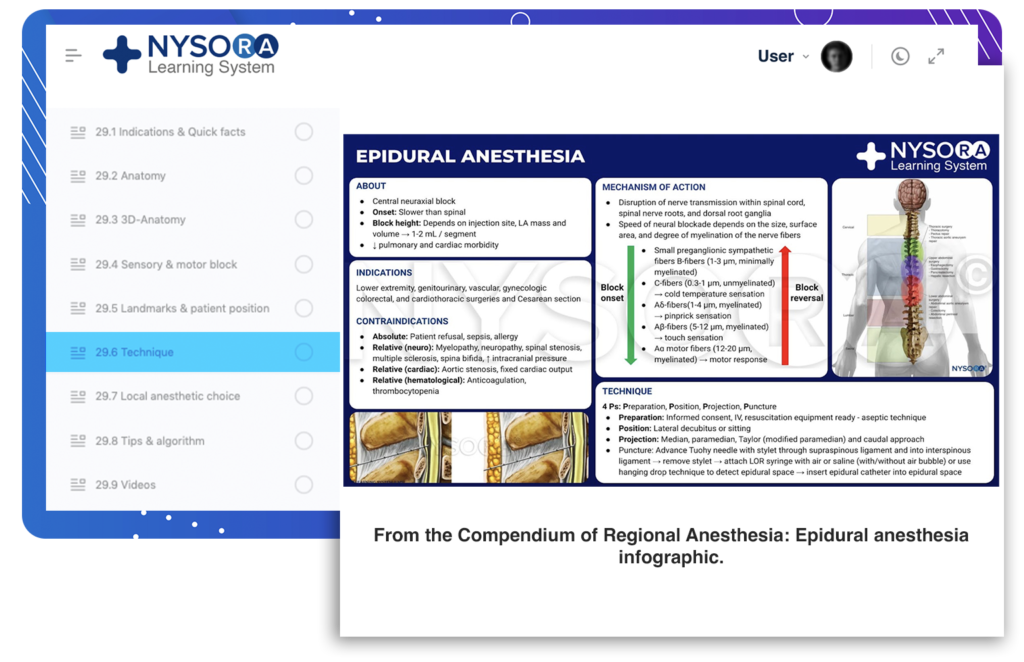
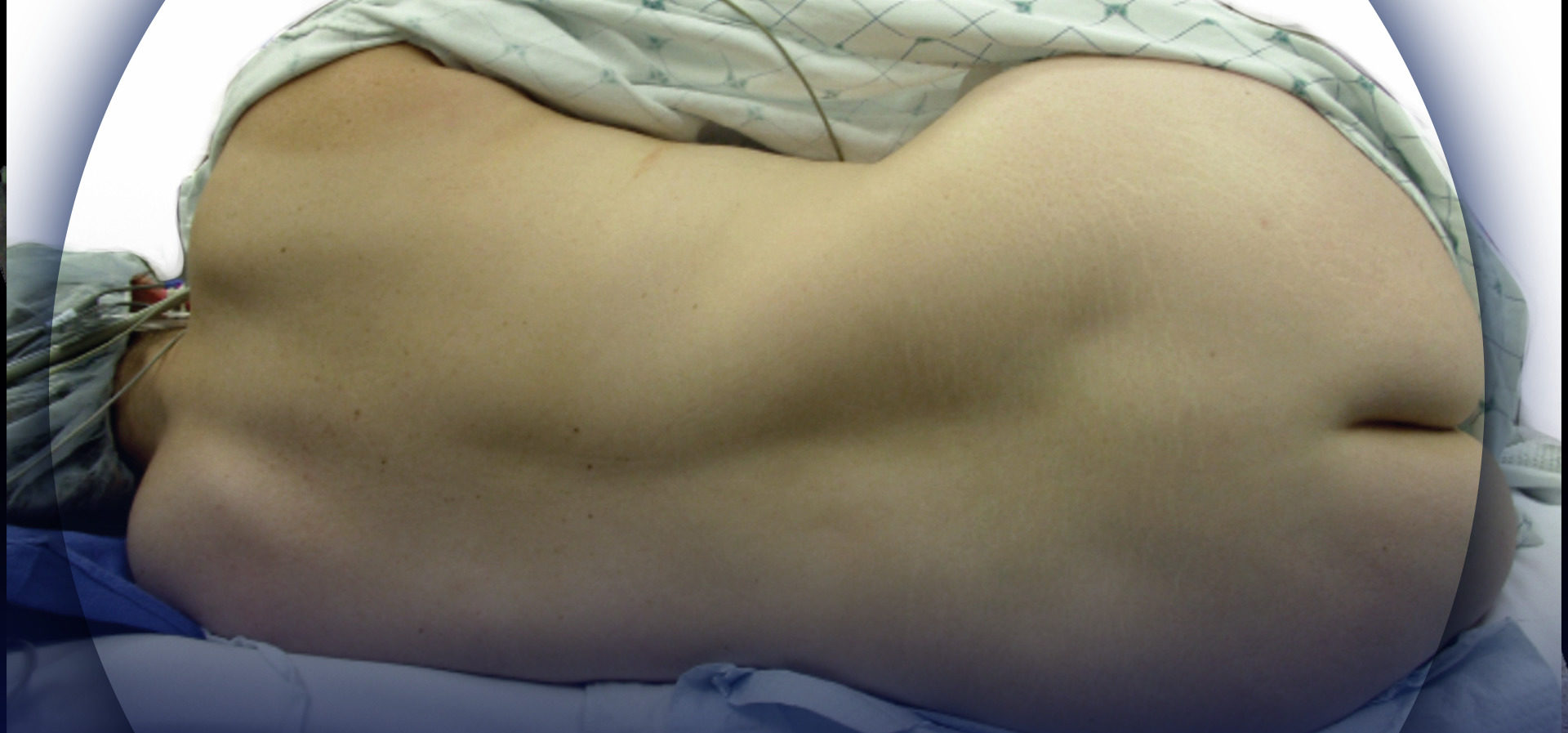
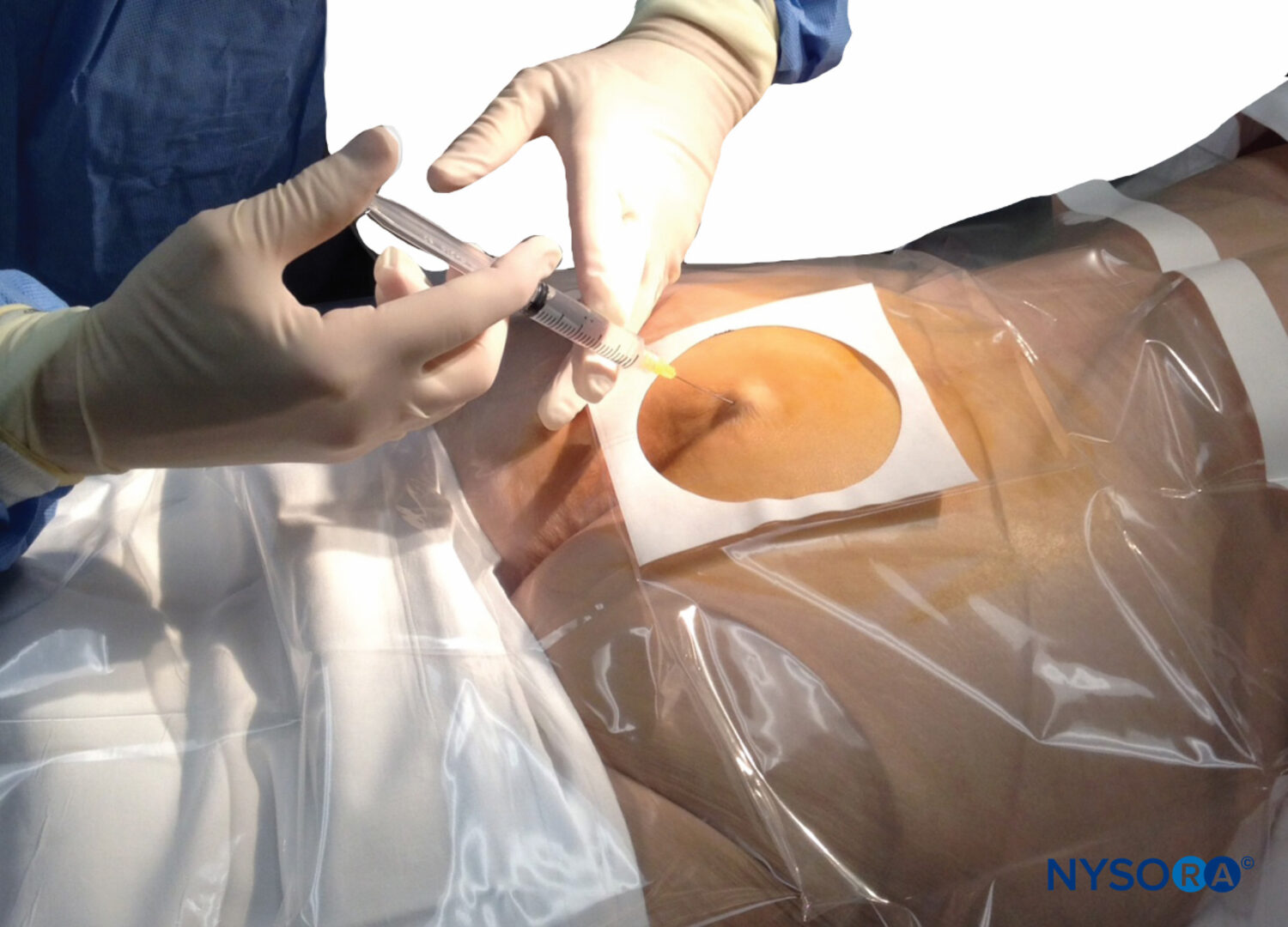
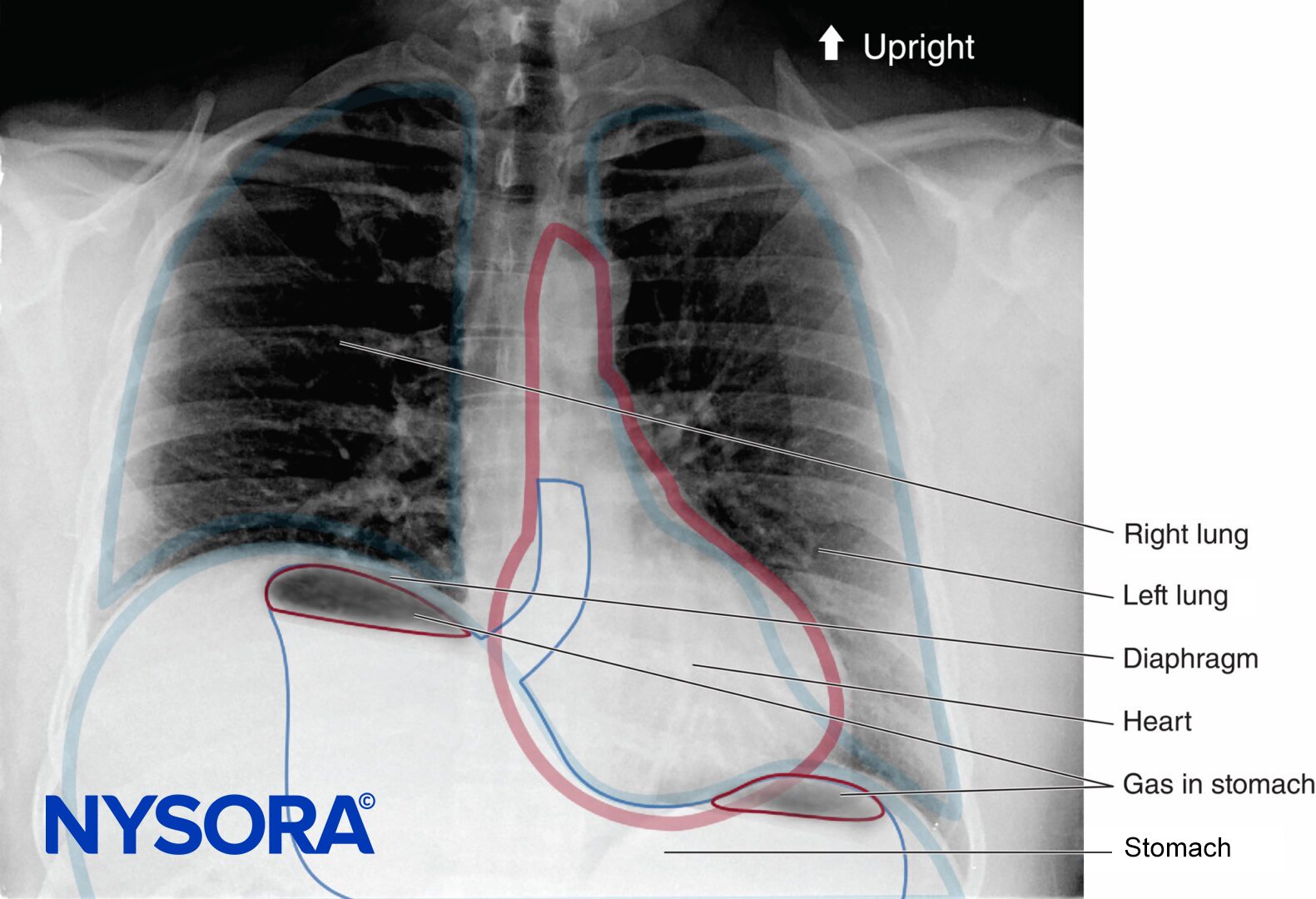
Note: If you are looking for information regarding Epidural Anesthesia and Analgesia for patients, click here.
Roulhac D. Toledano and Marc Van de Velde*
*The authors would like to thank Michael A. Maloney, MB, BAO, ChB, for his help with the tables and figures.
INTRODUCTION
Clinical indications for epidural anesthesia and analgesia have expanded significantly over the past several decades. Epidural analgesia is often used to supplement general anesthesia (GA) for surgical procedures in patients of all ages with moderate-to severe comorbid disease; provide analgesia in the intraoperative, postoperative, peripartum, and end-of-life settings; and can be used as the primary anesthetic for surgeries from the mediastinum to the lower extremities. In addition, epidural techniques are used increasingly for diagnostic procedures, acute pain therapy, and management of chronic pain. Epidural block may also reduce the surgical stress response, the risk of cancer recurrence, the incidence of perioperative thromboembolic events, and, possibly, the morbidity and mortality associated with major surgery.
This chapter covers the essentials of epidural anesthesia and analgesia. After a brief history of the transformation from single-shot to continuous epidural catheter techniques, it reviews (1) indications for and contraindications to epidural block; (2) basic anatomic considerations for epidural placement; (3) physiologic effects of epidural block; (4) pharmacology of drugs used for epidural anesthesia and analgesia; (5) techniques for successful epidural placement; and (6) major and minor