin. Using a highfrequency linear array transducer (6–13 MHz), the ultrasound probe is placed over the ASIS initially, with the long-axis view of the inguinal ligament, and is then moved distally. The ASIS is visualized a hyperechoic structure with posterior acoustic shadowing (Fig. 2). The sartorius muscle will be seen as an inverted triangular-shaped structure. Attention is paid to the orientation of the probe to the course of the nerve. The LFCN will appear as one or more hypoechoic structures in the short-axis view superficial to the sartorius muscle. In some situation, it will be in a more medial position sandwiched between the fascia lata and fascia iliaca (Fig. 2). When the nerve cannot be found in this area, one can look for the LFCN in the angle between the tensor of the fascia lata and the sartorius muscle. Once the LFCN has been identified, a 22-G 2.5-in. needle is advanced in plane with the ultrasound probe. Alternatively, the needle can be advanced out of plane using a nerve-stimulating needle to confirm placement.
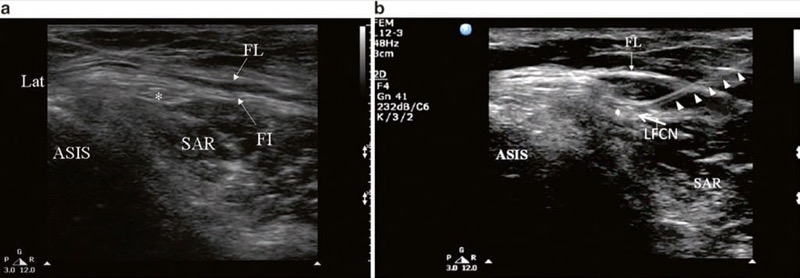
Fig. 2 Ultrasonographic image of lateral femoral cutaneous nerve (LFCN) (a) before and (b) after injection. FL fascia lata, FI fascia iliaca, SAR sartorius muscle, ASIS anterior superior iliac spine. Solid arrow head indicates the path of the needle; LFCN is indicated by asterisk. (Reproduced with permission from Lippincott Williams & Wilkins)
If it is difficult to identify the LFCN, two other methods can be employed. One is to inject dextrose 5% solution to hydro-dissect the plane between the fascia lata and the fascia over the sartorius and iliacus muscles [15]. The other is to locate the nerve with a transdermal nerve stimulator or to use a stimulating needle [13]. Once the nerve is identified, injection is commenced. The injectate should be visualized by ultrasound as it spreads around the nerve circumferentially and in a cephalad manner, and a total volume of 5–10 ml is usually adequate to ensure complete block.
5. SUPRASCAPULAR NERVE BLOCK
First described in 1941 [17], SSN block has been performed over the years by anesthesiologists, rheumatologists, and pain specialists for the management of acute and chronic shoulder pain [1, 18, 19]. Indications for performing this block in interventional pain practice include adhesive capsulitis, frozen shoulder, rotator cuff tear, and glenohumeral arthritis secondary to degeneration or inflammation [20]. There has been renewed interest in the technique of performing SSN block under ultrasound guidance, and descriptions of this method have appeared in recent published medical literature [21–23].
6. ANATOMY
The SSN, a mixed sensory and motor nerve, originates from the superior trunk of the brachial plexus (formed by the union of the fifth and sixth cervical nerves), runs parallel to the omohyoid muscle, and courses under the trapezius (Fig. 3) before it passes under the transverse scapular ligament in the suprascapular notch. It then passes beneath the supraspinatus and curves around the lateral border of the spine of the scapula (spinoglenoid notch) to the infraspinatus fossa (Fig. 4). In the supraspinatous fossa, it gives off two branches to the supraspinatus muscle and an articular branch to the shoulder joint; and in the infraspinatus fossa, it gives off two branches to the infraspinatus muscle, besides some branches to the shoulder joint and scapula. The sensory component of the SSN provides fibers to about 70% of the shoulder joint.
