Kenneth D. Candido, Anthony R. Tharian, and Alon P. Winnie
INTRODUCTION
Caudal anesthesia was described at the turn of last century by two French physicians, Fernand Cathelin and Jean-Anthanase Sicard. The technique pre-dated the lumbar approach to epidural nerve block by several years. Caudal anesthesia, however, did not gain in popularity immediately following its inception. One of the major reasons caudal anesthesia was not embraced is the wide anatomical variations of sacral bones and the consequent failure rate associated with attempts to locate the sacral hiatus. The failure rate of 5% to 10% made caudal epidural anesthesia unpopular until a resurgence of interest in the 1940s, led by Hingson and colleagues, who used it in obstetrical anesthesia. Caudal epidural anesthesia has many applications, including surgical anesthesia in children and adults, as well as the management of acute and chronic pain conditions. Success rates of 98%–100% can be achieved in infants and young children before the age of puberty, as well as in lean adults. The technique of caudal epidural nerve block in pain management has been greatly enhanced by the use of fluoroscopic guidance and epidurography, in which high success rates can be attained.
Unfortunately, clinical indications and especially therapeutic interventions for the relief of chronic pain in individuals with failed back surgery syndrome are often most prevalent in patients with difficult caudal landmarks. It has been suggested that traditional lumbar peridural nerve block should not be attempted employing an approach requiring needle placement through a spinal surgery scar due to the likelihood of tearing the dura and the possibility of inducing hematoma formation over the cauda equina when blood from the procedure becomes trapped between the layers of scar and connective tissues. Under these circumstances, it is recommended that fluoroscopically guided caudal epidural nerve block be performed in lieu of the traditional palpation approach. Alternatively, the use of ultrasound may be appropriate to identify the sacral hiatus, and this technique has recently been described. The second resurgence in popularity of caudal anesthesia has paralleled the increasing need to find safe alternatives to conventional lumbar epidural nerve block in selected patient populations, such as individuals with failed back surgery syndrome.
ANATOMIC CONSIDERATIONS
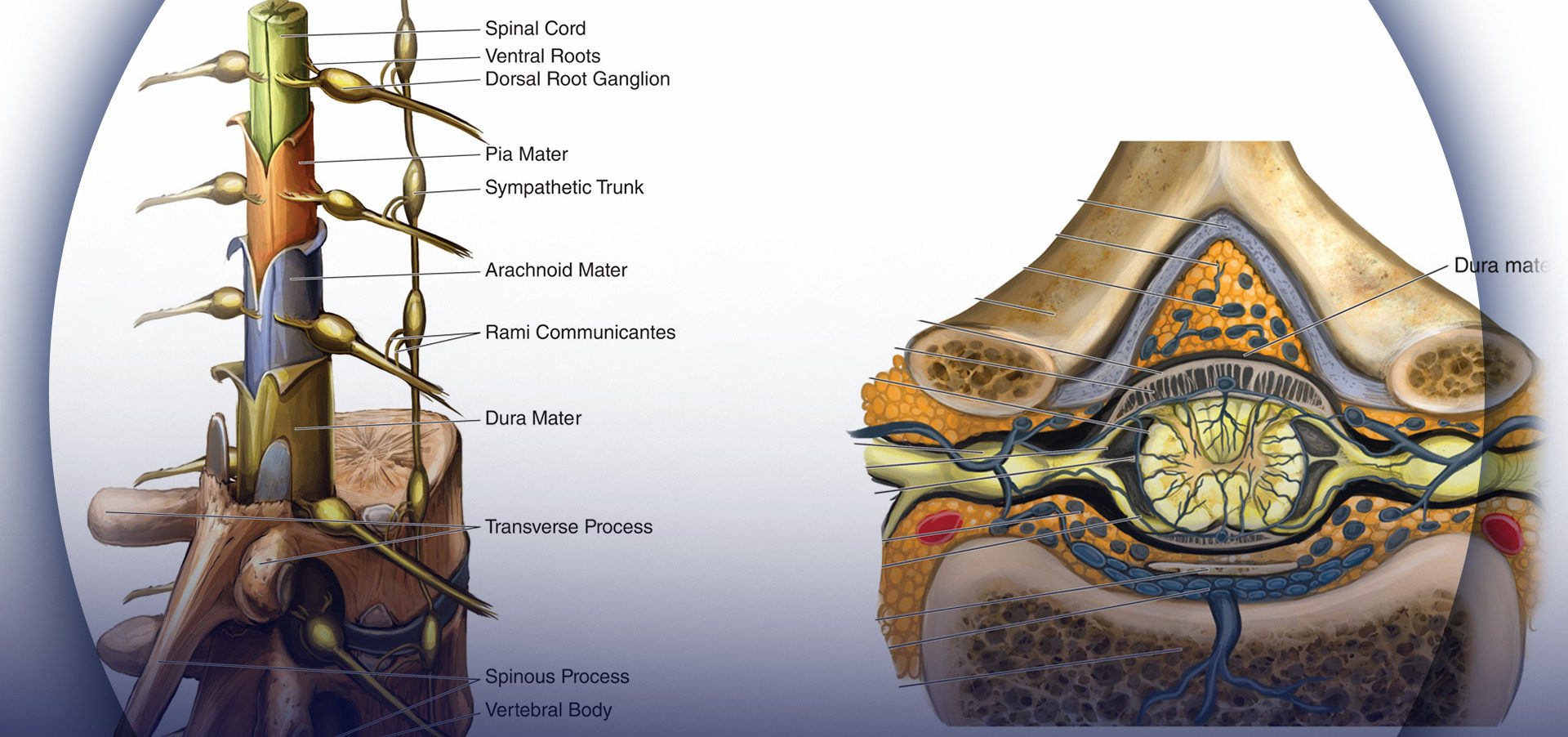
The sacrum is a large triangularly shaped bone formed by the fusion of the five sacral vertebrae. It has a blunted, caudal apex that articulates with the coccyx. Its superior, wide base articulates with the fifth lumbar vertebra at the lumbosacral angle (see Figure 1A). Its dorsal surface is convex and has a raised interrupted median crest with four (sometimes three) spinous tubercles representing fused sacral spines. Flanking the median crest, the posterior surface is formed by fused laminae. Lateral to the median crest, four pairs of dorsal foramina lead into the sacral canal through intervertebral foramina, each of which transmits the dorsal ramus of a sacral spinal nerve (see Figure 1A). Below the fourth (or third) spinous tubercle, an arched sacral hiatus is identified in the posterior wall of the sacral canal due to the failure of the fifth pair of laminae to meet, exposing the dorsal surface of the fifth sacral vertebral body. The caudal opening of the canal is the sacral hiatus (see Figures 1B and 1C) roofed by the firm elastic membrane, the sacrococcygeal ligament, which is an extension of the ligamentum flavum. The fifth inferior articular processes project caudally and flank the sacral hiatus as sacral cornua, connected to the coccygeal cornua by intercornual ligaments.
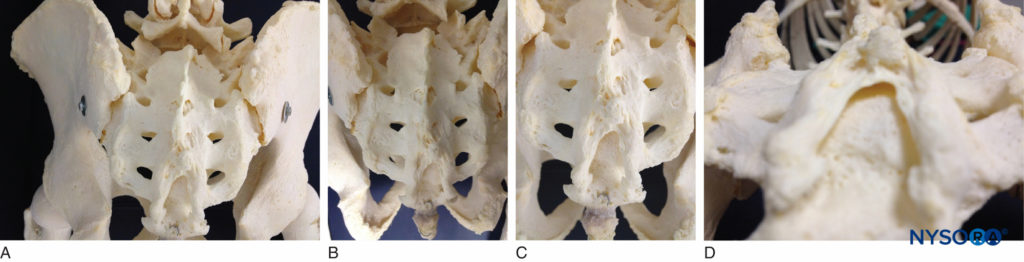
FIGURE 1. A: Skeletal specimen of the sacrum viewed from craniad to caudad demonstrating the four pairs of dorsal foramina, situated bilaterally. B: Skeletal model demonstrating the sacral hiatus and its relationship to the coccyx and the sacrum. The fifth inferior articular processes project caudally and flank the sacral hiatus as sacral cornua. C: Skeletal model showing the sacral hiatus with the exposed dorsal surface of the fifth sacral vertebral body. D: Skeletal specimen viewed from inferior to the sacral hiatus
The sacral canal is triangular in shape. It is a continuation of the lumbar spinal canal. Each lateral wall presents four intervertebral foramina, through which the canal is in contiguous with the pelvic and dorsal sacral foramina. The posterior sacral foramina are smaller than their anterior counterparts. The sacral canal contains the cauda equina (including the filum terminale) and the spinal meninges. Near its midlevel (typically the middle one-third of S2, but varying from the midpoint of S1 to the midpoint of S3), the subarachnoid and subdural spaces cease to exist, and the lower sacral spinal roots and filum terminale pierce the arachnoid and dura mater. However, variations in the termination of the dural sac as well as pathologic conditions like sacral meningocele or sacral perineural cysts can increase the chances of inadvertent dural puncture when performing caudal nerve block in such patients with abnormal anatomy.
The lowest margin of the filum terminale emerges at the sacral hiatus and traverses the dorsal surface of the fifth sacral vertebra and sacrococcygeal joint to reach the coccyx. The fifth sacral nerve roots also emerge through the hiatus medial to each of the sacral cornua. The sacral canal contains the epidural venous plexus, which generally terminates at S4 but which may continue more caudally. Most of these vessels are concentrated in the anterolateral portion of the canal. The remainder of the sacral canal is filled with adipose tissue, which is subject to an age-related decrease in its density. This change may be responsible for the transition from the predictable spread of local anesthetics administered for caudal anesthesia in children to the limited and unpredictable segmental spread seen in adults.
Considerable variability occurs in sacral hiatus anatomy among individuals of seemingly similar backgrounds, race, and stature. As individuals age, the overlying ligaments and the cornua thicken significantly. The hiatal margins often defy recognition by even skilled fingertips. The practical problems related to caudal anesthesia are mainly attributable to wide anatomical variations in size, shape, and orientation of the sacrum. Trotter summarized the major anatomical variations of the sacrum. The sacral hiatus may be almost closed, asymmetrically open, or widely open secondary to anomalies in the pattern of fusion of the laminae of the sacral arches. Sacral spina bifida was noted in about 2% of males and in 0.3% of females. The anteroposterior depth of the sacral canal may vary from less than 2 mm to greater than 1 cm. Individuals with sacral canals having anterior-posterior (A-P) diameters less than about 3 mm may not be able to accommodate anything larger than a 21-gauge needle (5% of the population).
In addition, the lateral width of the sacral canal varies significantly. Because the depth and width may vary, the volume of the canal itself may also vary. Trotter found that sacral volumes varied between 12 and 65 mL, with a mean volume of 33 mL. A magnetic resonance imaging (MRI) study in 37 adult patients found the volume (excluding the foramina and dural sac) was 14.4 mL, with a range of 9.5 to 26.6 mL. Patients with smaller capacities may not be able to accommodate the typical volumes of local anesthetics administered for epidural anesthesia via the caudal route. In a cadaver study of 53 specimens, the mean distance between the tip of the dural sac and the upper edge of the sacral hiatus as denoted by the sacrococcygeal membrane was 45 mm, with a range of 16–75 mm. In the MRI study mentioned, the mean distance was found to be 60.5 mm, with a range of 34–80 mm.
The sacrococcygeal membrane could not be identified in 10.8% of subjects using MRI. An anatomical evaluation of 92 isolated sacra found that 42% of cases had both a hiatus and cornua; 4% of the cases showed an absent hiatus. The apex of the sacral hiatus, in that study, was noted in 64% of cases to exit at the S4 level. The hiatus was closed in 3% of cases.
The sacral foramina afford anatomical passages that permit the spread of injected solutions, such as local anesthetics and adjuvants (see Figure 1A). The posterior sacral foramina are essentially sealed by the multifidus and sacrospinalis muscles, but the anterior foramina are unobstructed by muscles and ligaments, permitting ready egress of solutions through them. The sacral curvature varies substantially. In a cadaver study, looking at the anatomy of caudal epidural space, the sacral cornua were not palpable bilaterally in 14.3% and palpable unilaterally in 24.5% of specimens. The level of maximum curvature of the sacrum was at S3 in 69.4% of cases. This variability tends to be more severe in males than in females.