complications associated with epidural block. This chapter also addresses several areas of controversy concerning epidural techniques. These include controversies about epidural space anatomy, the traditional epinephrine test dose, methods used to identify the epidural space, and whether particular clinical outcomes may be improved with epidural techniques when compared to GA. More detailed information about local anesthetics (LAs), the mechanism of neuraxial block, the combined spinal-epidural (CSE) technique, obstetric anesthesia, and complications of central neuraxial block is provided following the links.
BRIEF HISTORY
The neurologist J. Leonard Corning proposed injecting an anesthetic solution into the epidural space in the 1880s, but devoted his research primarily to subarachnoid nerve blocks. Despite coining the term spinal anesthesia, he may unknowingly have been investigating the epidural space. The French physicians Jean Sicard and Fernand Cathelin are credited with the first intentional administration of epidural anesthesia. At the turn of the 20th century, they independently introduced single-shot caudal nerve blocks with cocaine for neurologic and genitourinary procedures, respectively.
Nineteen years later, the Spanish surgeon Fidel Pagés Miravé described a single-shot thoracolumbar approach to “peridural” anesthesia, identifying the epidural space through subtle tactile distinctions in the ligaments. Within a decade and seemingly without the knowledge of Pagés’s work, the Italian surgeon Achille Dogliotti popularized a reproducible loss-of-resistance (LOR) technique to identify the epidural space. Contemporaneously, the Argentine surgeon Alberto Gutiérrez described the “sign of the drop” for identification of the epidural space.