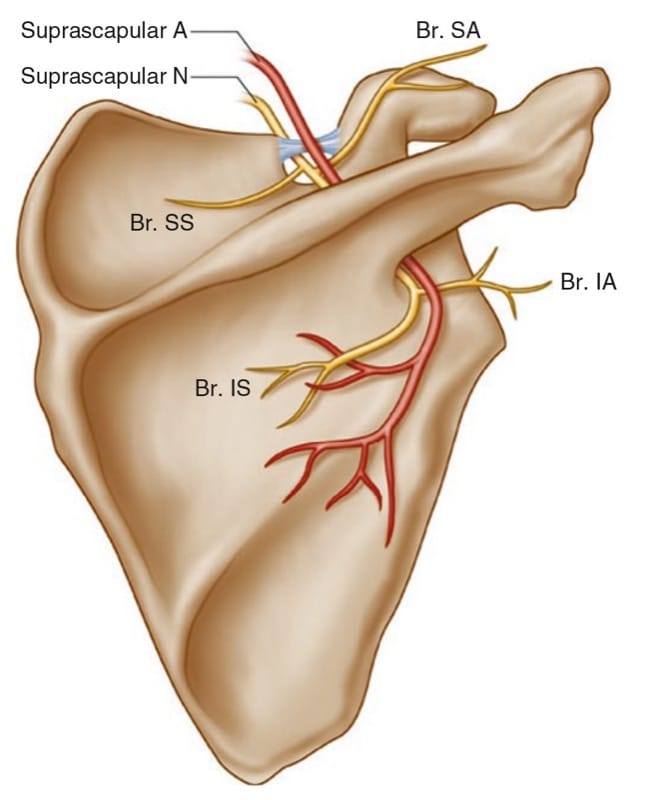
Fig.3 Suprascapular nerve and its branches. Superior articular branch (Br. SA) supplies the coracohumeral ligament, subacromial bursa, and posterior aspect of the acromioclavicular joint capsule; inferior articular branch (Br. IA) supplies the posterior joint capsule; Br. SS branch to the supraspinatus muscle, Br. IS branch to the infraspinatus muscle
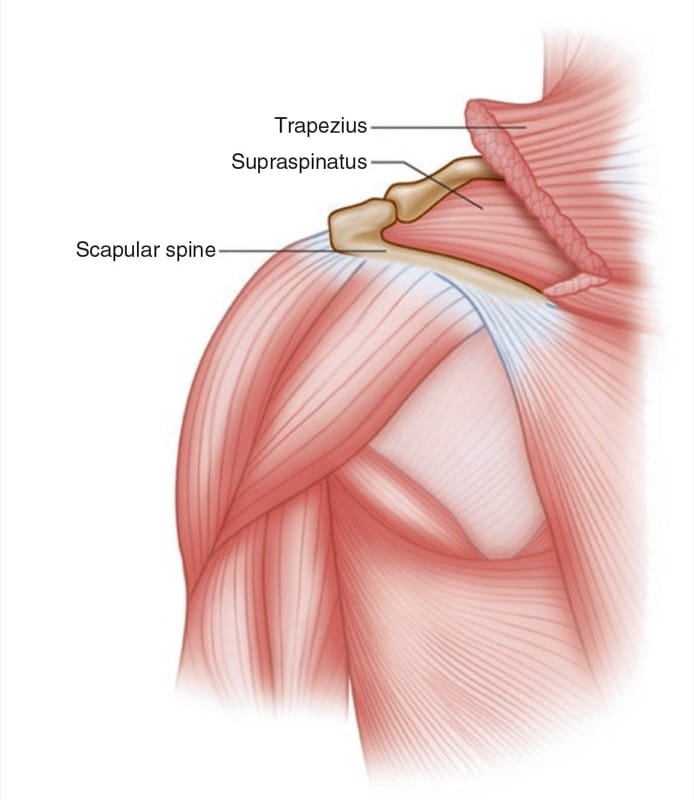
Fig.4 Left shoulder showing the muscle layers in the suprascapular fossa
The “U-” or “V-“shaped suprascapular notch is located on the superior margin of the scapula, medial to the coracoid process (Fig.5). However, the notch is absent in up to 8% of cadavers [24]. Above the notch run the suprascapular artery and vein, although rarely the artery travels along with the SSN through the notch [25]. The supraspinous fossa is bordered by the spine of the scapula dorsally, by the plate of the scapula ventrally, and by the supraspinous fascia superiorly, forming a classic compartment, the only exit through which is the suprascapular fossa [26, 27].