The clinical significance of this finding is that a non-curving epidural needle will more likely pass easily into the canal of females than males. The angle between the axis of the lumbar canal and the sacral canal varies between 7° and 70° in subjects with marked lordosis. The clinical implication of this finding is that the cephalad flow of caudally injected solutions may be more limited in lordotic patients with exaggerated lumbosacral angles than in those with flatter lumbosacral angles, in whom the axes of the lumbar and sacral canals are more closely aligned.
NYSORA Tips
- Considerable variability occurs in sacral hiatus anatomy.
- With advancing age, the overlying ligaments and the cornua thicken; consequently, identification of the sacral hiatal margins becomes more challenging.
- The success rate of caudal anesthesia is largely dependent on anatomic variations in size, shape, and orientation of the sacrum.
Learn more about Neuraxial Anatomy.
INDICATIONS FOR CAUDAL EPIDURAL NERVE BLOCK
The indications for caudal epidural nerve block are essentially the same as for lumbar epidural nerve block, but its use may be preferred when sacral nerve spread of anesthetics and adjuvants is preferred over lumbar nerve spread. The unpredictability cephalad spread of anesthetics administered into the caudal canal limits the use of this technique in situations where it is essential to provide lower thoracic and upper abdominal neuraxial block. Although this modality is described for perioperative use (diminishing role) and for managing chronic pain scenarios in adults (increasing role), caudal nerve block has a wide range of indications (Table 1). Successful treatment of postdural puncture headache by performing an epidural blood patch through a caudally placed needle has been reported.
TABLE 1. Indications for caudal epidural nerve block.
| Surgical, Obstetric, Diagnostic, and Prognostic | |
|---|---|
| 1. | Surgical anesthesia |
| 2. | Obstetric anesthesia |
| 3. | Differential neural block to evaluate pelvic, bladder, perineal, genital, rectal, anal, and lower extremity pain |
| 4. | Prognostic indicator before destruction of sacral nerves |
| Acute Pain | |
|---|---|
| 1. | Acute low back pain |
| 2. | Acute lumbar radiculopathy |
| 3. | Palliation in acute pain emergencies |
| 4. | Postoperative pain |
| 5. | Pelvic and lower extremity pain secondary to trauma |
| 6. | Pain of acute herpes zoster |
| 7. | Acute vascular insufficiency of the lower extremities |
| 8. | Hidradenitis suppurativa |
| Chronic Benign Pain | |
|---|---|
| 1. | Lumbar radiculopathy |
| 2. | Spinal stenosis |
| 3. | Low back syndrome |
| 4. | Vertebral compression fractures |
| 5. | Diabetic polyneuropathy |
| 6. | Postherpetic neuralgia |
| 7. | Reflex sympathetic dystrophy |
| 8. | Orchialgia |
| 9. | Proctalgia |
| 10. | Pelvic pain syndromes |
| Cancer Pain | |
|---|---|
| 1. | Pain secondary to pelvic, perineal, genital, or rectal malignancy |
| 2. | Bony metastases to pelvis |
| 3. | Chemotherapy-related peripheral neuropathy |
| Special Situations | |
|---|---|
| 1. | Patients with previous lumbar spine surgery |
| 2. | Patients who are “anticoagulated” or have coagulopathy |
NYSORA Tips
- The indications for caudal epidural nerve block are essentially the same as those for lumbar epidural nerve block.
- Caudal nerve block may be preferred over lumbar epidural nerve block when sacral nerve spread of anesthetics and adjuvants is preferred over lumbar nerve spread.
- The unpredictability of ascertaining consistent cephalad spread of anesthetics administered through the caudal canal limits the usefulness of this technique when it is essential to provide lower thoracic and upper abdominal neuraxial block.
Other, newer indications in adults deserve mention and are described further in this chapter, including the performance of percutaneous epidural neuroplasty; the use of caudal analgesia following lumbar spinal surgery; caudal analgesia after orthopedic lower extremity surgery; local anesthetic adjuvants for postoperative analgesia; and caudal nerve block for performing neurolysis for treatment intractable cancer pain.
THE TECHNIQUE OF CAUDAL EPIDURAL NERVE BLOCK
The classical technique of caudal epidural nerve block involves palpation, identification, and puncture. Patients are evaluated as for any epidural nerve block, and the indications and relative and absolute contraindications to its performance are identical. A full complement of noninvasive monitors is applied, and baseline vital signs are assessed. One must decide whether a continuous or single-shot technique will be employed. For continuous techniques, a Tuohy-type needle with a lateral-facing orifice is preferred.
Patient Positioning
Several positions can be used in adults, compared with the lateral decubitus position in neonates and children. The lateral position is often preferred in pediatrics because it permits easy access to the airway when general anesthesia or heavy sedation has been administered prior to performing the nerve block. In pediatric patients, nerve blocks may be performed with the patient fully anesthetized; this is not recommended for older children and adults. In adults, the prone position is the most frequently utilized, but the lateral decubitus position or the knee-chest (also known as “knee-elbow”) position may be employed. In the prone position, the procedure table or operating room table should be flexed, or a pillow may be placed beneath the symphysis pubis and iliac crests to produce slight flexion of the hips. This maneuver makes palpation of the caudal canal easier. The legs are separated with the heels rotated outward to smooth out the upper part of the anal cleft, while simultaneously relaxing the gluteal muscles. For placement of caudal epidural nerve block in the parturient, the lateral (Sim’s position) and the knee-elbow position are most commonly used.
Anatomical Landmarks
A dry gauze swab is placed in the intergluteal cleft to protect the anal area and genitalia from povidone iodine or other disinfectants (especially alcohol) used to disinfect the skin. Anatomical landmarks are next assessed. The skinfolds of the buttocks are useful guides in locating the underlying sacral hiatus. Alternatively, a triangle may be marked on the skin over the sacrum, using the posterior superior iliac spines (PSISs) as the base, with the apex pointing inferiorly (caudally). Normally, this apex sits over or immediately adjacent to the sacral hiatus. However, a recent study indicated that identification of the sacral hiatus using this method may be inaccurate because the actual triangle formed by the sacral hiatus and PSISs is not equiangular. Once the hiatus is marked, the tip of the index finger is placed on the tip of the coccyx in the intergluteal cleft while the thumb of the same hand palpates the two sacral cornua located 3–4 cm more rostrally at the upper end of the intergluteal cleft. The sacral cornua may be identified by gently moving the palpating index finger from side to side (Figure 2). The palpating thumb should sink into the hollow between the two cornua, as if between two knuckles of a fist. Sterile skin preparation and draping of the entire region are performed in the usual fashion.
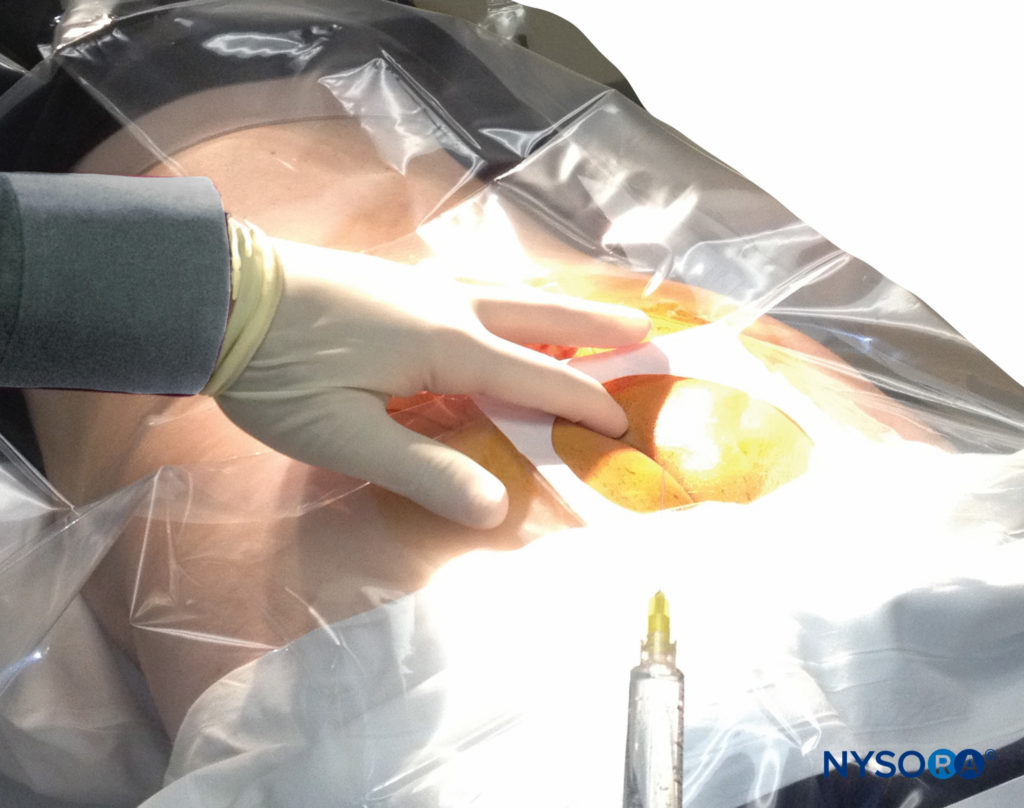
FIGURE 2. Technique of palpating the midline over the sacral hiatus. The palpating index and middle fingers are spread over the fifth sacral vertebral body. The sacrococcygeal ligament lies directly beneath the palpating finger.
Technique Using Fluoroscopy
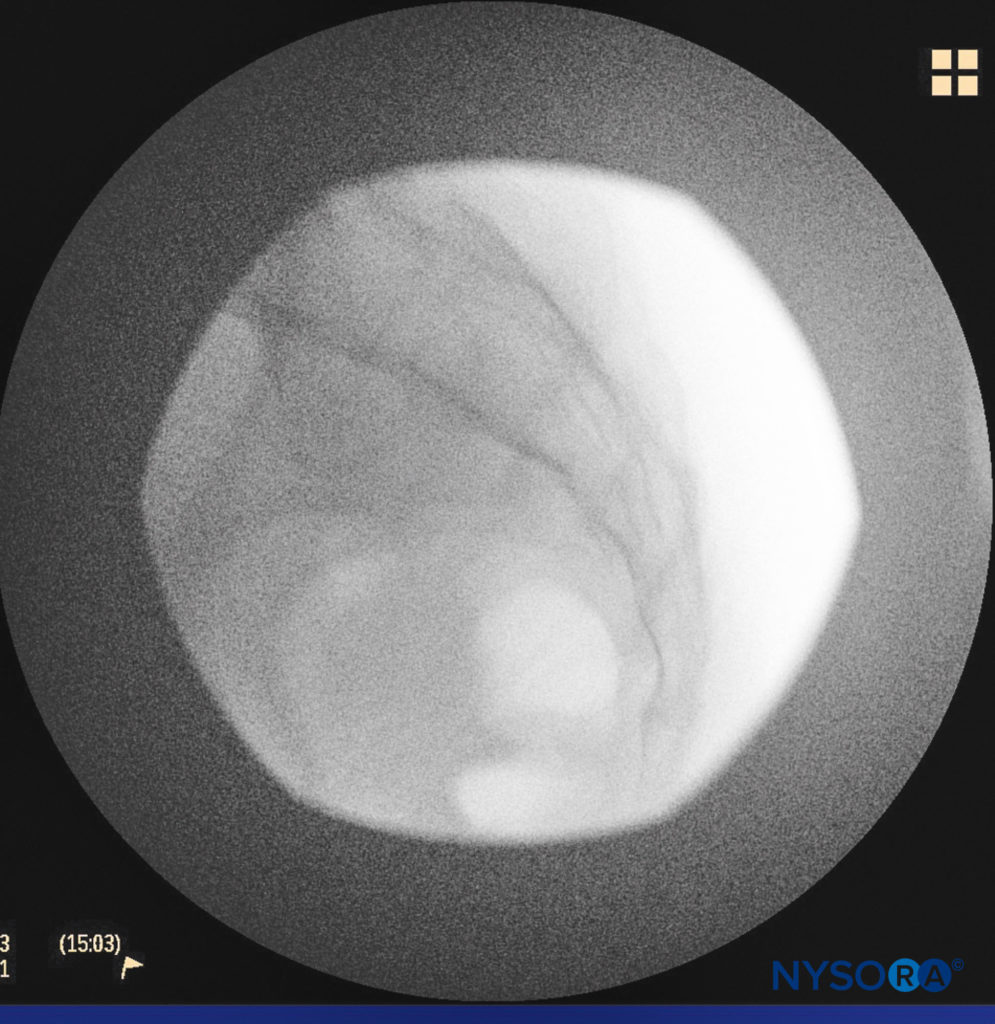
A small-gauge 1.5-inch needle is then utilized to infiltrate the skin over the sacral hiatus using 3-5 mL of 1%-1.5% plain lidocaine HCl (Figure 3). If fluoroscopy is utilized, a lateral view is obtained to demonstrate the anatomic boundaries of the sacral canal. We routinely leave the local anesthetic infiltration needle in situ for this view because it demonstrates whether the approach is at the appropriate level for subsequent advancement of the epidural needle. With fluoroscopy, the caudal canal appears as a translucent layer posterior to the sacral segments. The median sacral crest is visualized as an opaque line posterior to the caudal canal. The sacral hiatus is usually visualized as a translucent opening at the base of the caudal canal. The coccyx may be seen articulating with the inferior surface of the sacrum (Figure 4).