A number of innovations by Eugene Aburel, Robert Hingson, Waldo Edwards, and James Southworth, among others, attempted to prolong the single-shot epidural technique. However, Cuban anesthesiologist Manual Martinez Curbelo is credited with adapting Edward Tuohy’s continuous subarachnoid technique for the epidural space in 1947. His efforts were facilitated by an extensive knowledge of anatomy, a first-hand experience observing Tuohy at the Mayo Clinic, and the availability of 16-gauge Tuohy needles and small, gradated 3.5-French ureteral catheters, which curved as they exited the tip of the needle. Several modifications of the Tuohy needle, itself a modification of the Huber needle, have since emerged.
The epidural catheter has also evolved from its origins as a modified ureteral catheter. Several manufacturers currently use nylon blends to produce thin, kink-resistant catheters of appropriate tensile strength and stiffness. The wire-reinforced catheter represents the most recent technological advance in epidural catheter design. The addition of a circumferential stainless steel coil within a nylon or polyurethane catheter confers greater flexibility compared to standard nylon catheters and may decrease the incidence of venous cannulation, intrathecal placement, catheter migration, and paresthesias.
INDICATIONS
This section presents common and controversial indications for the use of lumbar and thoracic epidural block in lower extremity, genitourinary, vascular, gynecologic, colorectal, and cardiothoracic surgery. It also reviews less common and novel indications for epidural anesthesia and analgesia, including for the treatment of patients with sepsis and uncommon medical disorders (Table 1).
TABLE 1. Examples of applications for epidural block.
| Specialty | Surgical Procedure |
|---|---|
| Orthopedic surgery | Major hip and knee surgery, pelvic fractures |
| Obstetric surgery | Cesarean delivery, labor analgesia |
| Gynecologic surgery | Hysterectomy, pelvic floor procedures |
| General surgery | Breast, hepatic, gastric, colonic surgery |
| Pediatric surgery | Inguinal hernia repair, orthopedic surgery |
| Ambulatory surgery | Foot, knee, hip, anorectal surgery |
| Cardiothoracic surgery | Thoracotomy, esophagectomy, thymectomy, coronary artery bypass grafting (on and off pump) |
| Urologic surgery | Prostatectomy, cystectomy, lithotripsy, nephrectomy |
| Vascular surgery | Amputation of lower extremity, revascularization procedures |
| Medical conditions | Autonomic hyperreflexia, myasthenia gravis, pheochromocytoma, known or suspected malignant hyperthermia |
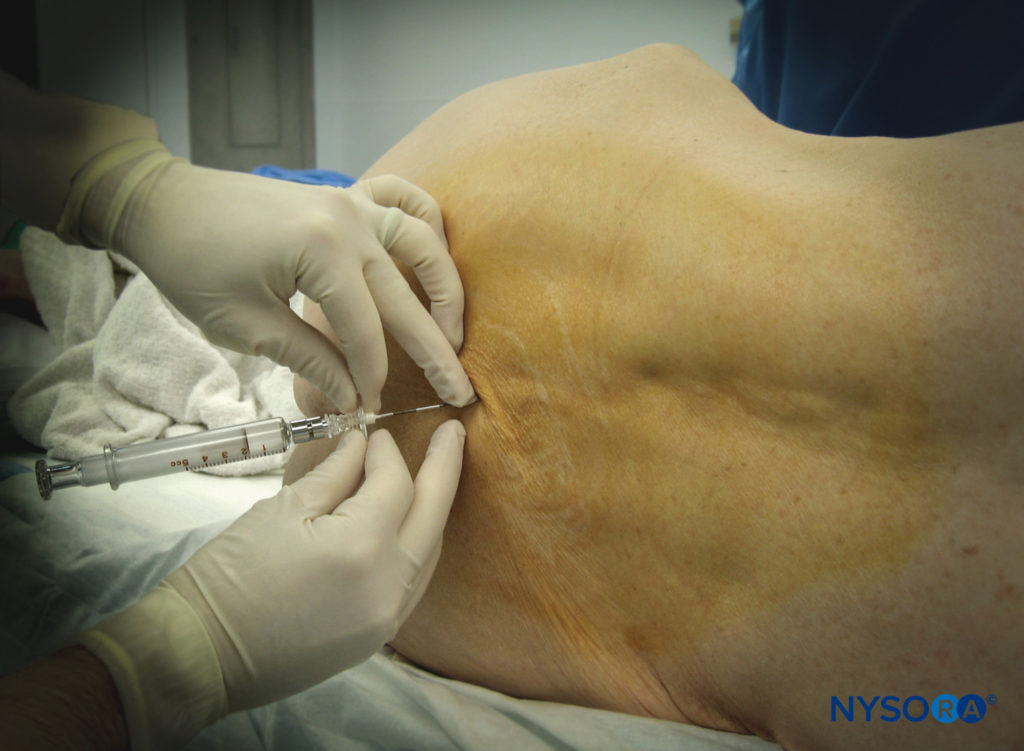
Lumbar Epidural block
Epidural anesthesia has been administered most commonly for procedures involving the lower limbs, pelvis, perineum, and lower abdomen but is increasingly being used as the sole anesthetic or as a complement to GA for a greater diversity of procedures. This section examines several common indications for lumbar epidural block, including lower extremity orthopedic surgery, infrainguinal vascular procedures, and genitourinary and vaginal gynecologic surgeries. When applicable, it reviews the benefits and drawbacks of the use of neuraxial techniques versus GA for specific procedures.
Lower Extremity Major Orthopedic Surgery
Both perioperative anticoagulant thromboprophylaxis and the increasing reliance on peripheral nerve blocks have influenced the current use of continuous lumbar epidural block for lower extremity surgery. Nonetheless, neuraxial block as a sole anesthetic or as a supplement to either GA or peripheral techniques is still widely used for major orthopedic surgeries of the lower extremities. The effective postoperative pain control provided by either peripheral or neuraxial nerve blocks, or a combination of the two techniques, improves patient satisfaction, permits early ambulation, accelerates functional recuperation, and may shorten hospital stay, particularly after major knee surgery. Other potential benefits of the use of neuraxial block in lieu of GA include the reduced incidence of deep vein thrombosis (DVT) in patients undergoing total hip and knee replacement surgery, improved postoperative cognitive function, and decreased intraoperative blood loss and transfusion requirements. A recent meta-analysis also demonstrated a statistically significant reduction in operative time when neuraxial block was used in patients undergoing elective total hip replacement, although the authors did not distinguish between spinal and epidural techniques.
Major orthopedic procedures that can be performed under epidural, CSE, or integrated epidural and GA include primary hip or knee arthroplasty, surgery for hip fracture, revision arthroplasty, bilateral total knee arthroplasty, acetabular bone grafting, and insertion of long-stem femoral prostheses (Table 2). Spinal anesthesia may be the preferred technique in some of these cases, particularly if anticipated postoperative pain is slight or negligible (eg, total hip arthroplasty) or if a supplemental peripheral nerve block is planned.
TABLE 2. Orthopedic surgeries suitable for epidural, combined spinal-epidural, or integrated epidural–general anesthesia.
| Procedure | Sensory Level Required |
|---|---|
| Closed reduction and external fixation of pelvis | Neuraxial technique seldom adequate for surgery; epidural useful for postoperative analgesia |
| Hip arthroplasty, arthrodesis, synovectomy | T10 |
| Open reduction internal fixation of acetabular fracture | T10 |
| Open reduction internal fixation of femur, tibia, ankle, or foot | T12 |
| Closed reduction and external fixation of femur and tibia | T12 |
| Above- and below-knee amputation | T12 (T8 with tourniquet) |
| Knee arthrotomy | T12 (T8 with tourniquet) |
| Arthroscopy of knee | T12 |
| Repair/reconstruction of knee ligaments | T12 |
| Total knee replacement | T12 (T8 with tourniquet) |
| Distal tibia, ankle, and foot procedures | T12 |
| Ankle arthroscopy, arthrotomy, arthrodesis | T12 |
| Transmetatarsal amputation | T12 |
Anesthesia to T10 with needle placement at L3 to L4 is adequate for most of these procedures.