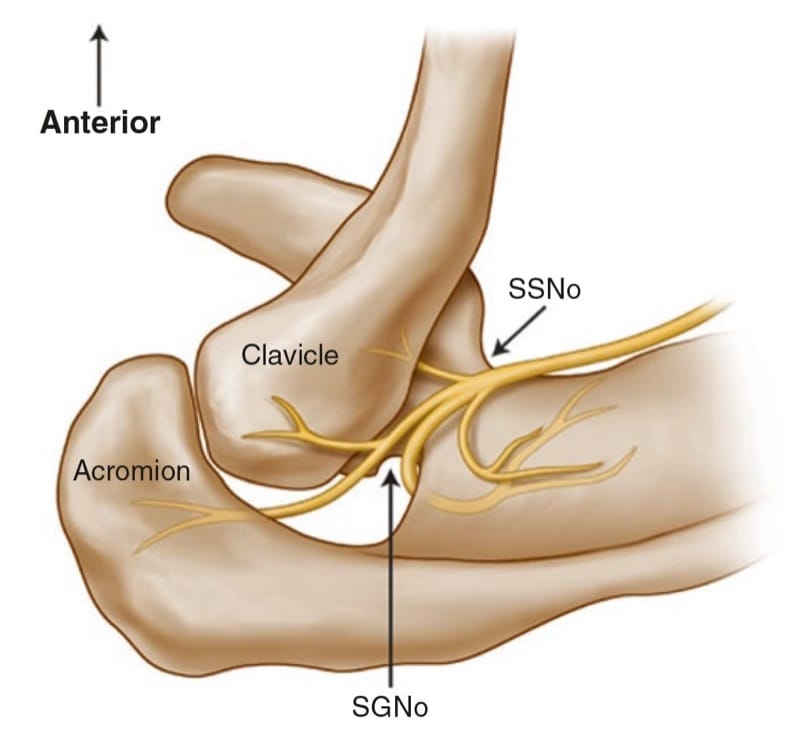
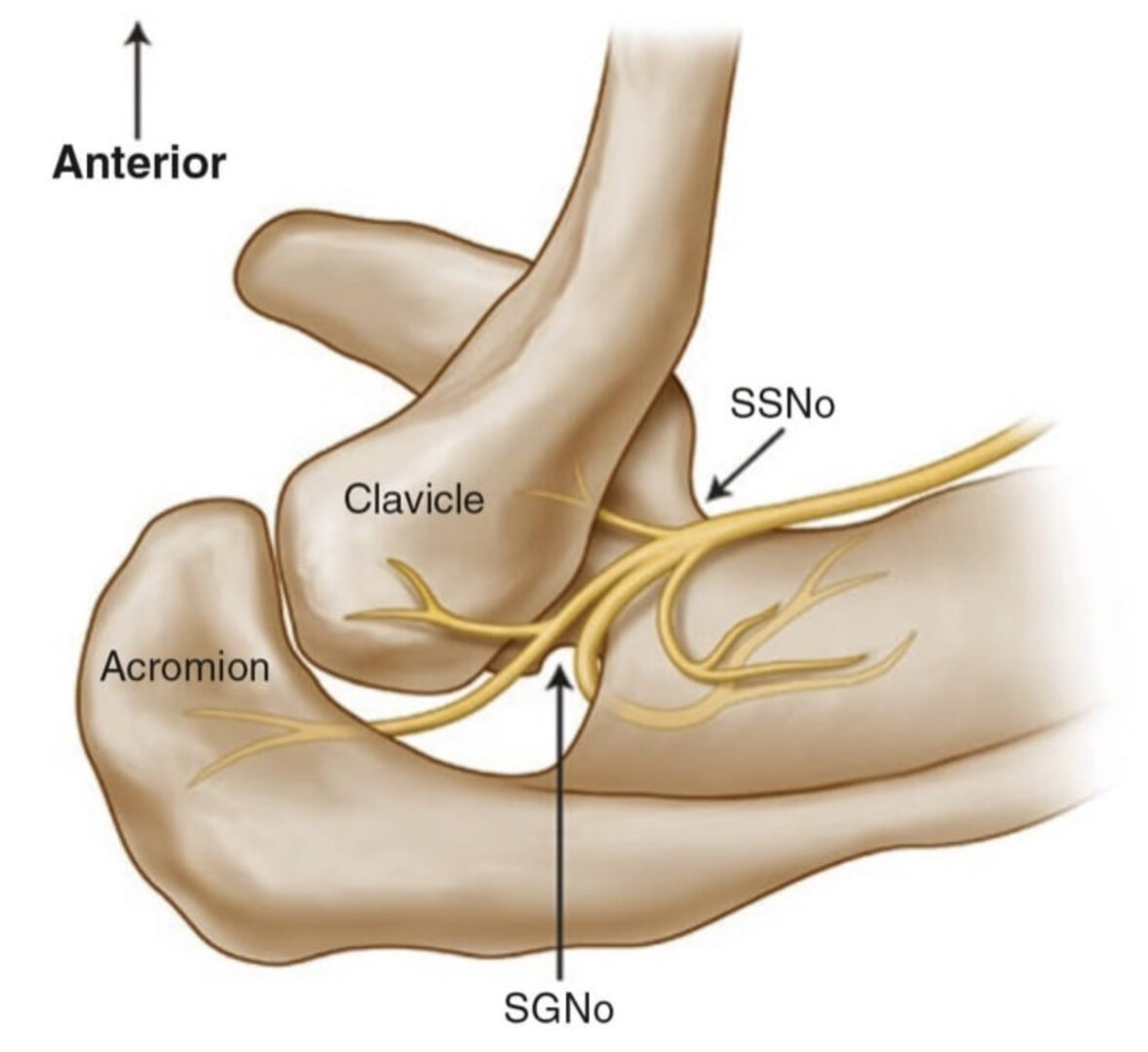
Fig.5 Superior view of the left shoulder. The course of the suprascapular nerve enters the suprascapular fossa through the suprascapular notch (SSNo) and then enters the infrascapular fossa through the spino-glenoid notch (SGNo)
7. LITERATURE REVIEW OF INJECTION TECHNIQUES
The targets for most of the techniques are either at the suprascapular notch or on the floor of the scapular spine. Without image guidance, techniques relying on identification of the suprascapular notch have the potential for SSN block failure and/or adverse effects. The risk of pneumothorax is approximately 1%, and this complication usually arises from the needle being inserted too deep [28, 29]. If the needle is placed blindly into the notch, the needle tip is unlikely to approximate the notch as demonstrated by a study using CT to confirm the position of the needle [30]. With the use of fluoroscopy, the position of the needle in the notch can be assured. However, there is a potential of spilling of local anesthetic to the brachial plexus [26]. A superior approach has been described in which the needle is inserted vertically into the suprascapular fossa. Large volumes of solution (10 ml or more) will accomplish this, but according to a recent study in cadavers, there will be spread to the axillary fossa in a minority of these cases [27].
Thus, the ideal site to perform the SSN injection is at the floor of the scapular spine between the suprascapular notch and spinoglenoid notch (Figs. 5 and 6). First, this technique is independent of the notch as a target. Thus, it avoids the risk of pneumothorax if