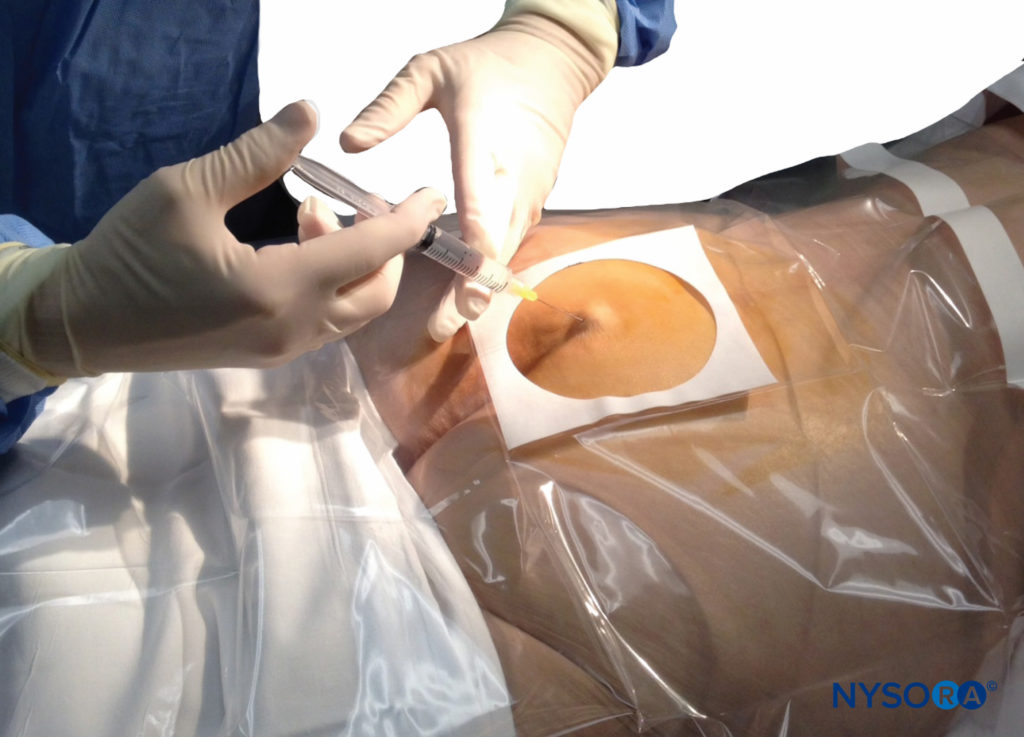
FIGURE 3. Technique of skin infiltration with local anesthetic. The needle is inserted first above and then into the substance of the sacrococcygeal ligament.
Once the tissues overlying the hiatus have been anesthetized, a 17- or 18-gauge Tuohy-type needle is inserted either in the midline or, using a lateral approach, into the caudal canal (Figure 5, Figure 6). A feeling of a slight “snap” may be appreciated when the advancing needle pierces the sacrococcygeal ligament. Once the needle reaches the ventral wall of the sacral canal, it is slowly withdrawn and reoriented, directing it more cranially (by depressing hub and advancing) for further insertion into the canal.
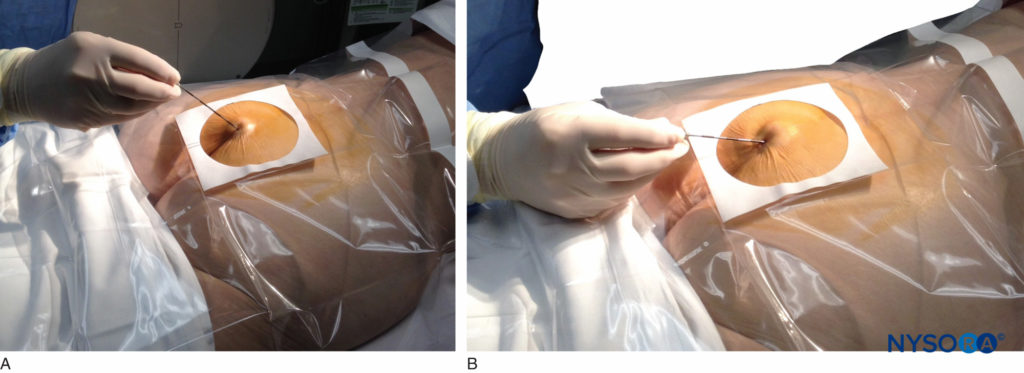
FIGURE 5. An 18-gauge, Tuohy-type needle is advanced from the skin into the sacral hiatus through the sacrococcygeal ligament. Usually, when fluoroscopy is not available to verify correct needle placement, a syringe loaded with air or saline is attached to the needle, and the loss-of-resistance technique is employed to identify the epidural space.
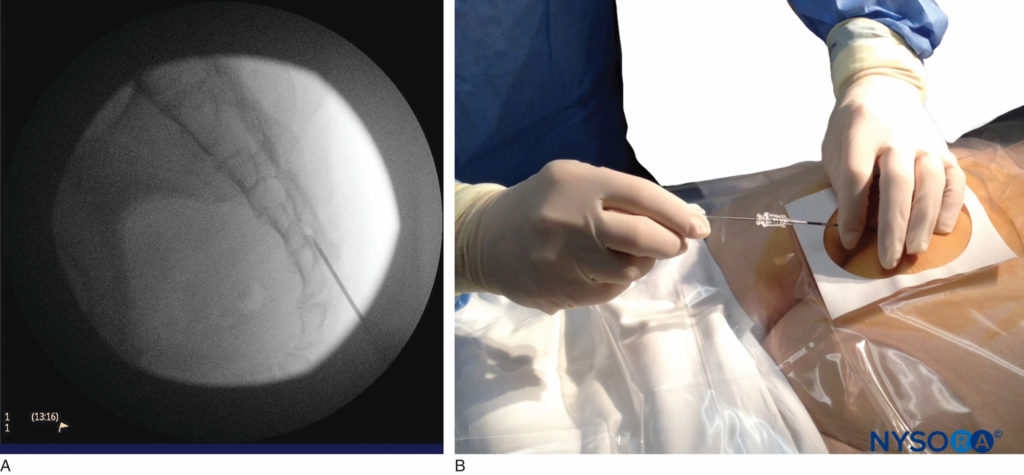
FIGURE 6. Lateral fluoroscopic image depicting the 18-gauge Tuohy needle correctly seated in the caudal epidural space.
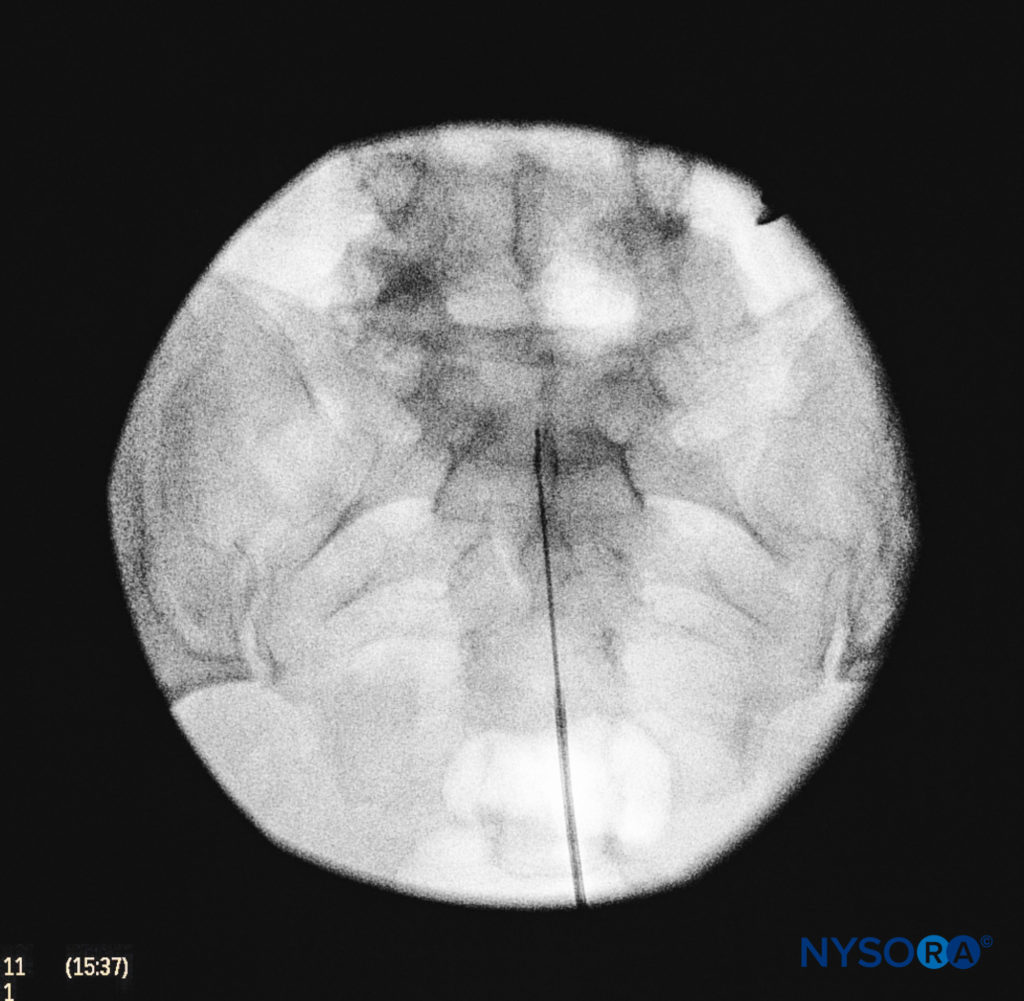
We utilize the anteroposterior view once the epidural needle is safely situated within the canal, and the epidural catheter is advanced cephalad (Figure 7, Figure 8). In this projection, the intermediate sacral crests appear as opaque vertical lines on either side of the midline. The sacral foramina are visualized as translucent and nearly circular areas lateral to the intermediate sacral crests. The presence of intestinal gas may obscure the recognition of these structures. A syringe loaded with either air or saline containing a small air bubble is then attached to the needle, and the loss-of-resistance technique is used to establish entry into the epidural space.

FIGURE 7. A continuous catheter with a stylet in place is being advanced through the 18-gauge Tuohy needle placed in the canal.
NYSORA Tips
- The needle tip should stay below the S2 level to avoid tearing the dura.
- The needle should never be advanced in the space too deep.