The use of neuraxial anesthesia for major orthopedic surgery is not without risks and challenges. Elderly patients, trauma victims, and individuals with hemophilia who develop complications from recurrent bleeding into their joints may not be appropriate candidates for regional block. In general, epidural procedures are well tolerated in patients with age-related comorbidities, such as restrictive pulmonary disease, prolonged hepatic clearance of drugs, hypertension (HTN), coronary artery disease (CAD), and renal insufficiency. Elderly patients may benefit from the decreased postoperative confusion and delirium associated with regional anesthesia, provided intraoperative hypotension is kept to a minimum. However, prevention of excessive sympathectomy-induced hemodynamic changes can be challenging, as these patients are both less capable of responding to hypotension and more prone to cardiac decompensation and pulmonary edema in response to rapid fluid administration. An epidural technique with a sensory level below T10, as appropriate for many orthopedic surgeries, and judicious administration of fluids and vasopressors may minimize these risks.
Elderly patients commonly present for surgery on anticoagulant or antiplatelet medications and may pose a risk for neurologic injury related to central neuraxial block. If an epidural technique is selected for these or other high-risk patients, appropriate timing of both block initiation and catheter removal relative to the timing of anticoagulant drug administration must be taken into account. For trauma patients, attaining proper positioning for administration of epidural anesthesia may present a challenge. Initiation of neuraxial block in the lateral position may improve chances of success. Intraoperatively, tourniquet pain can be anticipated with either spinal or epidural block, but occurs more frequently with the latter. While the mechanism remains poorly understood, it commonly presents within an hour of tourniquet inflation, increases in intensity over time, and is accompanied by tachycardia and elevated blood pressure. The administration of intrathecal or epidural preservative-free morphine may delay the onset of tourniquet pain.
Lower Limb Vascular Surgery
There are several potential benefits of the use of neuraxial anesthesia and analgesia for lower extremity vascular procedures.
Patients undergoing vascular surgery commonly have multiple major systemic diseases, such as CAD, cerebrovascular disease (CVD), diabetes mellitus (DM), chronic renal insufficiency, chronic HTN, and chronic obstructive pulmonary disease (COPD). Patients who present for arterial embolectomy may also have conditions that predispose them to intracardiac thrombus formation, such as mitral stenosis or atrial fibrillation. Avoiding GA in this high-risk patient population possibly enhances graft patency, reducing the need for reexploration and reducing the risk of thromboembolic complications; these are some of the advantages of using regional anesthesia. However, management of these individuals is often complicated by the high probability that they are taking presurgical antiplatelet or anticoagulant medications and will require additional systemic anticoagulation intraoperatively and postoperatively. Thus, these patients are considered at an increased risk for epidural hematoma; a careful risk-benefit analysis is necessary prior to initiate epidural block.
Consideration must also be given to the type of vascular procedure to be performed, the anticipated length of the procedure, the possible need for invasive monitoring, and the timely removal of the epidural catheter before transitioni ng to oral anticoagulation therapy. Maintaining normothermia, ensuring that motor strength can be promptly assessed postoperatively, and providing appropriate sedation during lengthy procedures are additional challenges.
Infrainguinal vascular procedures that are suitable for epidural block include arterial bypass surgeries, arterial embolectomy, and venous thrombectomy or vein excision (Table 3).
TABLE 3. Examples of vascular procedures performed with epidural block.
| Abdominal aortic aneurysm repair (neuraxial technique seldom adequate as sole anesthetic) |
| Aortofemoral bypass |
| Renal artery bypass |
| Mesenteric artery bypass |
| Infrainguinal arterial bypass with saphenous vein or synthetic graft |
| Embolectomy |
| Thrombectomy |
| Endovascular procedures (intraluminal balloon dilation with stent placement; aneurysm repair) |
Slow titration of LAs to attain a T8–T10 level, while maintaining hemodynamic stability, is optimal. The addition of epinephrine to LAs is controversial due to concerns that its vasoconstrictive effect may jeopardize an already-tenuous blood supply to the spinal cord. Studies to date have failed to demonstrate a difference in cardiovascular and pulmonary morbidity and mortality with the use of epidural anesthesia as compared with GA for these procedures, although epidural techniques may be superior for promoting graft survival.
Lower Genitourinary Procedures
Lumbar epidural block as either a primary anesthetic or as an adjunct to GA is an appropriate option for a variety of genitourinary procedures. Epidural anesthesia with a T9–T10 sensory level can be used for transurethral resection of the prostate (TURP), although spinal anesthesia may be preferred due to its improved sacral coverage, denser sensory block, and shorter duration. Both techniques are considered superior to GA for several reasons, including earlier detection of mental status changes associated with TURP syndrome; the ability of the patient to communicate breakthrough pain if an untoward complication such as perforation of the prostatic capsule or bladder occurs; the potential for decreased bleeding; and the decreased risks of perioperative thromboembolic events and fluid overload (Table 4). In addition, patients presenting for this and other prostate surgeries are generally elderly, with multiple comorbidities, and have a low risk for certain complications of neuraxial block, such as postdural puncture headache (PDPH).
TABLE 4. Benefits of central neuraxial block versus general anesthesia for transurethral resection of the prostate.
| Early detection of mental status changes |
| Early detection of breakthrough pain (indicative of capsular/bladder perforation) |
| Reduced blood loss |
| Decreased incidence of deep vein thrombosis |
| Decreased incidence of circulatory overload |
| Improved postoperative pain control |
Other transurethral procedures, such as cystoscopy and ureteral stone extraction, can be performed under GA, topical anesthesia, or neuraxial block, depending on the extent and complexity of the procedure, patient comorbidities, and patient, anesthesiologist, and surgeon preference. Of note, paraplegic and quadriplegic patients comprise a subset of patients who present for repeated cystoscopies and stone extraction procedures; neuraxial anesthesia is often preferred in these patients because of the risk of autonomic hyperreflexia (AH) (see separate section on this topic).
Because these procedures are done on an outpatient basis, lengthy residual epidural block should be avoided. Although there is some interindividual variability, a sensory level as high as T8 is required for procedures involving the ureters, while a T9–T10 sensory level is appropriate for procedures involving the bladder (Table 5).
TABLE 5. Sensory level required for genitourinary procedures.
| Procedure | Sensory Level Required |
|---|---|
| Nephrectomy | Consider combined general-epidural anesthesia |
| Cystectomy | T4 |
| Extracorporeal shock wave lithotripsy | T6 |
| Open prostatectomy | T8 |
| Ureteral stone extraction | T8 |
| Cystoscopy | T9 |
| Transurethral resection of prostate | T9 |
| Surgery involving testes | T10 |
| Surgery involving penis | L1 |
| Urethral procedures | Sacral block |
Vaginal Gynecologic Surgeries
Several vaginal gyneacologic surgeries can be performed with epidural block, although single-shot spinal or GA and, in some cases, paracervical nerve block or topical anesthesia may be more appropriate (Table 6). A dilation and curettage (D&C) can be performed under paracervical nerve block, GA, or neuraxial block.
TABLE 6. Vaginal gynecologic procedures suitable for epidural block.
| Dilation and curettage |
| Hysteroscopy (with or without distention media) |
| Urinary incontinence procedures |
| Hysterectomy |
If neuraxial anesthesia is selected, a T10 sensory level is appropriate. While outpatient diagnostic hysteroscopy can be performed under LA, hysteroscopy with distention media typically requires general or neuraxial anesthesia.
Epidural anesthesia may have the disadvantage of increased glycine absorption compared to GA. However, mental status changes related to absorption of the hypotonic irrigation solution are more easily detected in awake patients. For urinary incontinence procedures, epidural anesthesia offers the advantage of permitting the patient to participate in the intraoperative cough test, which theoretically decreases the risk of postoperative voiding dysfunction, although the incidence of this untoward outcome does not appear to be increased under GA. A T10 sensory level provides sufficient anesthesia for bladder procedures, but the level should be extended to T4 if the peritoneum is opened. Vaginal hysterectomy can be performed under general or neuraxial (most commonly spinal) anesthesia. A T4–T6 sensory level is appropriate for uterine procedures.