one considers the direction of the needle. This technique is also feasible in individuals without a suprascapular notch (8% of the population). Second, the suprascapular fossa forms a compartment and retains the local anesthetic around the nerve. One of the easiest ways to visualize this soft tissue plane is by the use of ultrasound [31].
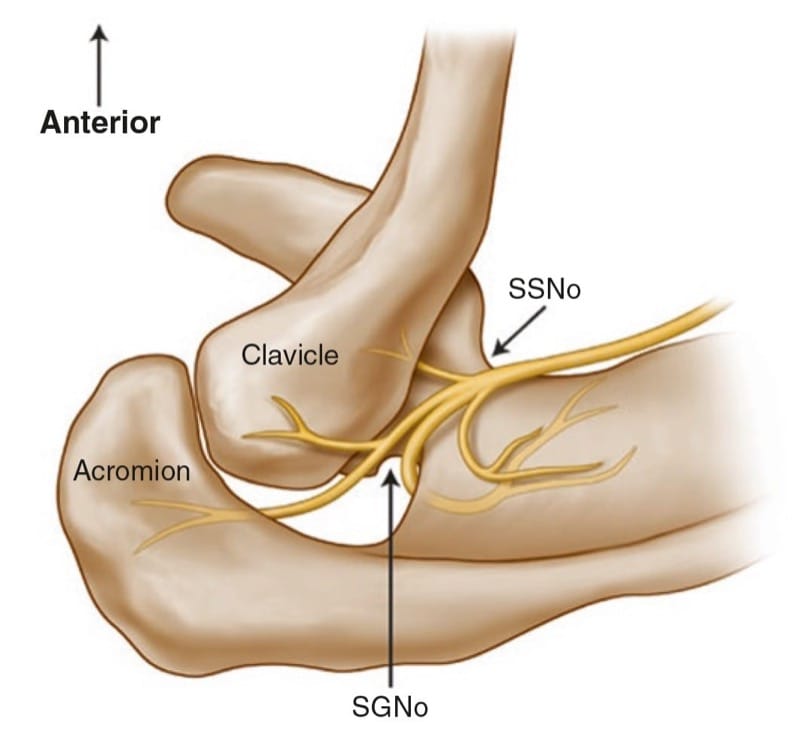
Fig.5 Superior view of the left shoulder. The course of the supra-scapular nerve enters the suprascapular fossa through the suprascapular notch (SSNo) and then enters the infrascapular fossa through the spino-glenoid notch (SGNo)
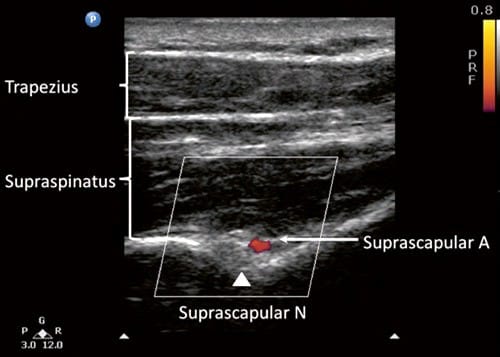
Fig.6 Ultrasonographic image of the suprascapular nerve on the floor of the scapular spine between suprascapular notch and spinogle-noid notch. Both suprascapular nerve and artery run underneath the fascia of supraspinatus muscle. (Reproduced with permission from Philip Peng Educational Series)
To date there is one case series evaluating the ultrasonographic morphology of the suprascapular notch [23]. This series reported results of measurement of the notch width, depth, and distance between the skin and notch based on 50 volunteers. The authors were able to visualize the transverse scapular ligament in 96% and the artery-vein complex in 86% of the volunteers. Although visualization of the transverse scapular ligament is feasible, the probe has to be steady in a very narrow angle, making the needle advancement a very challenging technique (Figs. 7 and 8). It is also important to recognize the sonographic appearance of the floor of the scapular spine in between the scapular notch and spinoglenoid notch (Fig. 6).