- The skin corresponding to about 1 cm inferior to the PSISs indicates the S2 level (caudal-most extension of the dura mater).
- The dural sac extends lower in children than in adults, and epidural needles should be carefully advanced no deeper than the S3 or S4 level.
An acoustic test, also called the “whoosh” test has been described for identifying correct needle placement in the caudal canal. This characteristic sound has been noted during auscultation of the thoracolumbar region during the injection of 2–3 mL of air into the caudal epidural space. The “swoosh test,” described by Orme and Berg, substitutes saline or local anesthetic instead of air as the injectate.
Of the 108 patients with a successful nerve block in one study, 98 had a positive test, with no false-positive results. Once the correct placement of the needle is confirmed, a catheter is inserted into the desired location (depth) and its position confirmed fluoroscopically when desired (Figure 9). Color Doppler ultrasonography has also been described for guiding the needle insertion into the epidural space and to confirm any intravascular injection.
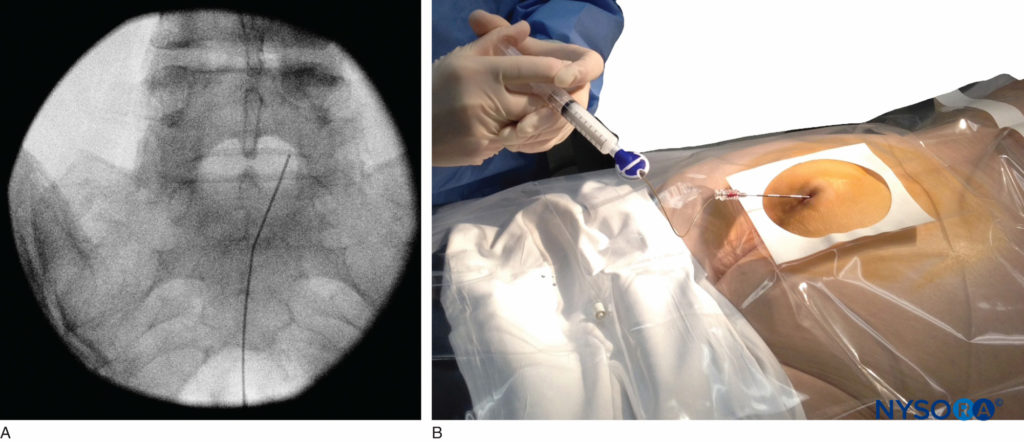
FIGURE 9. Anteroposterior fluoroscopic image depicting the catheter advanced to the L5–S1 interspace.
ULTRASOUND-GUIDED CAUDAL NERVE BLOCK
Ultrasound has played an increasing role in regional anesthesia and pain management, ultrasound guidance can be utilized to identify the sacral hiatus, thereby facilitating needle entry into the hiatus, as well as to visualize the advancing needle in the caudal canal. Because caudal nerve blocks are relatively easy to perform in the pediatric population, ultrasound may not be significantly advantageous over landmark-based techniques. However, in adult patients, for whom the anatomy of the sacral hiatus, caudal canal, and dural sac are variable, ultrasound guidance may prove to be helpful in reducing the risk of complications such as tissue trauma, dural puncture, local anesthetic toxicity, and vascular compromise.
Technique
With the patient in the prone position, the sacral cornua are identified by palpation using the technique described in the section on anatomic landmarks. Sterile skin preparation and draping of the entire region are performed. A low-frequency curvilinear probe is placed in the transverse plane across the two sacral cornua (Figure 10). The sacral cornua can be visualized as two symmetric hyperechoic arches, with a hypoechoic shade underneath both lines, bridging these two structures. Traversing this hypoechoic area are two hyperechoic lines, the superficial one being the sacrococcygeal ligament and the deeper one line being the dorsal bony surface of the sacrum (Figure 11). A 22-gauge needle is inserted into the space between the two cornua. A distinct “pop” is felt as the needle tip penetrates the sacrococcygeal ligament.
FIGURE 10. A low-frequency curvilinear ultrasound probe ensheathed in a sterile probe cover containing gel being placed in the transverse plane across the two sacral cornua.
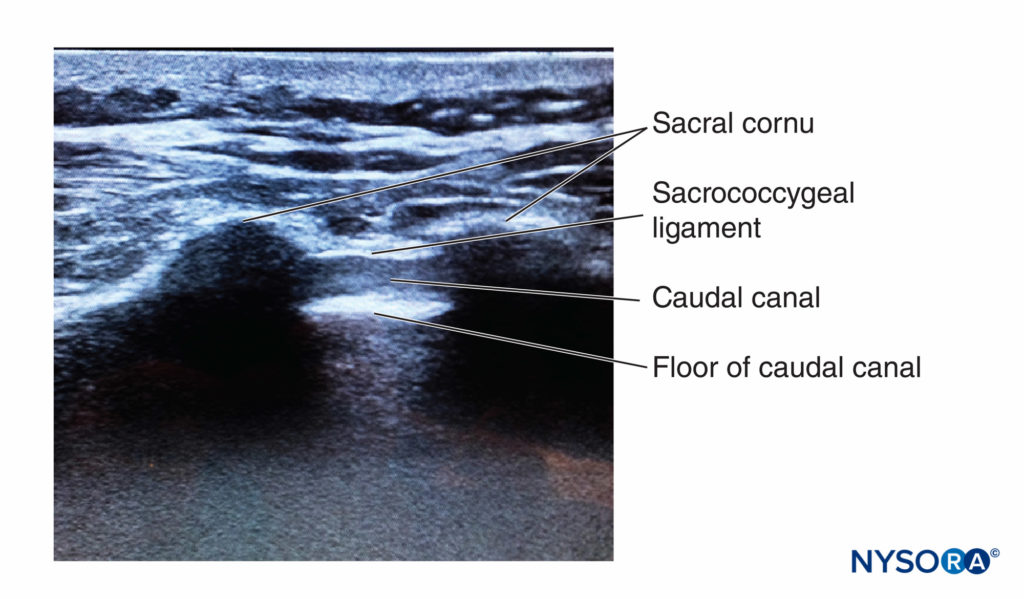
FIGURE 11. Ultrasound image along the short axis of the caudal canal, depicting the sacral cornua, sacrococcygeal ligament, and the caudal canal.
At this point, the orientation of the probe is changed into the sagittal plane (Figure 12), and the caudal canal is identified as a hypoechoic canal tapering off caudally and bordered by dorsal and ventral hyperechoic bands. The dorsal band is formed by the dorsal bony aspect of the caudal canal cranially and the sacrococcygeal ligament caudally. The ventral band is formed by the ventral bony surface of the caudal canal (Figure 13).
FIGURE 12. The low-frequency curvilinear probe placed in the sagittal plane along the long axis of the caudal canal.
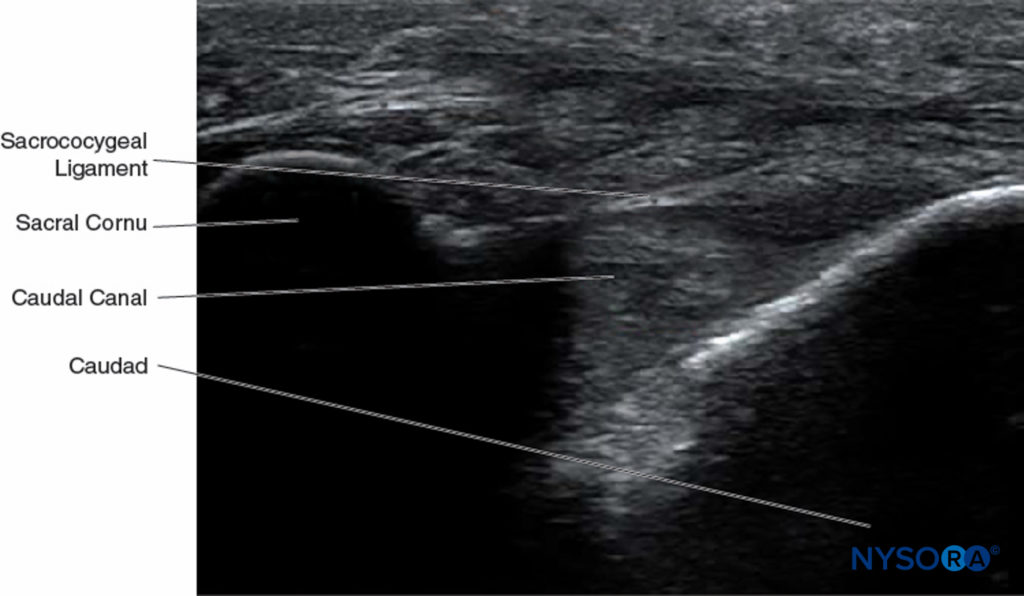
FIGURE 13. Ultrasonographic image along the long axis of the caudal canal depicting the long-axis view of the caudal canal.
NYSORA Tips
- In pediatric patients, electrical stimulation has been used to ascertain correct needle placement in the caudal canal. Anal sphincter contraction (corresponding to stimulation of S2–S4) is sought with 1- to 10-mA currents.
- If the needle has been inserted correctly, moving the hub from side to side, while the shaft is held at the sacrococcygeal membrane will allow the tip swinging freely in the sacral canal like a fulcrum.
- If cerebrospinal fluid (CSF) is obtained after aspiration, the needle should be withdrawn and injection should not be undertaken.
- If blood is aspirated, the needle should be withdrawn and reinserted until no blood is apparent at the hub.
- When injection of air (or saline) for the loss-of-resistance technique results in bulging over the sacrum, the needle tip most probably lies dorsal to the sacrum in the subcutaneous tissues.
- If the needle tip is subperiosteal, the injection will meet with significant resistance, resulting in significant patient discomfort. The cortical layer of the sacral bone is often thin, particularly in infants and older subjects, and its penetration can easily occur especially if force is exerted while advancing the needle. The sensation of entering cancellous bone is not unlike penetrating the sacrococcygeal membrane; there is a feeling of resistance that is suddenly overcome, and the needle advances more freely and subsequent injection is unhampered.
Several complications related to the technique procedure have been described. Injected solutions may be absorbed rapidly from bone marrow, and toxic drug reactions result. In this situation, pain is typically noted over the caudal part of the sacrum during the injection. If this occurs, the needle should be withdrawn slightly and rotated on its axis until it can be reinserted in a slightly different direction.
If the injection is made anterior to the sacrum, it is possible to perforate the rectum, or, in parturients, the baby’s head may be injured. This limits the use of caudal nerve block in laboring women once the presenting part has descended into the perineum. Inadvertent venous puncture may also occur, and the incidence of this has been reported to be about 0.6%.
Caudal nerve block may be used with a single-shot or continuous catheter technique. For continuous nerve block, the catheter may be advanced anterograde (conventionally) or retrograde. Continuous caudal nerve block may be performed in retrograde fashion using needle insertion into the lumbar epidural space, but directed inferiorly instead of superiorly. In one study of 10 patients, epidural catheters were advanced through 18-gauge Tuohy-type epidural needles in retrograde fashion from the L4–L5 interspace. This technique was associated with a 20% failure rate, with the catheter going into the paravertebral or retrorectal spaces, despite easy epidural space entry. Using the conventional approach, a Huber-tipped Tuohy needle is used as a conduit to pass the epidural catheter into the canal. This needle has a curved bevel tip that limits it being caught or snagged on the sacral periosteum. The needle is inserted with its shoulder facing anteriorly and its orifice dorsally. Alternatively, a standard 16- or 17-gauge catheter-over-needle assemblage (angiocatheter) may serve as the introducing needle for subsequent catheter placement. The catheter is advanced under fluoroscopic guidance, especially when it is performed for chronic pain management in failed back surgery syndrome.
The catheters should be advanced gently because there have been reports of dural puncture with rapid or aggressive advancement. A lateral and anteroposterior fluoroscopic views should be obtained to demonstrate placement of the catheter in the epidural space (Figures 14 and 9) and to follow its path in a cephalad or cephalolateral direction. When the desired level is attained, iodinated nonionic contrast media may be injected, followed by the injection of local anesthetics, corticosteroids, or adjuncts (Figure 15). Usually, the catheter is not advanced higher than the level of L4 vertebral body, although it could be advanced to the L1 or L2 levels for specific indications. Some authors recommend not to advance the catheter more than 8–12 cm cephalad.