Thoracic Epidural Anesthesia and Analgesia
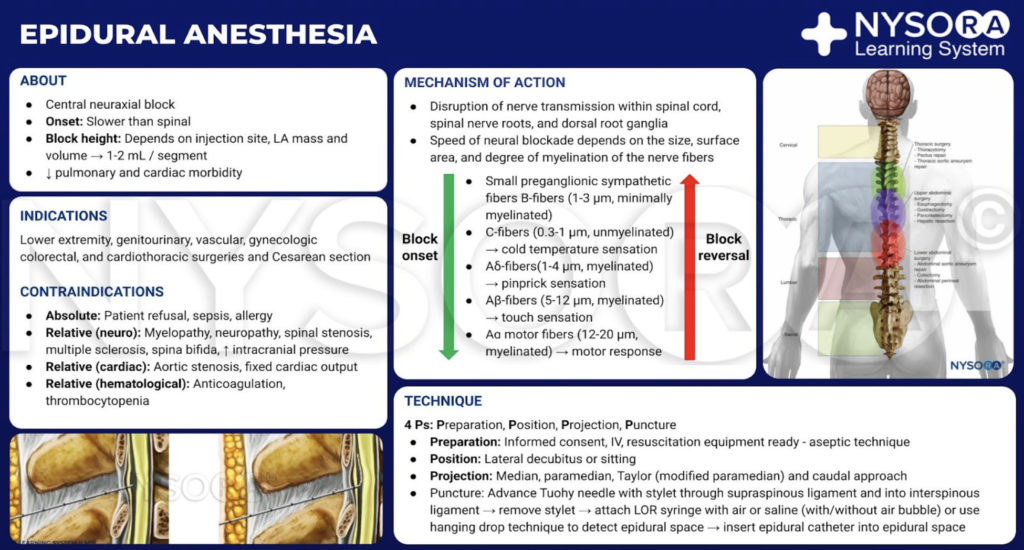
The benefits of and indications for thoracic epidural anesthesia (TEA) are expanding (Table 7). TEA offers superior perioperative analgesia compared with systemic opioids, decreases postoperative pulmonary complications, decreases the duration of postoperative ileus, and decreases mortality in patients with multiple rib fractures, among other things. This section explores the role of TEA as either a primary anesthetic or as an adjuvant to GA for cardiac, thoracic, abdominal, colorectal, genitourinary, and gynecologic surgery (Figure 1). It also reviews the expanding role of TEA for video-assisted thoracic surgery (VATS) and laparoscopic surgery.
TABLE 7. Benefits of thoracic epidural anesthesia and analgesia.
| Improved perioperative analgesia compared with other modalities |
| Decreased postoperative pulmonary complications |
| Decreased duration of postoperative ileus |
| Decreased duration of mechanical ventilation |
| Decreased mortality in patients with rib fractures |
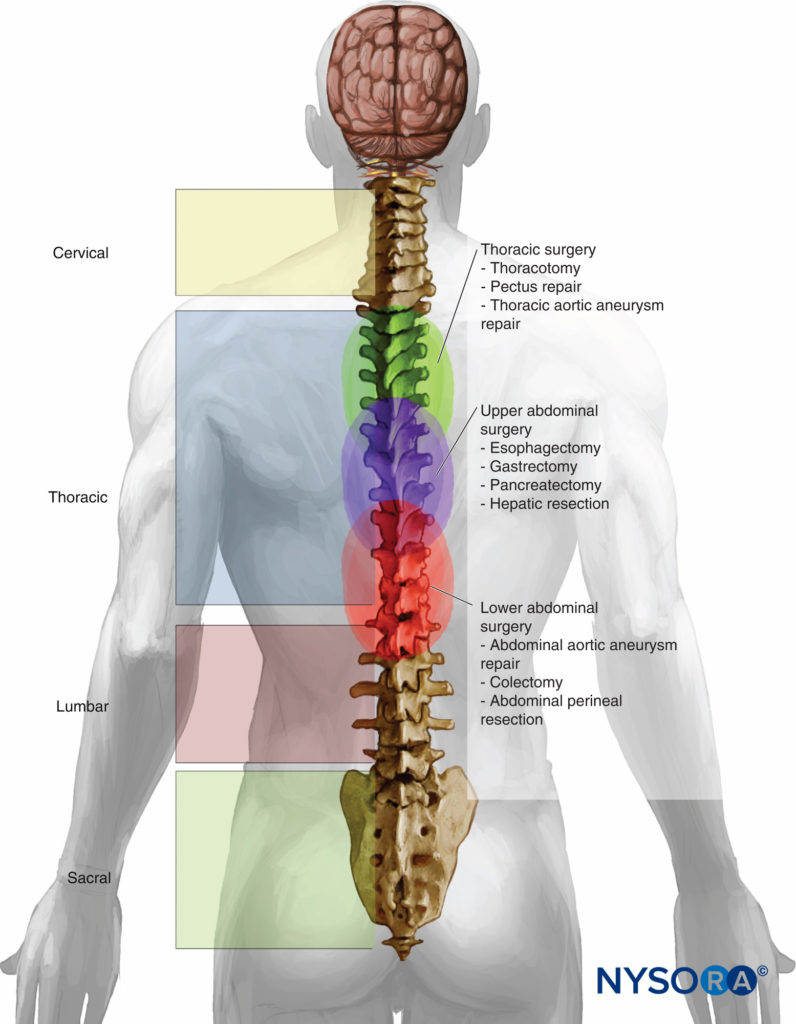
Figure 1. Level of placement in surgeries performed with thoracic epidural anesthesia and analgesia.
Cardiac Surgery
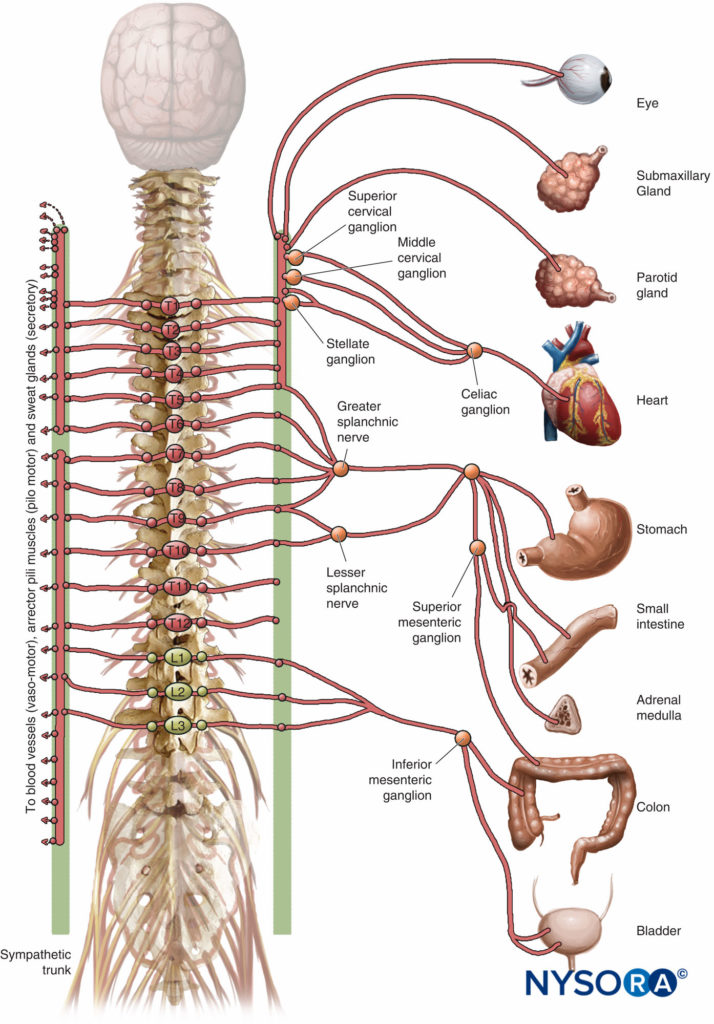
High TEA (block of the upper five thoracic segments) as an adjuvant to GA in cardiac surgery with cardiopulmonary bypass (CPB) has gained interest over the past several decades. Purported benefits include improved distribution of coronary blood flow, reduced oxygen demand, improved regional left ventricular function, a reduction in the incidence of supraventricular arrhythmias, attenuation of the surgical stress response, improved intraoperative hemodynamic stability, faster recovery of awareness, improved postoperative analgesia, and a reduction of postoperative renal and pulmonary complications.
Several of these potential benefits can be attributed to selective block of cardiac sympathetic innervation (the T1–T4 spinal segments). However, the insertion of an epidural catheter in patients requiring full heparinization for CPB carries the risk of epidural hematoma.