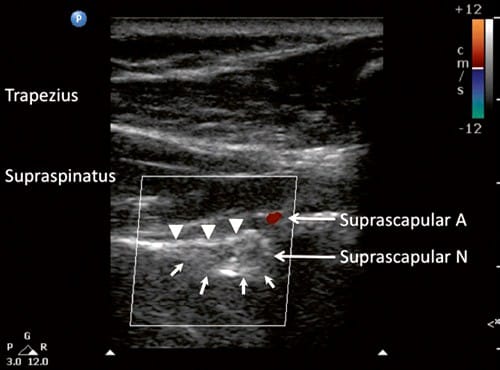
Fig.7 Ultrasonographic image of suprascapular nerve in the suprascapular notch (indicated by line arrows). Note that at this level, the suprascapular artery is above the transverse scapular ligament (solid arrow heads). A artery, N nerve. (Reproduced with permission from Philip Peng Educational Series)
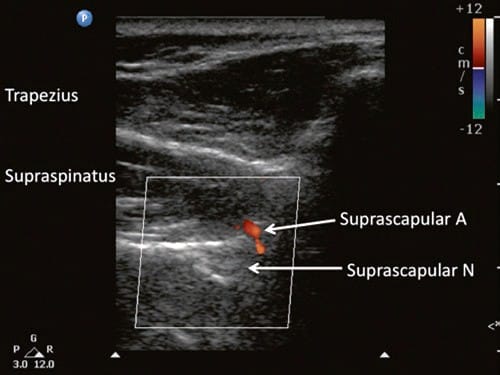
Fig.8 Ultrasonographic image of the suprascapular nerve slightly posterior to the plane obtained in Fig. 7. The suprascapular artery can be seen running toward the floor of the scapular spine. (Reproduced with permission from Philip Peng Educational Series)
8. ULTRASOUND-GUIDED BLOCK TECHNIQUE
The patient can be in sitting (or in prone) position. The scapula spine, coracoid process, and acromion are used as landmarks. Ultrasound scanning is performed with a linear ultrasound probe (7–13 MHz) placed in a coronal plane over the suprascapular fossa with a slight anterior tilt. The probe is placed in an orientation such that it is in the short axis to the line joining coracoid process and acromion (reflecting the position of the spinoglenoid notch) [1]. This line corresponds to the course of the suprascapular nerve between the scapular notch and the spinoglenoid notch. The supraspinatus and trapezius muscles and the bony fossa underneath them should come into view (Fig. 6). By adjusting the angle of the ultrasound probe in a cephalocaudal direction, the SSN and artery should be brought into view in the trough of the floor. The nerve can sometimes be difficult to visualize as it has an approximate diameter of 25 mm. A 22-G, 80-mm needle is inserted either in-plane from the medial aspect of the probe as the presence of the acromion process on the lateral side makes it difficult to angulate the needle. Because of the proximity of the nerve, an injectate volume of 5–8 ml is usually sufficient.
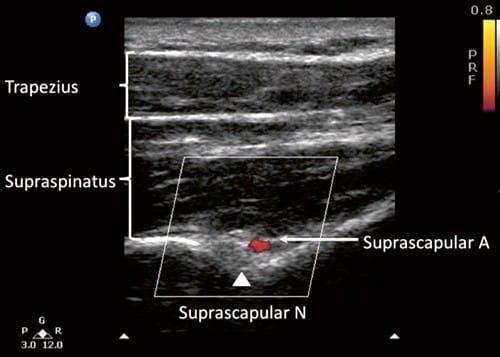
Fig.6 Ultrasonographic image of the suprascapular nerve on the floor of the scapular spine between suprascapular notch and spinogle-noid notch. Both suprascapular nerve and artery run underneath the fascia of supraspinatus muscle. (Reproduced with permission from Philip Peng Educational Series)
9. INTERCOSTAL NERVE BLOCK
The ICNs supply the skin and musculature of the chest and abdominal wall. ICN block is performed for the treatment of acute and chronic pain conditions affecting the thorax and upper abdomen [32, 33]. ICN block provides excellent analgesia for pain from rib fractures [34] and from chest and upper abdominal surgery [35]. Neurolytic ICNB may be used to manage chronic pain conditions such as postmastectomy and postthoracotomy pain [36] and pain from rib metastasis [37].