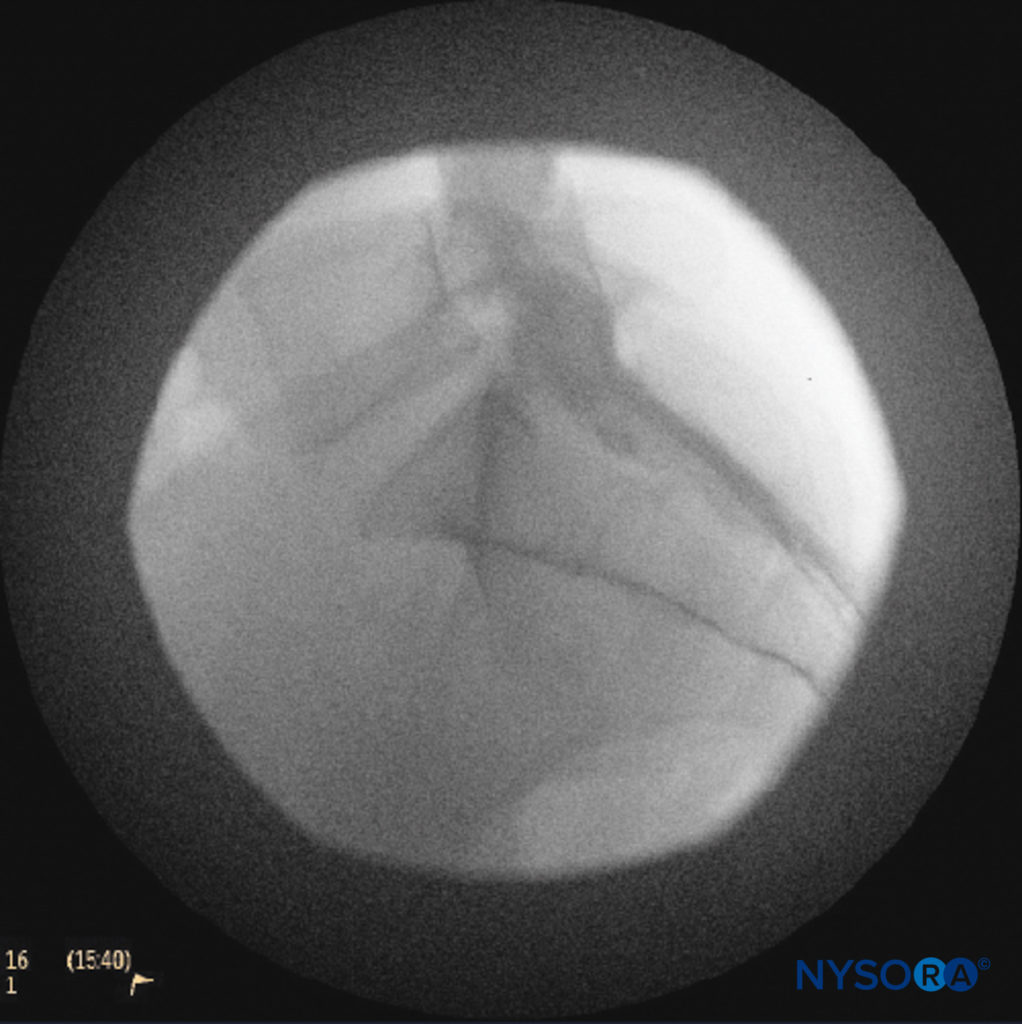
FIGURE 14. Lateral fluoroscopic image depicting radiopaque contrast medium in the caudal and lower lumbar epidural spaces. The image shows considerable spread, both anteriorly and posteriorly, following the injection of 2 mL of dye.
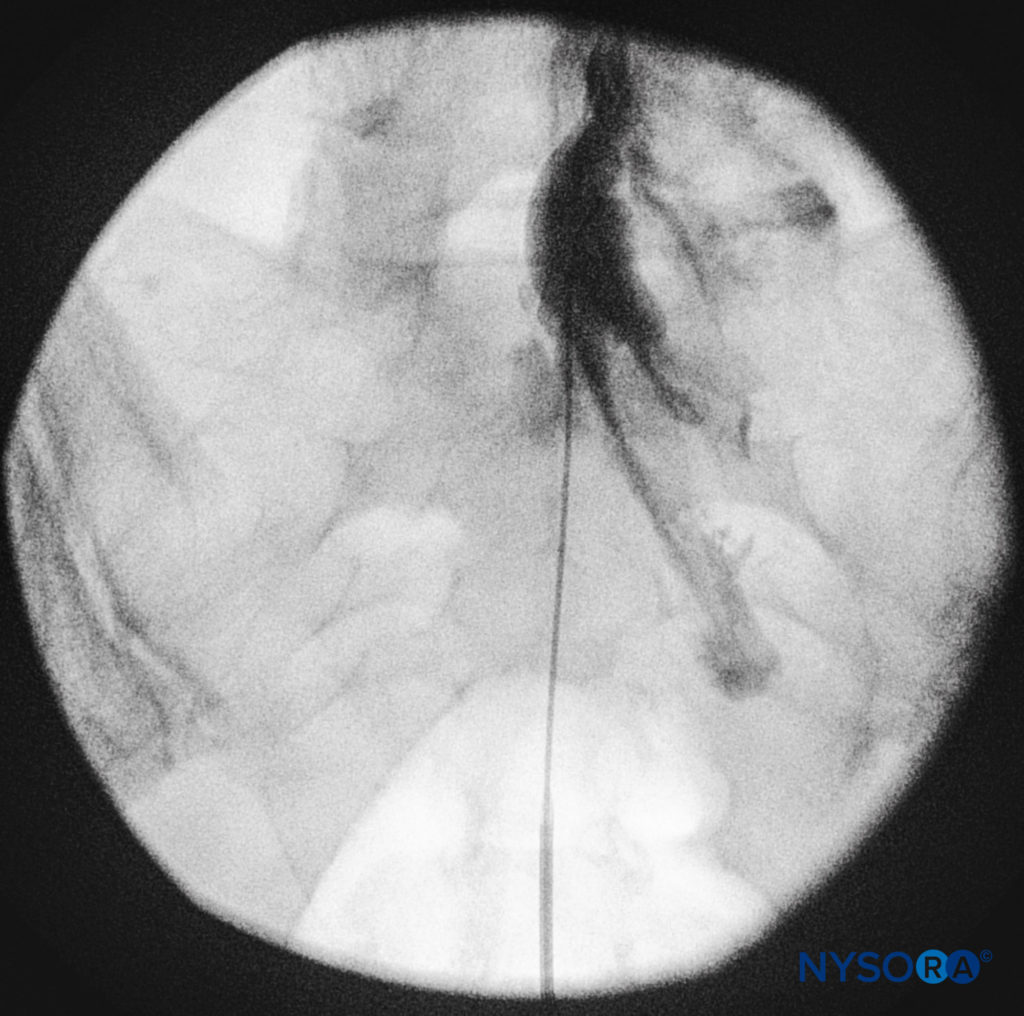
FIGURE 15. Anteroposterior fluoroscopic image depicting radiopaque contrast medium in the epidural space
NYSORA Tips
- Spread of the local anesthetic solutions injected into the caudal epidural space in adults is influenced by injected volume, speed of injection, and patient positioning.
- There is no correlation between the speed of injection and spread of the local anesthetic in children undergoing caudal anesthesia.
CHARACTERISTICS OF CAUDAL EPIDURAL NERVE BLOCK IN ADULTS
Caudal epidural nerve block results in sensory and motor nerve block of the sacral roots and limited autonomic nerve block. The sacral contribution of the parasympathetic nervous system is blocked causing loss of visceromotor function of the bladder and intestines distal to the colonic splenic flexure. Sympathetic nerve block, although limited compared to the lumbar or thoracic epidural nerve block, does occur. However, the sympathetic outflow from the preganglionic sympathetic fibers of the spinal cord ends at the L2 level; therefore, caudal nerve block should not routinely result in peripheral vasodilatation of the lower extremities to the degree witnessed with lumbar epidural block. Caudal epidural local anesthetic nerve block in adults may be chosen for surgeries of the lower abdomen, perineum, or lower extremities. The local anesthetic mixtures and doses are similar to those for lumbar epidural nerve block (Table 2). One recent study looking at the effect of gender on the minimal local anesthetic concentrations (MLACs) of ropivacaine for caudal anesthesia in patients undergoing anorectal surgery found that the MLAC for caudal anesthesia in female patients is 31% higher than in male patients.
TABLE 2. Local anesthetics commonly used for caudal anesthesia in adults
| Agent | Concentration | Dose (mg) | Sensory Onset (4-Segment Spread) | Duration (2-Segment Regression) |
|---|---|---|---|---|
| Lidocaine | 1.5%–2% | 300–600 | 10–20 min | 90–150 min |
| Chloroprocaine | 2%–3% | 400–900 | 8–15 min | 45–80 min |
| Mepivacaine | 2% | 400–600 | 10–20 min | 90–240 min |
| Ropivacaine | 0.75%–1% | 150–300 | 15–25 min | 120–210 min |
| Bupivacaine/levobupivacaine | 0.5%–0.75% | 100–225 | 10–25 min | 180–270 min |
Spread of Local Anesthetic Solutions
The large capacity of the sacral canal accommodates correspondingly large volumes of solution; significant volumes may be lost through the wide anterior sacral foramina. Therefore, the caudal dose requirements of local anesthetics to achieve the same segmental spread are significantly larger than are the corresponding lumbar doses. Roughly twice the lumbar epidural local anesthetic dose is needed for caudal block to attain similar levels of analgesia and anesthesia, and solutions injected in the caudal space take longer to spread (Table 2). Bromage noted that age is not correlated with caudal segmental spread in adults, and the upper level of analgesia resulting from 20-mL doses of local anesthetic solution varies widely between S2 and T8. This unpredictability limits the usefulness of applying caudal anesthesia for surgical procedures that require cephalad analgesia levels above the pelvic level or the umbilicus. A more recent study confirmed Bromage’s findings. In 172 women undergoing minor gynecologic surgery using caudal anesthesia with 20 mL of 1.5% lidocaine, the highest sensory dermatome level reached was below T10.
NYSORA Tips
- The sacral canal contains the cauda equina (including the filum terminale), the spinal meninges, adipose tissue, and sacral venous plexus.
- The volume of the sacral canal averages 14.4 mL.
- The indications for caudal epidural nerve block are the same as for a lumbar epidural nerve block.
- Percutaneous epidural neuroplasty is a technique of administering local anesthetics, corticosteroids, hyaluronidase, and hypertonic saline through a caudal catheter for the purpose of lysing epidural adhesions.
- Adult patients are typically placed prone for the nerve block, whereas the lateral decubitus position is preferred for pediatrics.
- Caudal block in pediatrics is used primarily for perioperative pain control, whereas in adults it is primarily for chronic pain management.
- In adults, roughly twice the local anesthetic dose is required to attain the same segmental spread with caudal nerve block compared with the dose used for lumbar epidural nerve block.
Indications for Caudal Epidural Analgesia in Adults
A caudal epidural nerve block is indicated whenever the area of surgery involves the sacral and lower lumbar nerve roots. The technique is suitable for anal surgery (hemorrhoidectomy and anal dilation), gynecologic procedures, surgery on the penis or scrotum, and lower limb surgeries. Using a catheter technique, it is possible to use the caudal epidural nerve block for cesarean section, vaginal hysterectomy, and inguinal herniorrhaphy.
A caudal epidural nerve block is used less frequently than the lumbar or thoracic epidural nerve block for perioperative analgesia in adults. The pelvis enlarges markedly in puberty, while the epidural fat in the lumbosacral region undergoes compaction and increased fibrous content. This hinders cephalad spread of solutions, particularly when compared with the spread in children.