The evidence in support of high TEA for cardiac surgery is not conclusive. A study by Liu and colleagues comparing TEA with traditional opioid-based GA for coronary artery bypass grafting (CABG) with CPB found no difference in the rates of mortality or myocardial infarction, but demonstrated a statistically significant reduction in the risk of postoperative cardiac arrhythmias and pulmonary complications, improved pain scores, and earlier tracheal extubation in the TEA group. In contrast, a recent randomized control trial comparing the clinical effects of fast-track GA with TEA versus fast-track GA alone in over 600 patients undergoing elective cardiac surgery (both on pump and off pump) found no statistically significant difference in 30-day survival free from myocardial infarction, pulmonary complications, renal failure, or stroke. The duration of mechanical ventilation, length of intensive care unit (ICU) stay, length of hospital stay, and quality of life at 30-day follow-up were also similar for the two groups. Overall, the role of TEA as an adjuvant to GA for cardiac surgery with CPB remains controversial.
The role of high TEA in off-pump coronary artery bypass (OPCAB) surgery is also debated in the literature. TEA offers the advantages of avoiding intubation of the trachea in selected CABG cases, earlier extubation in patients receiving GA, and reduced postoperative pain and morbidity. But, concerns remain about compromised ventilation with a high sensory block, hypotension due to sympathicolysis, and epidural hematoma, despite the vastly reduced heparin dose compared with CPB cases. A recent prospective, randomized controlled trial of more than 200 patients undergoing OPCAB surgery found that the addition of high TEA to GA significantly reduced the incidence of postoperative arrhythmias, improved pain control, and improved the quality of recovery. Until more definitive outcome data are available, the role of neuraxial techniques in OPCAB surgery remains uncertain.
Thoracic and Upper Abdominal Surgical Procedures
Epidural anesthesia and analgesia are commonly used for upper abdominal and thoracic surgery, including gastrectomy, esophagectomy, lobectomy, and descending thoracic aorta procedures (Table 8).
TABLE 8. Indications for thoracic epidural anesthesia and analgesia.
| Anatomic Region | Procedure |
|---|---|
| Thorax | Thoracotomy |
| Pectus repair |
|
| Thoracic aneurysm repair |
|
| Thymectomy |
|
| Video-assisted thoracic surgery | |
| Upper abdomen | Esophagectomy |
| Gastrectomy |
|
| Pancreatectomy |
|
| Cholecystecomy |
|
| Hepatic resection | |
| Lower abdomen | Abdominal aortic aneurysm repair |
| Colectomy |
|
| Bowel resection |
|
| Abdominal perineal resection | |
| Urogenital/ gynecologic | Cystectomy |
| Nephrectomy |
|
| Ureteral repair |
|
| Radical abdominal prostatectomy |
|
| Ovarian tumor debulking |
|
| Pelvic exenteration |
|
| Total abdominal hysterectomy |
It is less commonly used for VATS, unless conversion to an open procedure is highly anticipated or if the patient is at high risk for complications from GA. Epidural block for many of these procedures commonly serves as an adjuvant to GA and as an essential component of postoperative pain management. Concurrent administration of high TEA with GA, however, carries risks of intraoperative bradycardia, hypotension, and changes in airway resistance. There is some debate regarding whether intraoperative activation of epidural block is required to appreciate the analgesic benefits of TEA or if postoperative activation produces equivalent benefits. A systematic review by Møiniche and colleagues found that the timing of several types of analgesia, including epidurals, intravenous opioids, and peripheral LAs, did not influence the quality of postoperative pain control.
Thoracic epidural anesthesia initiated at the mid- to upper thoracic region can also be used for breast procedures. Benefits may include superior postoperative analgesia, decreased incidence of postoperative nausea and vomiting (PONV), improved patient satisfaction, and avoiding tracheal intubation in patients with moderate-to-severe comorbidities. The sensory level required depends on the procedure: A level extending from T1–T7 is adequate for breast augmentation; C5–T7 is required for modified radical mastectomy; and C5–L1 is required for mastectomy with transverse rectus abdominis myocutaneous (TRAM) flap reconstruction (Table 9). The epidural catheter can be introduced at T2–T4 to achieve segmental block of the thoracic dermatomes for most breast procedures; placement at T8–T10 is appropriate for TRAM flap reconstruction.
TABLE 9. Sensory level required for breast procedures.
| Surgery | Segmental block |
|---|---|
| Modified radical mastectomy | C5–T7 |
| Mastectomy with transverse rectus abdominus flap | C5–L1 |
| Partial mastectomy; breast augmentation | T1–T7 |
Epidural block provides a useful adjuvant to GA for procedures within the thoracic cavity, such as lung and esophageal surgery. The benefits of TEA for these procedures include enhanced postoperative analgesia; reduced pulmonary morbidity (eg, atelectasis, pneumonia, and hypoxemia); swift resolution of postoperative ileus; and decreased postoperative catabolism, which may spare muscle mass. Segmental epidural block of T1–T10 provides sensory block of the thoracotomy incision and the chest tube insertion site.
Upper abdominal surgeries that can be performed with epidural anesthesia and analgesia include esophagectomy, gastrectomy, pancreatectomy, hepatic resection, and cholecystectomy. Laparoscopic cholecystectomy with epidural block30 and distal gastrectomy with a combined general-epidural anesthetic have also been reported. Midthoracic epidural catheter placement with segmental block extending from T5 (T4 for laparoscopic surgery) to T8 is appropriate for most upper abdominal procedures and, due to lumbar and sacral nerve root sparing, has minimal risk of lower extremity motor deficits, urinary retention, hypotension, and other sequelae of lumbar epidural anesthesia.
Suprainguinal Vascular Procedures
An upper midthoracic epidural can be used as an adjuvant to GA for surgeries of the abdominal aorta and its major branches. Epidural block for aortofemoral bypass, renal artery bypass, and repair of abdominal aortic aneurysms may provide superior postoperative pain control, facilitate early extubation of the trachea, permit early ambulation, and decrease the risk of thromboembolic events in patients who are at particularly high risk for this untoward complication. However, intraoperative epidural block may complicate management of hemodynamic changes associated with aortic cross-clamping and unclamping, as well as compromise early assessment of motor function in the immediate postoperative period. A sensory level from T6 to T12 is necessary for an extensive abdominal incision; a level extending from T4–T12 is required to attain denervation of the viscera.
Extracorporeal Shock Wave Lithotripsy,Prostatectomy, Cystectomy, Nephrectomy
Extracorporeal shock wave lithotripsy (ESWL) with or without water immersion can be performed under general or neuraxial anesthesia. A T6–T12 sensory level is necessary when neuraxial techniques are selected. Epidural block is associated with less intraoperative hypotension than a single-shot spinal, although both techniques serve to avoid GA in potentially high-risk patients.
Open prostate surgery, radical cystectomy and urinary diversion, and simple, partial, and radical nephrectomy can be performed under neuraxial block, either alone or in combination with GA, depending on the procedure. Some potential advantages of neuraxial compared with GA for radical retropubic prostatectomy include decreased intraoperative blood loss and transfusions, a decreased incidence of postoperative thromboembolic events, improved analgesia and level of activity up to 9 weeks postoperatively, faster return of bowel function, and several other still-disputed advantages of neuraxial anesthesia, such as faster time to hospital discharge and reduced hospital costs. For the open procedure, patients may require generous sedation in the absence of a combined general-neuraxial technique. A T6 sensory level is required, with catheter placement in the midthoracic region. Radical cystectomy is performed on patients with invasive bladder cancer and may have improved outcomes with a combined general-epidural anesthetic compared to GA alone.
Epidural block can provide controlled hypotension intraoperatively, contributing to decreased blood loss, and optimize postoperative pain relief. A midthoracic epidural with a T6 sensory level is appropriate. Although GA is often required for radical nephrectomy due to concerns for patient positioning, intraoperative hypotension, and the potential for significant intraoperative blood loss, epidural analgesia provides more effective postoperative pain relief than systemic opioids while avoiding the adverse effects of the latter.
Several other urologic-related surgeries can be performed with neuraxial block as the sole anesthetic or as an adjuvant to GA. The use of a combined GA-epidural technique in patients with functional adrenal tumors undergoing laparoscopic adrenalectomy is safe and effective and may have the added benefit of minimizing fluctuations in hormone levels. Of note, however, epidural block may not diminish the pressor effects of direct tumor stimulation. The use of epidural anesthesia for retroperitoneal laparoscopic biopsy for patients who are not candidates for percutaneous biopsy has also been reported.