10. ANATOMY
The ICNs originate from the first 12 thoracic nerves. Emerging from their respective intervertebral foramen, the thoracic nerves divide into posterior cutaneous rami that supply the skin and muscle in the paravertebral region and ventral rami that become the ICNs (Fig. 9). ICNs are mixed sensory-motor nerves. After exiting from the spine, it is located between the pleura and the posterior intercostal membrane and subsequently traverses the membrane to lie deep to or in the internal intercostal muscle (Fig. 10). The intercostal vein and artery run in close proximity in this groove, just superior to the nerve (Fig. 11) [38]. The neurovascular bundle lies in the intercostal space but runs deep to the subcostal grove at the angle of the rib. At a distance of about 5–8 cm anterior to the angle of the rib, the groove ends and blends into the surface of the lower edge of the rib [39]. The lateral cutaneous branch of the ICN, which supplies the skin of the chest, branches off and pierces the external intercostal muscle in the region between the posterior and midaxillary line. As the ICNs approaches the midline anteriorly, it pierces the overlying muscles and skin to terminate as the anterior cutaneous branch.
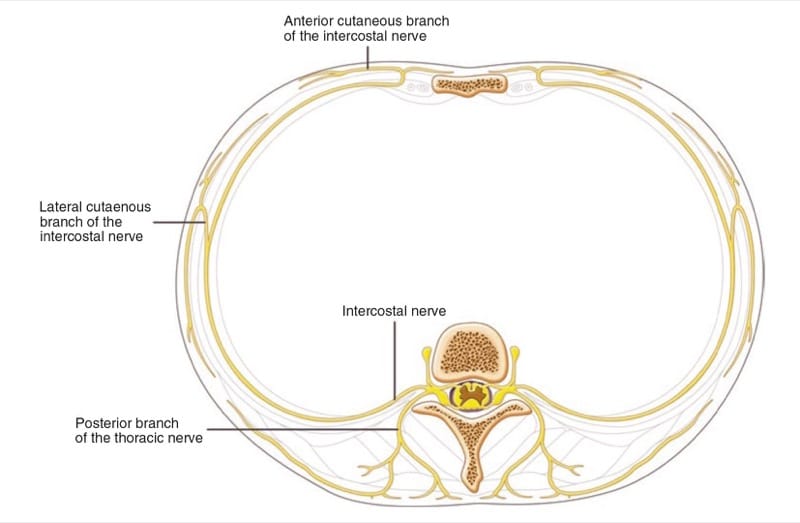
Fig.9 Branches of the typical intercostal nerves.
(Reproduced with permission from Philip Peng Educational Series)
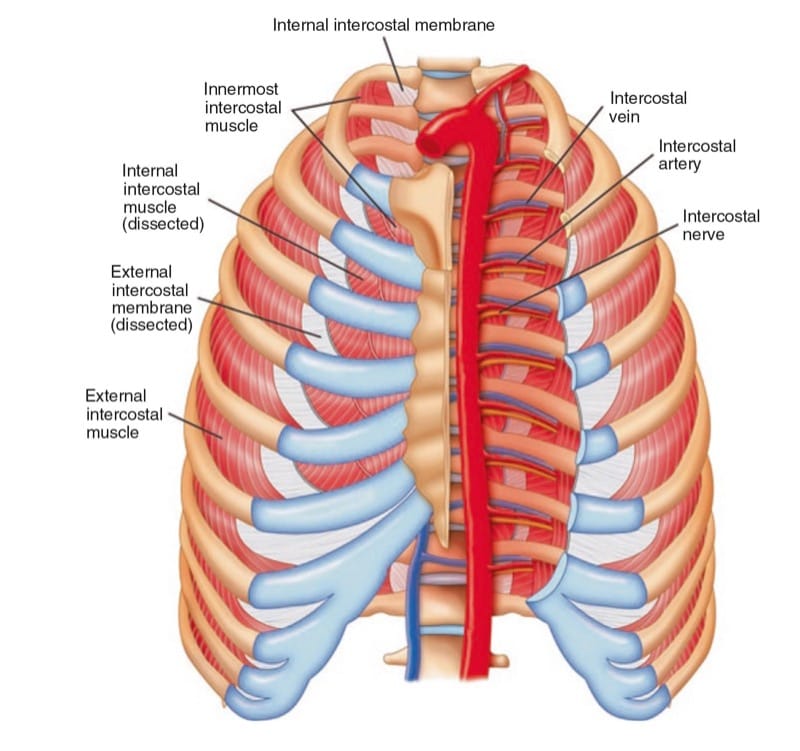
Fig.10 Intercostal muscles in the chest wall.