As an alternative to the caudal epidural nerve block in adults, one might consider a median approach to trans-sacral epidural nerve block. In the original description of that technique, 87% of nerve blocks were successful for transurethral resection of bladder tumors versus 100% success for sacral procedures. Anesthesia level, side effects, and hemodynamics were similar between the two groups studied in the initial report.
CAUDAL NERVE BLOCK FOR LABOR ANALGESIA
The sacral canal shares in the general engorgement of extradural veins that occurs in late pregnancy or in any clinical condition in which the inferior vena cava (IVC) is partially obstructed. Because the effective volume of the caudal canal is markedly diminished during the latter part pregnancy, the caudal dosage should be reduced proportionately in women at term. The segmental spread of local anesthetics may increase substantially in pregnant women at term, necessitating a 28% to 33% decrease of dose requirement in this patient population. The choice of a continuous catheter or a single-shot technique during active labor is limited by the relative lack of sterility at the sacral hiatus, which may be contaminated by feces and meconium.
Rare cases of Horner syndrome have been reported when large doses of local anesthetics are injected caudally during labor analgesia administration. This is most likely to occur if the injection is made with the patient on her back (engorgement of epidural venous plexus and IVC compression are maximal).
The so-called dual technique (lumbar and caudal) of epidural nerve block for labor is no longer widely used. Because the pain of uterine contractions is mediated by sympathetic nervous system fibers originating from the T10 to L2 spinal segments, a lumbar epidural catheter suffices for both stage I and stage II of parturition, with dosage adjustments made depending on the exact circumstances and requirements (see Obstetric Regional Anesthesia).
INDICATIONS FOR CAUDAL EPIDURAL ANALGESIA IN CHILDREN
Characteristics of the block
The sacral hiatus is usually easy to palpate in infants and children, which makes this technique much easier and predictable. Consequently, in many institutions with large numbers of pediatric patients, caudal epidural nerve block is an integral part of the intra- and postoperative pain management for children undergoing a wide range of surgical procedures both below and above the diaphragm. The technique is easily learned; one study demonstrated an 80% success rate in resident trainees after completing 32 procedures performed without fluoroscopic guidance. In infants and small children, a 21-gauge, short-bevel, 1-inch needle may be used for single-injection techniques. For continuous nerve blocks, a standard epidural catheter may be advanced through an 18-gauge angiocatheter or through a thin-wall 18-gauge epidural needle. It has been noted that by 4 or 5 years of age the sacral canal is usually large enough to accept such a needle for passage of a catheter. The electrocardiogram has been used to verify appropriate thoracic catheter tip placement (epidural electrocardiography).
Spread of Local Anesthetic Solutions
The segmental spread of analgesia following caudal administration is more predictable in children up to about 12 years of age. Some studies suggested that the cephalad spread of caudal solutions in children is not hampered by the same anatomical constraints that develop from puberty onward. Before puberty, anatomical barriers at the lumbosacral junction have not yet developed to a marked degree, and caudal solutions can flow freely upward into the higher recesses of the spinal canal. As a consequence, the rostral spread of caudal anesthesia is more extensive and more predictable in children than in adults.
Indications in Children
In children, caudal nerve block is usually combined with light general anesthesia with spontaneous ventilation. During lower abdominal and genitourinary surgery in children, caudal nerve block with 0.25% bupivacaine (2 mg/kg) was shown to lower the metabolic and endocrine responses to stress, as measured by glucose concentrations; mean prolactin levels; insulin; and cortisol concentrations, as compared with general anesthesia alone. Thoracic placement of catheters is possible in neonates and small children. However, one radiographic study of 115 infants found 10 caudally placed catheters to be in the high thoracic or low cervical region, when their intended site was in the lower thoracic segments.
The three groups of indications for caudal epidural nerve block in children are the following:
- Patients requiring sacral nerve block (circumcision, anal surgery)
- Patients requiring lower thoracic nerve block (inguinal herniorrhaphy)
- Patients requiring analgesia of the upper thoracic dermatomes (thoracic surgery)
Pharmacologic Considerations for Caudal Epidural Anesthesia in Children
Caudal nerve block with bupivacaine (4 mg/kg) and morphine (150 μg/kg) was found to lower fentanyl requirements during cardiac surgery and shorten extubation times in a group of 30 pediatric patients randomized to receive general anesthesia alone or a combination of general or caudal nerve block.
Anesthetic dose requirements are about 0.1 mL/segment per year for 1% lidocaine or 0.25% bupivacaine.1 The dose may also be calculated based on body weight. The relationship between age and dose requirements is strictly linear, with a high degree of correlation up to 12 years old. A recent study using fluoroscopy with 0.2% ropivacaine containing radiopaque dye at a ratio of 1:4 showed that the weight-based doses for caudally administered 0.2% bupivacaine were applicable to 0.2% ropivacaine as well. Plasma bupivacaine concentrations in children receiving caudal nerve block with 0.2% of the local anesthetic (2 mg/kg) were less than equivalent doses administered via ilioinguinal-iliohypogastric nerve block for pain control following herniotomy or orchidopexy. In addition, the times to peak plasma concentrations were faster in the peripheral nerve block group, indicating that caudal nerve block is a safe alternative to local infiltration techniques in inguinal surgery.
In a study of children 1 to 6 years of age who underwent orchidopexy, a caudal nerve block using larger volumes of dilute bupivacaine (0.2%) was shown to be more effective than a smaller volume of the standard (0.25%) concentration for blocking the peritoneal response to spermatic cord traction, with no change in the quality of postoperative analgesia. In that study, the total bupivacaine dose was identical in both groups (20 mg). Ropivacaine 0.5% was shown to provide a significantly longer duration of analgesia following inguinal herniorrhaphy in children aged 1.5–7 years when compared to 0.25% ropivacaine or 0.25% bupivacaine. All children received 0.75 mL/kg of the local anesthetic. However, the times to first voiding and to standing were significantly delayed in the group receiving 0.5% ropivacaine, and there was one case of motor nerve block of the lower extremities.
Another study compared high-volume/low-concentration (1.5 mL/kg of 0.15% solution) and low-volume/high-concentration ropivacaine (1.0 mL/kg 0.225% solution) for caudal analgesia in children aged 1–5 years of age undergoing orchiopexy. This study showed that a larger volume of diluted ropivacaine provided better quality and longer duration of analgesia after discharge than a smaller volume of more concentrated ropivacaine.
Ropivacaine has also been used for caudal nerve block for hypospadias repair in a double-blind, randomized study in 26 children. The minimal effective local anesthetic concentration of ropivacaine was found to be 0.11%, to provide effective caudal analgesia in children under general anesthesia with 0.5 minimum alveolar concentration of enflurane.
Plasma concentrations of ropivacaine after caudal nerve block in 20 children 1 to 8 years of age, using 2 mg/mL, 1 mL/kg, demonstrated free fractions of 5%, clearance of 7.4 mL/kg/min, and terminal half-life of 3.2 hours, well below those associated with toxic symptoms in adults. Another study looking at the minimum local analgesic concentration of ropivacaine for intraoperative caudal analgesia in preschool and school-age children undergoing hypospadias repair found that a higher concentration of ropivacaine was needed for school-age children than preschool age children to provide intraoperative caudal analgesia when combined with general anesthesia. Of the three commonly used local anesthetics for single-shot caudal anesthesia (bupivacaine, ropivacaine, and levobupivacaine), no superiority in terms of clinical efficacy was found for any of these drugs in a meta-analysis of 17 randomized controlled trials that concerned single-shot pediatric caudal anesthesia with at least two of the three drugs in question. Bupivacaine and ropivacaine showed the highest and lowest incidence of motor nerve block, respectively.
A quantitative systematic review of randomized controlled trials looking at the safety and efficacy of clonidine as an additive for caudal regional anesthesia suggested that clonidine may provide an extended duration of analgesia with a decreased incidence for analgesic rescue requirements and few adverse effects compared to caudal local anesthetics alone. Data from 20 randomized controlled trials were used in this meta-analysis to assess the safety and efficacy of caudal clonidine added to local anesthetics versus local anesthetics alone in children undergoing urological, lower abdominal, or lower limb surgery. Dexmedetomidine (1 μg/kg) was shown to provide an extended duration of pain relief when added to bupivacaine 2.5 mg/mL, 1 mL/kg, when compared to an identical dose of bupivacaine alone in children aged 1–6 years undergoing unilateral inguinal hernia repair/orchidopexy. Another study compared the analgesic effects and side effects of dexmedetomidine and clonidine added to bupivacaine in pediatric patients undergoing lower abdominal surgeries. Sixty patients aged 6 months to 6 years were evenly and randomly assigned into three groups in a double-blinded manner. Each patient received a single caudal dose of bupivacaine 0.25% (1 mL/kg) combined with dexmedetomidine 2 μg/kg in normal saline 1 mL, clonidine 2 μg/ kg in normal saline 1 mL, or a corresponding volume of normal saline. The results demonstrated that while both additives increased the period of analgesia, there was no significant advantage of selecting dexmedetomidine over clonidine.
The local anesthetics typically administered for single-shot caudal nerve blocks in pediatric patients are listed in Table 3.
TABLE 3. Typical local anesthetics for caudal nerve block in pediatrics (single shot).
| Agent | Concentration (%) | Dose (mg) | Onset (minutes) | Duration of Action (minutes) |
|---|---|---|---|---|
| Ropivacaine74 | 0.2 | 2 mg/kg | 9 | 520 |
| Bupivacaine74 | 0.25 | 2 mg/kg | 12 | 253 |
| Ropivacaine75 | 0.2 | 0.7 mg/kg | 11.7 | 491 |
| Bupivacaine75 | 0.25 | 0.7 mg/kg | 13.1 | 457 |
| Ropivacaine76 | 0.2 | 1 mL/kg | 8.4 | Not available |
| Levobupivacaine76 | 0.25 | 1 mL/kg | 8.8 | Not available |
| Bupivacaine76 | 0.25 | 1 mL/kg | 8.8 | Not available |
NYSORA Tips
- Relaxation of the anal sphincter following local anesthetic injection may predict the success for a caudal nerve block.
- This is particularly useful in children because most caudal nerve blocks are performed while the child is anesthetized, and it is not possible to assess the effectiveness of the nerve block by testing for sensory analgesia levels.
- One study demonstrated that the presence of a lax anal sphincter at the termination of surgery correlated with the reduced need to administer opioids perioperatively.
Other Considerations for Use of Caudal Epidural Anesthesia in Children
Although the caudal nerve block is a mainstay of perioperative pain management in pediatric surgery and represents probably 60% of all regional anesthetic techniques performed in this patient population, not all studies have demonstrated a marked benefit of caudal nerve block for postoperative analgesia when compared with other modalities. Following unilateral inguinal herniorrhaphy, the caudal nerve block was shown to provide effective, but not superior, pain management when compared with local wound infiltration in 54 children. The side effects and rescue analgesia requirements did not differ between the two groups. Caudal epidural nerve block in children may induce significant changes in descending aortic blood flow while maintaining heart rate and mean arterial blood pressure. In a study of 10 children aged 2 months to 5 years, a transesophageal Doppler probe was used to calculate hemodynamic variables after the injection of 1 mL/kg of 0.25% bupivacaine with epinephrine 5 μg/mL. The aortic ejection volume increased, and aortic vascular resistance decreased by about 40%. Another study that looked at peripheral hemodynamics using Doppler ultrasonography in sevoflurane-anesthetized children before and after a caudal nerve block showed significantly altered flow patterns after the nerve block. The peak velocity increased by 24%; volume flow increased by 76%, and the diameter of the dorsalis pedis artery increased by 20%. However, blood pressures and heart rates were not significantly affected by caudal nerve block. These data suggest that caudal nerve block results in vasodilatation secondary to sympathetic nervous system block.