Lower Abdominal and Gyneacologic Surgeries
Total abdominal hysterectomy is often performed under GA, a combined general-epidural anesthetic, or neuraxial anesthesia with or without sedation. Although still not routine, gynecologic laparoscopy is increasingly being performed under neuraxial anesthesia, commonly with decreased Trendelenburg tilt, reduced CO2 insufflation pressures (below 15 mm Hg), and supplemental opioids or nonsteroidal anti-inflammatory drugs (NSAIDs) to minimize referred shoulder pain. Epidural block for open procedures has the advantages of providing prolonged postoperative analgesia, decreasing the incidence of PONV and perioperative thromboembolic events, and potentially influencing perioperative immune function and, relatedly, the recurrence of cancer in patients undergoing hysterectomy for ovarian or related cancer. The proposed preemptive analgesia effect provided by neuraxial block during abdominal hysterectomy requires further investigation. A sensory level extending to T4 or T6 provides sufficient anesthesia for procedures involving the uterus. Either epidural catheter insertion in the lumbar region with high volumes of LAs to raise the sensory level or low- to midthoracic placement is appropriate. The visceral pain associated with bowel and peritoneal manipulation decreases as the level of the block is increased; a T3–T4 level may be optimal.
Open and laparoscopic colectomy, sigmoidectomy, and appendectomy are among other lower abdominal surgeries that can be performed under neuraxial anesthesia, with or without GA. Of particular interest in patients undergoing bowel surgery thoracic epidural block decreases the duration of postoperative ileus, possibly without affecting anastomotic healing and leakage. The superior postoperative analgesia associated with continuous epidural infusions, with or without opioids, most likely improves postoperative lung function in patients undergoing gastrointestinal (GI) surgery, although specific randomized controlled trials have not been conducted. In combination with early feeding and ambulation, TEA plays a role in early hospital discharge after certain GI surgeries. A similar outcome has been demonstrated after laparoscopic colonic resection, followed by epidural analgesia for 2 days and early oral nutrition and mobilization (ie, multimodal rehabilitation). Epidural catheter placement between T9 and T11 is usually appropriate for lower abdominal procedures; a sensory block extending to T7 or T9 is required for most colonic surgeries (sigmoid resection, ileotransversostomy, hemicolectomy).
Uncommon Medical Disorders and Clinical Scenarios
Epidural anesthesia and analgesia may also be indicated in the perioperative management of patients with specific medical conditions or coexisting disease, such as myasthenia gravis (MG), AH, malignant hyperthermia (MH), COPD, pheochromocytoma (see previous discussion), and sepsis. Several other subsets of patients may benefit from continuous epidural catheter techniques, including palliative care patients, parturients with comorbidities, and patients at risk for recurrent malignancy.
Myasthenia Gravis
Patients with MG pose particular challenges to anesthesiologists, including abnormal responses to depolarizing and nondepolarizing neuromuscular blocking agents; potential difficulty reversing residual neuromuscular block in patients taking cholinesterase inhibitors; prolonged postoperative mechanical ventilation requirements; risk of postsurgical respiratory failure; and postoperative pain management concerns. Epidural block eliminates the need for intraoperative muscle relaxants in myasthenic patients and provides superior postoperative pain relief compared with opioids, while minimizing the risk of opioid-induced respiratory depression and pulmonary dysfunction. Due to the possibility that ester LA metabolism may be prolonged in patients taking cholinesterase inhibitors, amide LAs may be preferred for the management of myasthenic patients. Reduced doses of LAs may also be appropriate. Concerns for compromising a myasthenic patient’s respiratory function with a high epidural appear to be unfounded.
Autonomic Hyperreflexia
Epidural techniques are appropriate for the perioperative management of patients with AH. AH occurs in up to 85% of patients with spinal cord injuries at or above T4–T7 as a result of uninhibited sympathetic activity. In response to visceral or cutaneous stimulation below the level of the lesion and in the absence of descending central inhibition, patients may develop acute, extreme sympathetic hyperactivity. Generally, intense vasoconstriction occurs below the level of the spinal cord lesion, with vasodilation above. Patients may experience sweating, nausea, flushing, pallor, shivering, nasal obstruction, blurred vision, headache, difficulty breathing, seizures, and cardiac arrhythmias. Reflex bradycardia is seen in the majority of cases. Severe life-threatening HTN can result in intracranial hemorrhage, myocardial ischemia, pulmonary edema, and death. Epidural block as the sole anesthetic, as a supplement to GA, or for labor analgesia attenuates the physiologic perturbations associated with AH, although incomplete nerve block of sacral segments or missed segments may contribute to a high failure rate. Spinal anesthesia, which nerve blocks the afferent limb of this potentially lethal reflex, and deep GA more reliably prevent AH.
Malignant Hyperthermia
The anesthetic management of MH presents a challenge to the anesthesiologist. MH is a clinical syndrome of markedly accelerated metabolism triggered primarily by volatile agents and the depolarizing agent succinylcholine. Susceptible patients may develop fever, tachycardia, hypercarbia, tachypnea, arrhythmias,hypoxemia, profuse sweating, HTN, myoglobinuria, mixed acidosis, and muscle rigidity in response to exposure to volatile agents or succinylcholine, although cases have been reported in which there is no evident triggering agent. Late complications may include consumptive coagulopathy, acute renal failure, muscle necrosis, pulmonary edema, and neurologic sequelae. Avoiding exposure to triggering agents is a cornerstone in the management of MH-susceptible patients.
Whenever suitable, local, peripheral, or central neuraxial nerve blocks are recommended, as these techniques are reported to be safer than the use of GA. Both ester and amide LAs are considered safe in MH-susceptible patients, as is epinephrine, although controversy remains in the literature.
Chronic Obstructive Pulmonary Disease
Epidural block is a reasonable anesthetic option for patients with COPD undergoing major surgery due to concerns for prolonged mechanical ventilation. However, whether epidural techniques reduce pulmonary complications in patients with COPD is not known. In a recent propensity-controlled analysis of more than 500 patients with COPD undergoing abdominal surgery, epidural analgesia as an adjuvant to GA was associated with a statistically significant reduction in the risk of postoperative pneumonia. Patients with the most severe type of COPD benefited disproportionately. The study also found a nonsignificant beneficial effect of epidural analgesia on 30-day mortality, a trend that has been demonstrated in other studies.
Pediatric Surgery
There is a considerable body of literature dedicated to the use of regional anesthesia for pediatric surgery in both the inpatient and the ambulatory settings. Advantages of neuraxial block for the pediatric population include optimal postoperative analgesia, which is particularly important in extensive scoliosis repair, repair of pectus excavatum, and major abdominal and thoracic procedures; decreased GA requirements; earlier awakening; and earlier discharge in the ambulatory setting. Certain subsets of pediatric patients, such as those with cystic fibrosis, a family history of MH, or a history of prematurity, also benefit from the use of neuraxial anesthesia in lieu of GA. However, parental refusal, concerns about performing regional nerve blocks in anesthetized patients, and airway concerns in patients with limited oxygen reserves pose challenges to the routine use of neuraxial block in this patient population.