(Reproduced with permission from Philip Peng Educational Series)
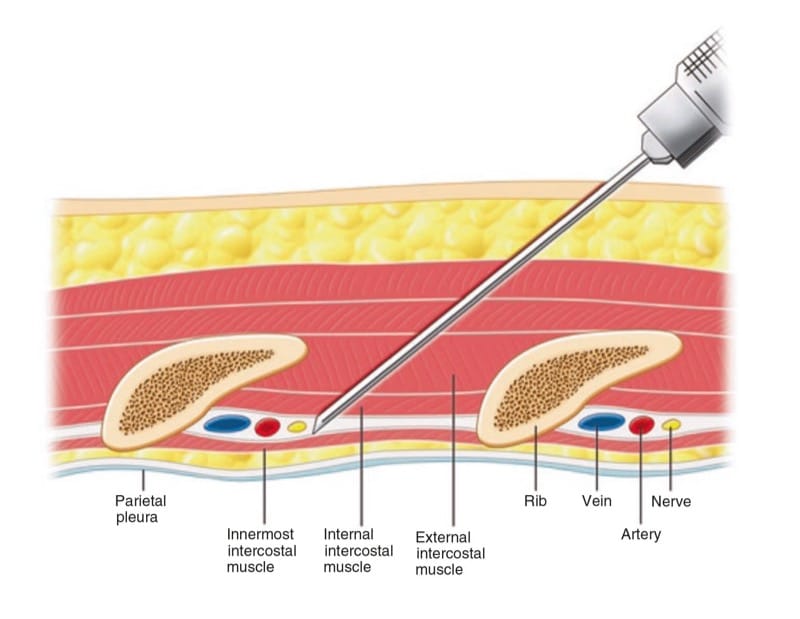
Fig.11 Cross section of chest wall showing intercostal muscles and neurovascular bundles. (Reproduced with permission from Philip Peng Educational Series)
However, there are some exceptions – the first ICN has no anterior cutaneous branch and usually has no lateral cutaneous branch, and most of its fibers leave the intercostal space by crossing the neck of the first rib to join those from C8, while a smaller bundle continues on as a genuine ICN to supply the muscles of the intercostal space. Some fibers of the second and third ICNs give rise to the intercostobrachial nerve, which innervates the axilla and the skin of the medial aspect of the upper arm as far distal as the elbow. The ventral rami of the 12th ICN are similar to the other ICNs but are called a subcostal nerve because it is not in between the two ribs.
11. LITERATURE REVIEW OF INJECTION TECHNIQUES
The classic landmark-based technique is performed with the patient in the sitting or prone position. ICN block is usually performed at the angle of the rib to ensure that the tissues innervated by the lateral cutaneous nerve are blocked. The needle is angled slight cephalad and walked off the inferior margin of the rib into the subcostal grove, where the needle is advanced 2–3 mm further. The small distance (as little as 0.5 cm) between the rib’s inferior margin and the pleura cannot be overemphasized [37]. The injection is performed after negative aspiration for air and blood, but this maneuver cannot reliably prevent pneumothorax and/or hemothorax. The incidence of pneumothorax ranges anywhere from 0.09% to 8.7% [33, 40, 41].
The fluoroscopic technique is performed with the patient in prone position. The appropriate rib is identified under fluoroscopic AP view, and the needle is introduced in the inferior margin of the rib. Following negative aspiration, a contrast injection is performed to ensure appropriate spread prior to injection [42]. This technique does not theoretically minimize the risk of pneumothorax because the pleura cannot be visualized with fluoroscopy.
The feasibility of US-guided ICN injection has been confirmed in a small cadaver study [43]. A small case series also confirmed the feasibility and technical advantages of US-guided cryoablation of the ICNs in four patients with postthoracotomy pain syndrome [36].
12. ULTRASOUND-GUIDED BLOCK TECHNIQUE
With the patient in prone position, a 6–13 MHz linear transducer is placed in the short axis to the ribs so that two consecutive ribs are simultaneously observed. The best site for injection is the angle of the rib (6–7.5 cm from the vertebral spinous process) where the costal groove is at its broadest and deepest and the lateral branch of the ICN has not yet branched [1]. The ribs are easily identified with their typical dorsal shadowing. The key structures in the scan are internal and external intercostal muscles, and the pleura appears as a prominent hyperechoic line with gliding action during respiration (Fig. 12).