APPLICATIONS OF CAUDAL EPIDURAL NERVE BLOCK IN ACUTE AND CHRONIC PAIN MANAGEMENT
Radiculopathy Refractory to Conventional Therapy
In cases of radiculopathy refractory to conventional analgesic therapies, caudal epidural analgesic techniques can significantly and reliably reduce pain. One such technique is percutaneous epidural neuroplasty: A caudal catheter is left in place for up to 3 days for the purpose of injecting hypertonic solutions into the epidural space to treat radiculopathy with low back pain and associated epidural scarring, typically from previous lumbar spinal surgery. In addition to local anesthetics and corticosteroids, hypertonic saline and hyaluronidase are added to the injectate. The technique requires the use of fluoroscopic guidance and caudal epidurography because of its efficacy in correlating a filling defect of injected iodinated nonionic contrast medium with a patient’s reported level of pain.
Injection of solutions into the epidural space of a patient with meningeal adhesions is usually painful because of stretching of affected nerve roots. Dexamethasone or betamethasone have been recommended instead of methylprednisolone or triamcinolone because particulate steroids may occlude an epidural catheter or possibly cause spinal ischemia following unintentional intravascular injection.
Hypertonic saline is used to prolong pain relief due to its local anesthetic effects and its ability to reduce edema in previously scarred or inflamed nerve roots. A lateral needle approach is recommended into the caudal canal, directing the needle and catheter toward the affected side. Lateral placement tends to minimize the likelihood of penetrating the dural sac or injecting subdural. When 5–10 mL of contrast media are injected into the caudal canal through an epidural catheter, a “Christmas tree” appearance develops as dye spreads into the perineural structures inside the bony canal and along the nerves as they exit the vertebral column. Epidural adhesions prevent the spread of the dye, so the involved nerves are not outlined by the contrast. Once correct catheter placement in the epidural space is ensured, 1500 units of hyaluronidase in 10 mL of preservative-free saline is injected rapidly. This is followed by an injection of 10 mL of 0.2% ropivacaine and 40 mg of triamcinolone. After these two injections, an additional volume of 9 mL of 10% hypertonic saline is infused over 20–30 minutes. On the second and third days, the local anesthetic (ropivacaine) injection is followed by the hypertonic saline solution.
A study compared the effectiveness of percutaneous epidural adhesiolysis utilizing an injection of 5 mL of preservative-free 2% lidocaine, followed by 6 mL of 10% sodium chloride solution and 6 mg of nonparticulate betamethasone via a fluoroscopically guided, targeted placement of a caudal catheter (group 1) versus an injection of the same solution with 6 mL of 0.9% sodium chloride solution instead of the hypertonic saline via a catheter placed in the caudal canal with its tip at the S3 level (group 2). The study found significant pain relief (76%) in the hypertonic saline group at 1-year follow-up, compared to 4% of patients in the normal saline group.
A prospective, double-blind, randomized study looking at the role of adding hyaluronidase to fluoroscopically guided caudal epidural steroid and hypertonic saline injections in patients with failed back surgery syndrome showed a significant improvement in long-term pain relief in patients who received hyaluronidase. A total of 38 patients were enrolled in the study. Twenty patients received fluoroscopically guided caudal injections of 10 mL of 0.25% bupivacaine solution containing 80 mg of methylprednisolone and 30 mL of 3% hypertonic saline (group 1). Eighteen patients received the same amount of local anesthetic and steroids, followed by 1500 IU of hyaluronidase (which was replaced by an equivalent volume of normal saline in group 1 patients) and 30 mL of 3% hypertonic saline (group 2).
Another randomized, controlled, double-blind trial of fluoroscopically guided caudal epidural injections compared 10 mL of lidocaine 0.5% and 9 mL of lidocaine 0.5% mixed with either 6 mg of betamethasone or 40 mg of methylprednisolone (total volume 10 mL). This study showed a potential superiority of steroids when compared with local anesthetic alone at 1-year follow-up.
Postoperative Analgesia in Patients Undergoing Lumbar Spine Surgery
Another unique application of caudal nerve block is the provision of postoperative analgesia in patients undergoing lumbar spine surgeries. In one case series, patients received 20 mL of 0.25% bupivacaine with 0.1 mg buprenorphine via the caudal epidural approach, performed prior to surgical incision. The patients underwent posterior interbody fusion and laminotomy for spinal stenosis, and postoperative pain control was compared in the caudal group with a group treated with conventional parenteral opioids. The caudal group required fewer rescue analgesic medication doses for the first 12 hours following surgery. A reduction in blood pressure in the caudal group patients undergoing laminotomy, but not fusion, was noted in the patients with prolonged duration (24 hours) of postoperative analgesia.
Other Applications
Caudal epidural nerve block has also been compared with intramuscular opioids in the treatment of pain after emergency lower extremity orthopedic surgery. The caudal group who received 20 mL of 0.5% bupivacaine had 8 hours of superior analgesia and also had a significant reduction in the need for rescue opioid medications.
Caudal injection of clonidine (75 μg with 7 mL bupivacaine 0.5% and 7 mL lidocaine 2% with epinephrine 5 μg/mL) has been used for postoperative analgesia after elective hemorrhoidectomy. Thirty-two adults received the clonidine-local combination, while a control group received local anesthetic alone.
Analgesia averaged 12 hours in the clonidine group, compared to less than 5 hours in the group receiving only local anesthetic. Bradycardia occurred in about 22% of patients in the clonidine group. This contrasts with the results of an evaluation of clonidine used as an adjunct for pediatric caudal anesthesia as noted previously.
Caudal injections of alcohol or phenol have been used to treat intractable pain due to cancer. In a study of 67 nerve blocks, it was found that the lower sacral roots were easily reached with the caudal injection and that the S1 and S2 roots (contribution from the lumbosacral plexus) were spared.
COMPLICATIONS ASSOCIATED WITH CAUDAL EPIDURAL NERVE BLOCK
The complications of caudal nerve block are the same as those occurring following lumbar epidural nerve block and may include those related to the technique itself and those related to the injectate (local anesthetic or other injected substance). Fortunately, serious complications occur infrequently. The list of potential complications includes epidural abscess, meningitis, epidural hematoma, dural puncture and post-dural puncture headache, subdural injection, pneumocephalus, and air embolism, back pain, and broken or knotted epidural catheters. Other rare complications that have been reported with caudal anesthesia include unilateral parotid swelling, persistent hiccups following continuous caudal infusion of 0.1% ropivacaine, and intracranial hypotension headache after uncomplicated caudal epidural injection.
Systemic Toxicity of Local Anesthetics
The incidence of local anesthetic-induced seizures following caudal nerve block seems to be higher that of lumbar or thoracic approaches. In a retrospective study of 25,697 patients who received brachial plexus nerve blocks, caudal epidural nerve blocks, or lumbar epidural nerve blocks from 1985 to 1992, Brown noted 26 seizure episodes. The frequency of seizures in adults by decreasing order was caudal, brachial plexus nerve block, lumbar, or thoracic epidural nerve block. Nine cases were attributed to caudal nerve blocks, eight occurring with chloroprocaine and one with lidocaine. There was a 70-fold increased incidence of local anesthetic toxic reactions with caudal epidural anesthesia (0.69%) than with lumbar or thoracic epidural anesthesia in adults.
NYSORA Tips
- The incidence of local anesthetic-induced seizures following caudal epidural nerve block is higher than following lumbar or thoracic approaches.
- The relative risk of local anesthetic toxicity follows this order: caudal > brachial plexus nerve block > lumbar or thoracic epidural nerve block.
- Elevation of heart rate by more than 10 beats per minute or an increase in systolic blood pressure of more than 15 mm Hg after injection of epinephrine-containing local anesthetic is suggestive of intravascular injection.
In children, however, one retrospective review identified only two toxic reactions (ie, local anesthetic-induced seizures) in 15,000 caudal nerve blocks. Dalens’s group found that unintentional intravascular injection occurs in up to 0.4% of pediatric caudal nerve blocks,70 demonstrating the importance of performing epinephrine-containing test dosing in this age group. It has been suggested that an elevation of heart rate by more than 10 beats per minute or an increase in systolic blood pressure of more than 15 mm Hg should be taken as indicative of systemic injection. T-wave changes on the ECG occur earliest following intravascular injection, followed by heart rate changes and finally by blood pressure changes. These changes may be delayed for up to 90 seconds following the injection. For more information, see Local Anesthetic Systemic Toxicity.
Occurrence of Total Spinal Anesthesia
Total spinal anesthesia can occur when an injected dose of local anesthetic intended for the epidural space gains access to the subarachnoid space. In the case report of an 18-month-old child weighing 10 kg who received a caudal nerve block postoperatively after undergoing emergency repair of a recurrent diaphragmatic hernia, 4 mL of 0.5% bupivacaine and 2.5 μg/kg of buprenorphine were injected in a total volume of 10 mL. Eye-opening and hand movement were delayed for 3 hours following this complication. In another infant undergoing revision of a Nissen fundoplication, a caudally placed catheter was unintentionally advanced to the cervical spinal region. Electrical stimulation of the catheter (Tsui test) resulted in phrenic nerve stimulation. The catheter was successfully repositioned and further care was uncomplicated. This case report illustrates the relative ease of passing the catheter to high vertebral levels in infants as opposed to adults.
Infection
One case report documented the rare occurrence of distant diskitis and vertebral osteomyelitis involving skip levels and without the development of epidural abscess formation in an elderly woman who received caudal epidural steroid and local anesthetic for degenerative spondylolisthesis. One month later, she developed an L2–L3 and L4–L5 infective diskitis, together with adjacent vertebral osteomyelitis. Cultures demonstrated Pseudomonas aeruginosa growth, which was treated with antibiotics.
SUMMARY
Caudal epidural nerve block is a technique of providing analgesia and anesthesia of the lumbosacral nerve roots that pre-dates conventional lumbar approaches. The nerve block has undergone several periods of acceptability, and although it is infrequently applied to routine surgical cases in adults, it is the most commonly performed regional anesthetic technique in infants and children. Caudal nerve block in adult patients has enjoyed a resurgence lately, mainly because it provides an alternative route to the lumbar epidural space when direct access is limited by previous surgeries and for performing epiduroscopy. Clinicians who routinely utilize fluoroscopy and ultrasound imaging will find that it has many applications, for both routine and complicated cases.
REFERENCES
- Bromage PR: Epidural Analgesia. Saunders, 1978, pp 258–282.
- Racz G: Personal communication, October 12, 2003, American Society of Anesthesiologists Annual Meeting, San Francisco.
- Trotter M: Variations of the sacral canal: Their significance in the administration of caudal analgesia. Anesth Analg 1947;26:192–202.
- MacDonald A, Chatrath P, Spector T, et al: Level of termination of the spinal cord and the dural sac: A magnetic resonance study. Clin Anat; 1999;12:149–152.
- Joo J, Kim J, Lee J: The prevalence of anatomical variations that can cause inadvertent dural puncture when performing caudal nerve block in Koreans: A study using magnetic resonance imaging. Anesthesia 2010;65 (1):23–26.
- Igarashi T, Hirabayashi Y, Shimizu R, et al: The lumbar extradural structure changes with increasing age. Br J Anaest 1997;78:149–152.
- Crighton I, Barry B, Hobbs G: A study of the anatomy of the caudal space using magnetic resonance imaging. Br J Anaesth 1997;78:391–395.
- Sekiguchi M, Yabuki S, Satoh K, et al: An anatomic study of the sacral hiatus. A basis for successful epidural nerve block. Clin J Pain 2004;20:51–54.
- Bryce-Smith R: The spread of solutions in the extradural space. Anaesthesia 1954;9:201–205.
- Brenner E: Sacral anesthesia. Ann Surg 1924;79:118–123.
- Aggarwal A, Kaur H, Batra YK, et al: Anatomic consideration of caudal epidural space: A cadaver study. Clin Anat 2009;22(6):730–737.
- Waldman S: Caudal epidural nerve block. In Waldman S (ed): Interventional Pain Management, 2nd ed. Saunders, 2001, p 520.
- Winnie A, Candido KD: Differential neural block for the diagnosis of pain. In Waldman S (ed): Interventional Pain Management, 2nd ed. Saunders, 2001, pp 162–173.
- Candido KD, Stevens RA: Intrathecal neurolytic blocks for the relief of cancer pain. Best Pract Res Clin Anaesthesiol 2003;17:407–428.
