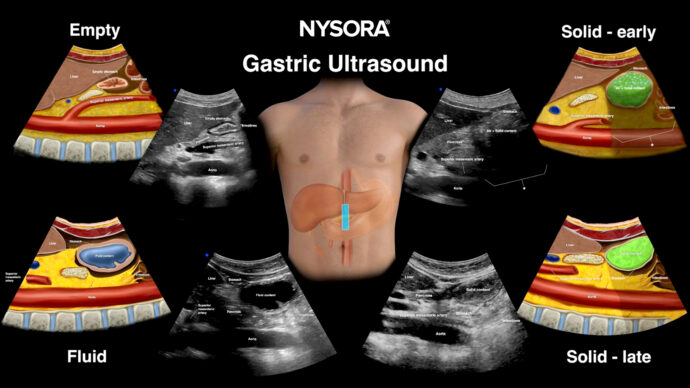
Understanding the deranged hemostasis and fibrinolysis balance in disseminated intravascular coagulation (DIC)
Disseminated Intravascular Coagulation (DIC) is a critical condition resulting from an imbalance between coagulation (blood clot formation) and fibrinolysis (clot breakdown). This complex disruption can lead to severe bleeding, thrombosis, and organ failure, making it essential to diagnose and manage DIC effectively across clinical settings such as sepsis, trauma, and obstetrics.
What is DIC and how does it occur?
- DIC occurs when an abnormal activation of coagulation pathways leads to widespread microthrombi (small blood clots) throughout the body, ultimately exhausting clotting factors and leading to severe bleeding.
- Pathophysiology: The condition arises from various triggers, such as sepsis or trauma, causing the coagulation cascade to activate excessively.
- Outcome: The abnormal clotting blocks blood flow to organs, leading to tissue damage, while the simultaneous depletion of clotting resources heightens bleeding risk.
Key components of hemostasis and fibrinolysis
- Hemostasis involves coagulation processes essential to stop bleeding and protect against infection.
- Fibrinolysis maintains vascular integrity by breaking down clots, preventing excessive clot formation.
- In DIC, the balance is disturbed, leading to either a pro-coagulant or fibrinolytic dominant state, both of which have significant clinical implications.
Clinical contexts and mechanisms leading to DIC
- Sepsis
- Sepsis-induced DIC is common, affecting up to 50% of septic patients, doubling mortality risk.
- Mechanisms include tissue factor activation, impaired anticoagulant pathways, and diminished fibrinolysis.
- Microorganisms and host inflammatory responses (e.g., cytokine release) amplify coagulation, creating microvascular obstructions that can cause multi-organ failure.
- Trauma
- Trauma patients often exhibit DIC due to systemic inflammatory responses and tissue injury.
- Hypercoagulability may follow an initial bleeding phase, evolving into DIC.
- Management often involves hemostatic resuscitation strategies, with whole-blood resuscitation and tranexamic acid (TXA) as key interventions.
- Obstetrics
- Pregnancy-related hemostatic adaptations increase coagulation potential, posing DIC risk in obstetric emergencies like placental abruption or amniotic fluid embolism.
- Diagnosis is challenging due to normal pregnancy changes in coagulation markers, underscoring the need for pregnancy-specific DIC scores and viscoelastic testing.
Diagnosing DIC: Laboratory tests and scoring systems
- Laboratory markers
- Common markers include elevated D-dimer, decreased platelet counts, and prolonged clotting times.
- Emerging biomarkers, such as syndecan-1 and thromboinflammation indicators, offer insights but are primarily research-based.
- Scoring systems
- International Society on Thrombosis and Haemostasis (ISTH) and the Japanese Association for Acute Medicine (JAAM) scoring systems aid diagnosis.
- The Sepsis-Induced Coagulopathy (SIC) score offers early detection, especially critical in septic patients.
Treatment and management strategies for DIC
- Treating the underlying cause
- Managing the primary cause, such as infection or trauma, is foundational in stabilizing DIC.
- Anticoagulation and fibrinolysis modulation
- Japanese guidelines endorse therapies like recombinant thrombomodulin for sepsis-related DIC, though broader consensus is lacking due to mixed clinical trial results.
- Antithrombin and thrombomodulin studies show potential benefits, especially when administered before overt DIC onset.
- Hemostatic resuscitation and blood product use
- In trauma, whole-blood transfusion and TXA help manage bleeding, with viscoelastic testing guiding transfusion needs.
- In obstetrics, algorithms using thromboelastometry are applied during severe hemorrhages, helping to reduce unnecessary transfusions.
Conclusion
DIC represents a dynamic interaction between coagulation and fibrinolysis that, when disrupted, can have life-threatening consequences. Understanding the pathophysiology of DIC across different clinical scenarios helps clinicians in early detection and management, ultimately aiming to balance hemostatic mechanisms and prevent fatal outcomes.
Stay current with Anesthesia Updates 2025—a concise, expert-reviewed guide covering the latest in anesthesiology. Essential insights and practical updates for busy clinicians. Order now on Amazon to enhance patient care and clinical practice.