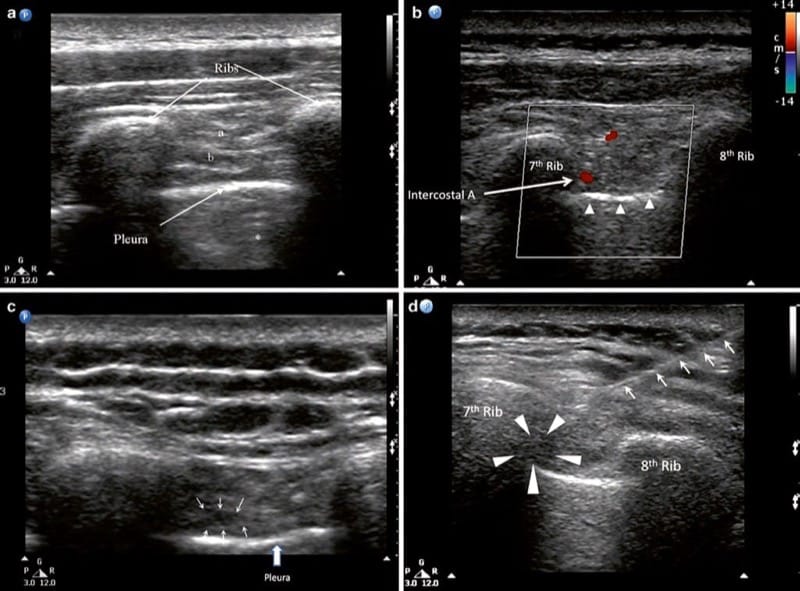
Fig.12 Ultrasonographic image showing the intercostal muscles and pleura at the angle of rib. (a) External intercostal muscle, (b) internal intercostal muscle, * reverberation artifact. (b) A similar image taken 2 cm medial to the angle of rib. The intercostal artery is seen in the intercostal space. The pleura, appears as a hyperechoic line, is indicated by the solid arrow heads. (c) Ultrasonographic image following injection. The small arrows outline the collection of local anesthetic. (d) Intercostal space following injection. The needle is indicated by the line arrows and the local anesthetic by the arrow heads. (Reproduced with permission from Philip Peng Educational Series)
The intercostal space of interest is located by scanning upward from the 12th rib. The needle target is the internal intercostal muscle as the innermost internal intercostal is an ill-defined layer of the muscle under ultrasonography. A 22-G needle can be inserted either inplane or out-of-plane to the plane just deep to the internal intercostal muscle or “into” the intercostal muscle. In-plane technique is the authors’ preferred technique since it will allow visualization of the needle tip, which needs to be placed 2–3 mm proximal to the pleura [44]. The needle entry site is the upper margin of the rib one level caudal to the targeted ICN. Because of the precision required, and the adverse consequences of advancing the needle too deep (i.e., pneumothorax), it is prudent to inject a small amount of solution upon reaching the external intercostal muscle to confirm needle tip position [1]. The needle is then advanced a few millimeters further into the internal intercostal muscle, and the spread of local anesthetic is visualized in real time as it is injected. If the injectate is seen pushing the external intercostal muscle upward, the needle position is still superficial. Usually, 2 ml of local anesthetic is sufficient to fill the intercostal space, which allows block of several ICNs with minimal risk of toxic effects.
Once the ICN block is complete, the probe is used to check for the absence of pneumothorax. The ultrasound probe should be placed in the nondependent area. Normally, the pleura appears to glide with respiratory movement. Artifacts presenting as horizontal lines parallel to the pleural interface and vertical “comet tails” are also seen. Comettail artifacts (CTAs) indicate the presence of an intact lung surface. When a pneumothorax is present, the pleura no longer glides with respiration (loss of “gliding sign”), and there is a loss of CTAs. Using these signs, the sensitivity and specificity of ultrasound for detecting pneumothorax approach 100% [45].
13. CONCLUSION
Application of ultrasound in the field of interventional pain management allows the visualization of soft tissues and vessels, which in turn improves the accuracy of the needle placement. Ultrasound in pain management faces many of the same challenges it faced, and continues to face, in the perioperative setting, namely, visualization of thin needles, poor image quality in obese patients, and the need to invest time and money in training so that the procedures are effective and safe. However, the benefits to be derived are likely to make ultrasound a very attractive option, and with further research and training, ultrasound may well become a standard of care.