- Lou L, Racz G, Heavner J: Percutaneous epidural neuroplasty. In Waldman S (ed): Interventional Pain Management, 2nd ed. Saunders, 2001, pp 434–445.
- Cook RA, Driver RP Jr: Epidural blood patch. W V Med J 2009;105(5): 28–29.
- Heavner J, Racz G, Raj P: Percutaneous epidural neuroplasty: Prospective evaluation of 0.9% NaCl versus 10% NaCl with or without hyaluronidase. Reg Anesth Pain Med 1999;24:202–207.
- Manchikanti L, Bakhit C, Pampati V: Role of epidurography in caudal neuroplasty. Pain Digest 1998;8:277–281.
- Kakiuchi M, Abe K: Pre-incisional caudal epidural block and the relief of pain after lumbar spine operations. Int Orthop 1997;21:62–66.
- McCrirrick A, Ramage D: Caudal block for postoperative analgesia: A useful adjunct to intramuscular opiates following emergency lower leg orthopaedic surgery. Anaesth Intensive Care 1991;19:551–554.
- Van Elstraete A, Pastureau F, Lebrun T, et al: Caudal clonidine for postoperative analgesia in adults. Br J Anaesth 2000;84:401–402.
- Porges P, Zdrahal F: [Intrathecal alcohol neurolysis of the lower sacral roots in inoperable rectal cancer]. Anaesthetist 1985;34:627–629.
- Ivani G, DeNegri P, Conio A, et al: Comparison of racemic bupivacaine, ropivacaine and levobupivacaine for pediatric caudal anesthesia. Effects on postoperative analgesia and motor block. Reg Anesth Pain Med 2002;27:157–161.
- Chan S, Tay H, Thomas E: “Whoosh” test as a teaching aid in caudal block. Anaesth Intensive Care 1993;21:414–415.
- Orme R, Berg S: The “swoosh” test—an evaluation of a modified “whoosh” test in children. Br J Aneaesth 2003;90:62–65.
- Kim MS, Han KH, Kim EM, et al: The myth of the equilateral triangle for identification of sacral hiatus in children disproved by ultrasonography. Reg Anesth Pain Med 2013;38(3):243–247.
- Yoon JS, Sim KH, Kim SJ, Kim WS, Koh SB, Kim BJ: The feasibility of color Doppler ultrasonography for caudal epidural steroid injection. Pain 2005;118:210–214.
- Tsui B, Tarkkila P, Gupta S, Kearney R: Confirmation of caudal needle placement using nerve stimulation. Anesthesiology 1999;91:374–378.
- Digiovanni A: Inadvertent interosseous injection—a hazard of caudal anesthesia. Anesthesiology 1971;34:92–94.
- Lofstrom B: Caudal anaesthesia. In Ejnar Eriksson (ed): Illustrated Handbook in Local Anaesthesia. Astra, 1969, pp 129–134.
- Caudal block. In Covino BG, Scott DB (eds): Handbook of Epidural Anaesthesia and Analgesia. Grune & Stratton, 1985, pp 104–108.
- Dawkins C: An analysis of the complications of extradural and caudal block. Anaesthesia 1969;24:554–563.
- Chung Y, Lin C, Pang W, et al: An alternative continuous caudal block with caudad catheterization via lower lumbar interspace in adult patients. Acta Anaesthesiol Scand 1998;36:221–227.
- Triffterer L, Machata AM, Latzke D, et al: Ultrasound assessment of cranial spread during caudal block in children: effect of the speed of injection of local anesthetics. Br J Anaesth 2012;108(4):670–674.
- Li Y, Zhou Y, Chen H, Feng Z: The effect of sex on the minimum local analgesic concentration of ropivacaine for caudal anesthesia in anorectal surgery. Anesth Analg 2010;110:1490–1493.
- Wong S, Li J, Chen C, et al: Caudal epidural block for minor gynecologic procedures in outpatient surgery. Chang Gung Med J 2004;27:116–121.
- Nishiyama T, Hanaoka K, Ochiai Y: The median approach to transsacral epidural block. Anesth Analg 2002;95:1067–1070.
- Schuepfer G, Konrad C, Schmeck J, et al: Generating a learning curve for pediatric caudal epidural blocks: An empirical evaluation of technical skills in novice and experienced anesthesiologists. Reg Anesth Pain Med 2000;25:385–388.
- Tsui B, Seal R, Koller J: Thoracic epidural catheter placement via the caudal approach in infants by using electrocardiographic guidance. Anesth Analg 2002;95:326–330.
- Lundblad M, Lonnqvist PA, Eksborg S, Marhofer P: Segmental distribution of high-volume caudal anesthesia in neonates, infants and toddlers as assessed by ultrasonography. Paediatr Anaesth 2011;21(2): 121–127.
- Tuncer S, Yosunkaya A, Reisli R, et al: Effect of caudal block on stress response in children. Pediatr Int 2004;46:53–57.
- Valairucha S, Seefelder C, Houck C: Thoracic epidural catheters placed by the caudal route in infants: The importance of radiographic conformation. Paediatr Anaest 2002;12:424–428.
- Rojas-Perez E, Castillo-Zamora C, Nava-Ocampo A: A randomized trial of caudal block with bupivacaine 4 mg x kg-l(1.8ml x kg-l) vs general anesthesia with fentanyl for cardiac surgery. Paediatr Anaesth 2003; 13:311–317.
- Koo BN, Hong JY, Kil HK: Spread of ropivacaine by a weight-based formula in a pediatric caudal block: A fluoroscopic examination. Acta Anaesthesiol Scand 2010;54(5):562–565.
- Stow P, Scott A, Phillips A, et al: Plasma bupivacaine concentrations during caudal analgesia and ilioinguinal-iliohypogastric nerve block in children. Anaesthesia 1998;43:650–653.
- Verghese S, Hannallah R, Rice LJ, et al: Caudal anesthesia in children: Effect of volume versus concentration of bupivacaine on blocking spermatic cord traction response during orchidopexy. Anesth Analg 2002;95: 1219–1223.
- Koinig H, Krenn C, Glaser C, et al: The dose-response of caudal ropivacaine in children. Anesthesiology 1999;90:1339–1344.
- Jeong-Yeon Hong MD, Sang W, Han MD, et al: A comparison of high volume/low concentration and low volume/high concentration ropivacaine in caudal analgesia for pediatric orchiopexy. Anesth Analg 2009;109; 1073–1078.
- Deng S, Xiao, W, Tang G, et al: The minimum local anesthetic concentration of ropivacaine for caudal analgesia in children. Anesth Analg 2002;94:1465–1468.
- Lonnqvist P, Westrin P, Larsson B, et al: Ropivacaine pharmacokinetics after caudal block in 1-8 year old children. Br J Anaesth 2000;85: 506–511.
- Deng XM, Xiao WJ, Tang GZ, Luo MP, Xu KL: Minimum local analgesic concentration of ropivacaine for intra-operative caudal analgesia in pre-school and school age children. Anesthesia 2010;65(10):991–995.
- Dobereiner EF, Cox RG, Ewen A, Lardner DR: Evidence-based clinical update: Which local anesthetic drug for pediatric caudal block provides optimal efficacy with the fewest side effects? Can J Anaesth 2010;57(12):1102–1110.
- Schnabel A, Poepping DM, Pogatzki-Zahn EM, Zahn PK: Efficacy and safety of clonidine as additive for caudal regional anesthesia: A quantitative systematic review of randomized controlled trials. Pediatr Anesth 2011;21:1219–1230.
- Saadawy I, Bolker A, Elshahawy MA, et al: Effect of dexmedetomidine on the characteristics of bupivacaine in a caudal block in pediatrics. Acta Anesthesiol Scand 2009;53:251–256.
- El-Hennawy M, Abd-Elwahab AM, Abd-Elmaksoud AM, et al: Addition of clonidine or dexmedetomidine to bupivacaine prolongs caudal analgesia in children. Br J Anaesth 2009;103(2):268–274.
- Verghese S, Mostello L, Patel R: Testing anal sphincter tone predicts the effectiveness of caudal analgesia in children. Anesth Analg 2002;94: 1161–1164.
- Schindler M, Swann M, Crawford M: A comparison of postoperative analgesia provided by wound infiltration or caudal analgesia. Anesth Intensive Care 1991;19:46–49.
- Larousse E, Asehnoune K, Dartayet B, et al: The hemodynamic effects of pediatric caudal anesthesia assessed by esophageal Doppler. Anesth Analg 2002;94:1165–1168.
- Hong JY, Ahn S, Kil HK: Changes of dorsalis pedis artery flow pattern after caudal block in children: observational study using a duplex sonography. Pediatr Anesth 2011;21:116–120.
- Manchikanti L, Cash KA, McManus CD, et al. The preliminary results of a comparative effectiveness evaluation of adhesiolysis and caudal epidural injections in managing chronic low back pain secondary to spinal stenosis: A randomized, equivalence controlled trial. Pain Physician 2009; 12:E341–E354.
- Yousef AA, El-Deen AS, Al-Deeb AE: The role of adding hyaluronidase to fluroscopically guided caudal steroid and hypertonic saline injection in patients with failed back surgery syndrome: A prospective, doubleblinded, randomized study. Pain Pract 2010;10(6):548–553.
- Manchikanti L, Singh V, Cash KA, Pampati V: A randomized, controlled, double-blind trial of fluoroscopic caudal epidural injections in the treatment of lumbar disc herniation and radiculitis. Spine 2011;36(23): 1897–1905.
- Joshi W, Connelly R, Freeman K, et al: Analgesic effect of clonidine added to bupivacaine 0.125% in pediatric caudal block. Paediatr Anaesth 2004;14:483–486.
- Valois T, Otis A, Ranger M, Muir JG: Incidence of self-limiting back pain in children following caudal block: an exploratory study. Pediatr Anesth 2010;20:844–850.
- Lin J, Zuo YX: Unilateral parotid swelling, following caudal block without airway device placement. Pediatr Anesth 2011;21:169–178.
- Bagdure DN, Reiter PD, Bhoite GR, et al: Persistent hiccups associated with epidural ropivacaine in a newborn. Ann Pharmacother 2011; 45(6):e35.
- Thomas R, Thanthulage S: Intracranial hypotension headache after uncomplicated caudal epidural injection. Anaesthesia 2012;67: 416–419.
- Brown D, Ransom D, Hall J, et al: Regional anesthesia and local anesthetic-induced systemic toxicity: Seizure frequency and accompanying cardiovascular changes. Anesth Analg 1995;81:321–328.
- Giaufre E, Dalens B, Gombert A: Epidemiology and morbidity of regional anesthesia in children: A one year prospective survey of the French– language Society of Pediatric Anesthesiologists. Anesth Analg 1996:83: 904–912.
- Dalens B, Hansanoui A: Caudal anesthesia in pediatric surgery: Success rate and adverse effects in 750 consecutive patients. Anesth Analg 1989;8:83–89.
- Afshan G, Khan F: Total spinal anaesthesia following caudal block with bupivacaine and buprenorphine. Paediatr Anaesth 1996;6:239–242.
- Tsui B, Malherbe S: Inadvertent cervical epidural catheter placement via the caudal route using electrical stimulation. Anesth Analg 2004;99: 259–261.
- Yue W, Tan S: Distant skip level discitis and vertebral osteomyelitis after caudal epidural injection: A case report of a rare complication of epidural injections. Spine 2003;1:209–211.
- Ivani G, Mereto N, Lampugnani E, et al: Ropivacaine in paediatric surgery: Preliminary results [Abstract]. Paediatr Anaesth 1998;8: 127–129.
- Ivani G, Lampugnani E, De Negri P, et al: Ropivacaine vs. bupivacaine in major surgery in infants [abstract]. Can J Anaesth 1999;46: 467–469.
- Ivani G, De Negri P, Conio A, et al: Comparison of racemic bupivacaine, ropivacaine and levobupivacaine for pediatric cauda anesthesia. Effects on postoperative analgesia and motor block. Reg Anesth Pain Med 2002;27:157–161.