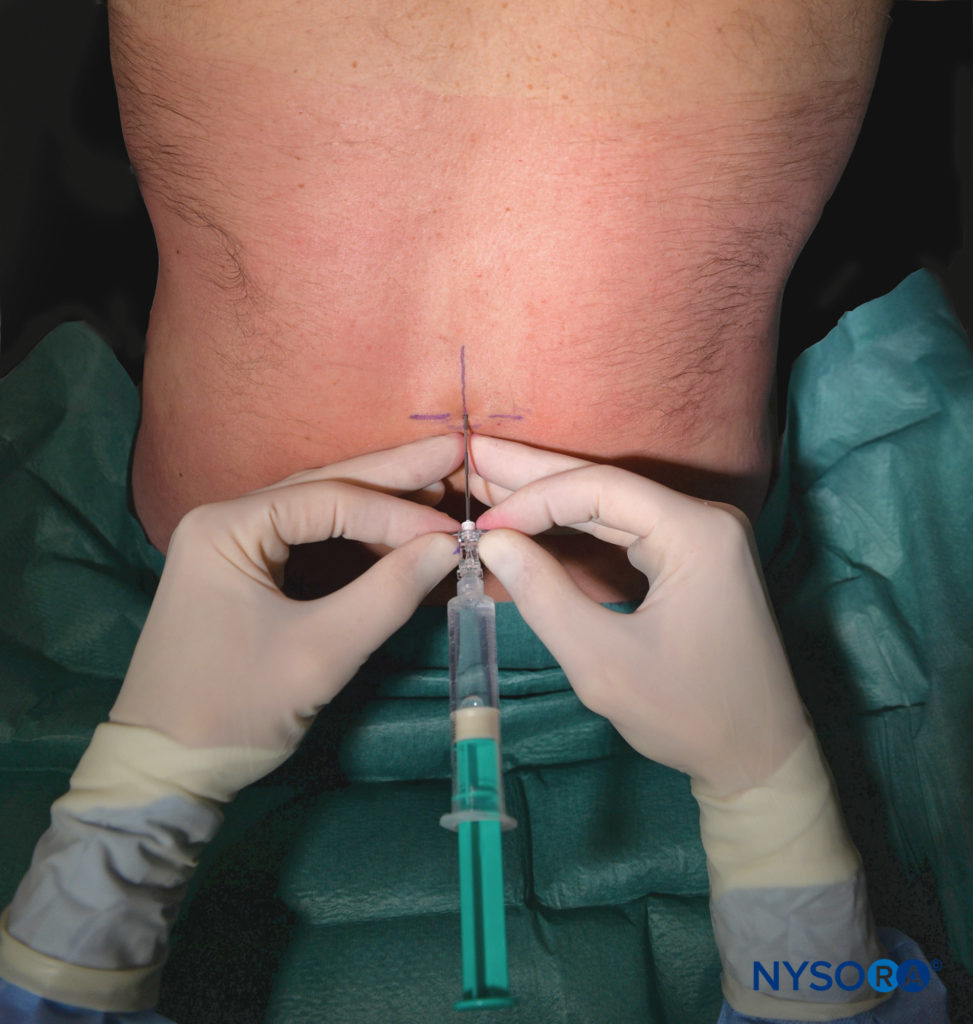
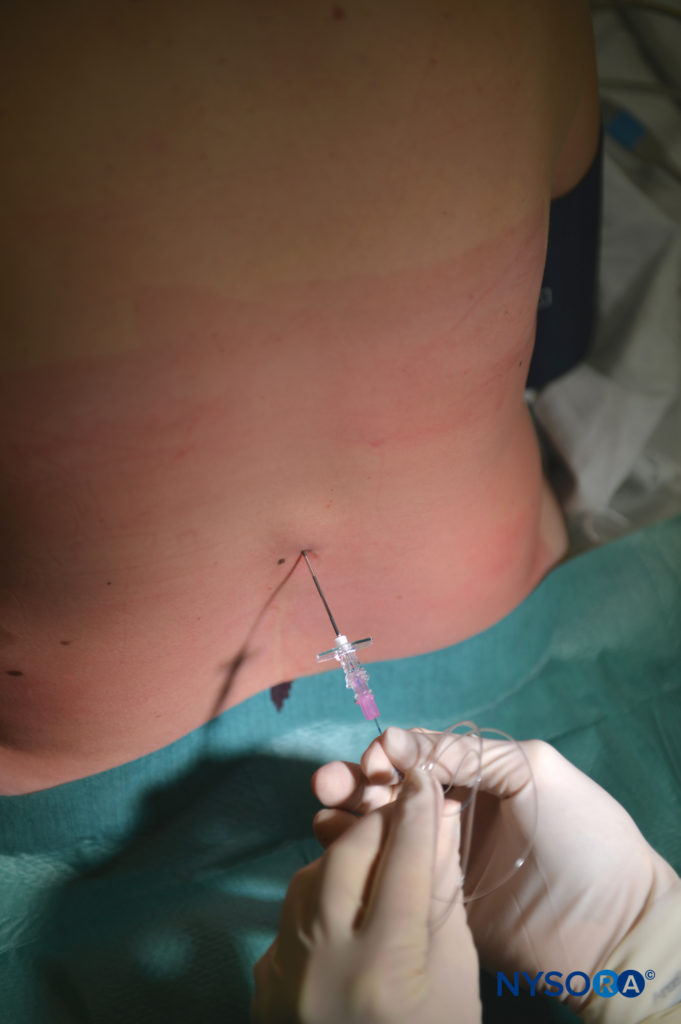
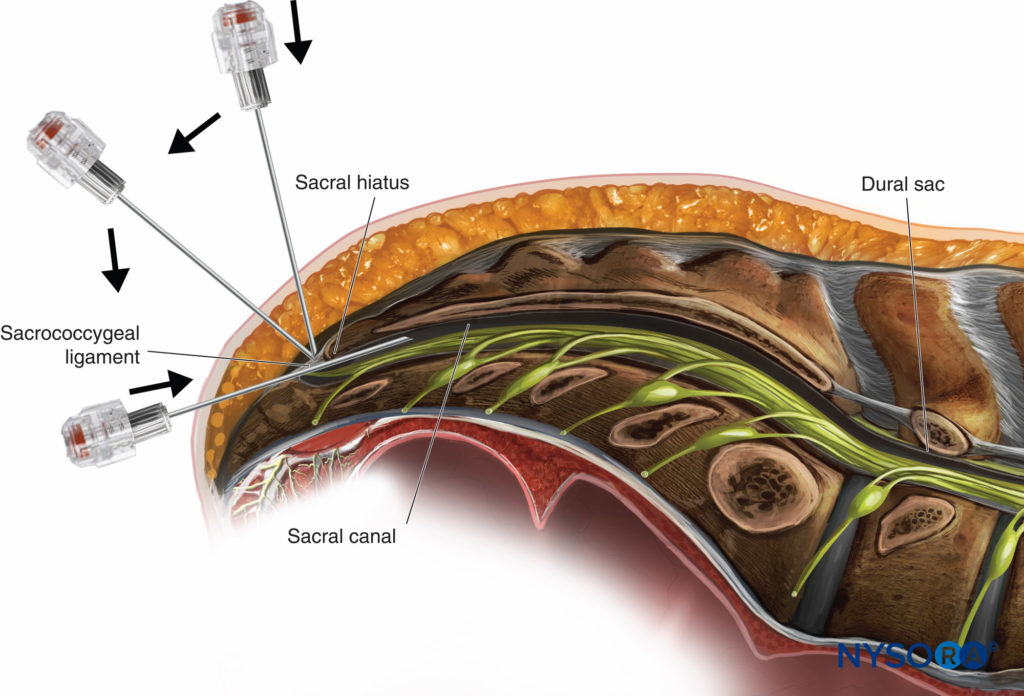
The single-shot caudal approach to the epidural space, with or without sedation, is commonly used in pediatric patients for a variety of surgeries, including circumcision, hypospadias repair, inguinal herniorrhaphy, and orchidopexy.
Continuous caudal catheters may be advanced cephalad to higher vertebral levels and used as the sole anesthetic or as an adjuvant to GA. Lumbar anesthesia and TEA provide a more reliable sensory block at higher segmental levels in older children. See “Regional Anesthesia in Pediatric Patients: General Considerations” for a more detailed discussion of caudal nerve blocks and Caudal Anesthesia.
Ambulatory Surgery
Spinal anesthesia or peripheral nerve blocks are preferred over epidural techniques for most clinical scenarios in the ambulatory setting due to concerns for the relatively slow onset of epidural block, urinary retention, prolonged immobility, PDPH, and delayed discharge. The use of short-acting LAs, when appropriate, may obviate these concerns. Epidural techniques have the advantages of permitting slow titration of LAs, the ability to tailor nerve block height and duration to the surgical procedure, and a decreased risk of transient neurologic symptoms (TNS) when compared with spinal anesthesia. Total hip arthroplasty, knee arthroscopy, foot surgery, inguinal herniorrhaphy, pelvic laparoscopy, and anorectal procedures are among the many outpatient surgeries that can be performed with neuraxial block as the primary anesthetic. For information about regional block in the ambulatory setting refer to: Peripheral Nerve Blocks for Outpatient Surgery.
Labor Analgesia and Anesthesia
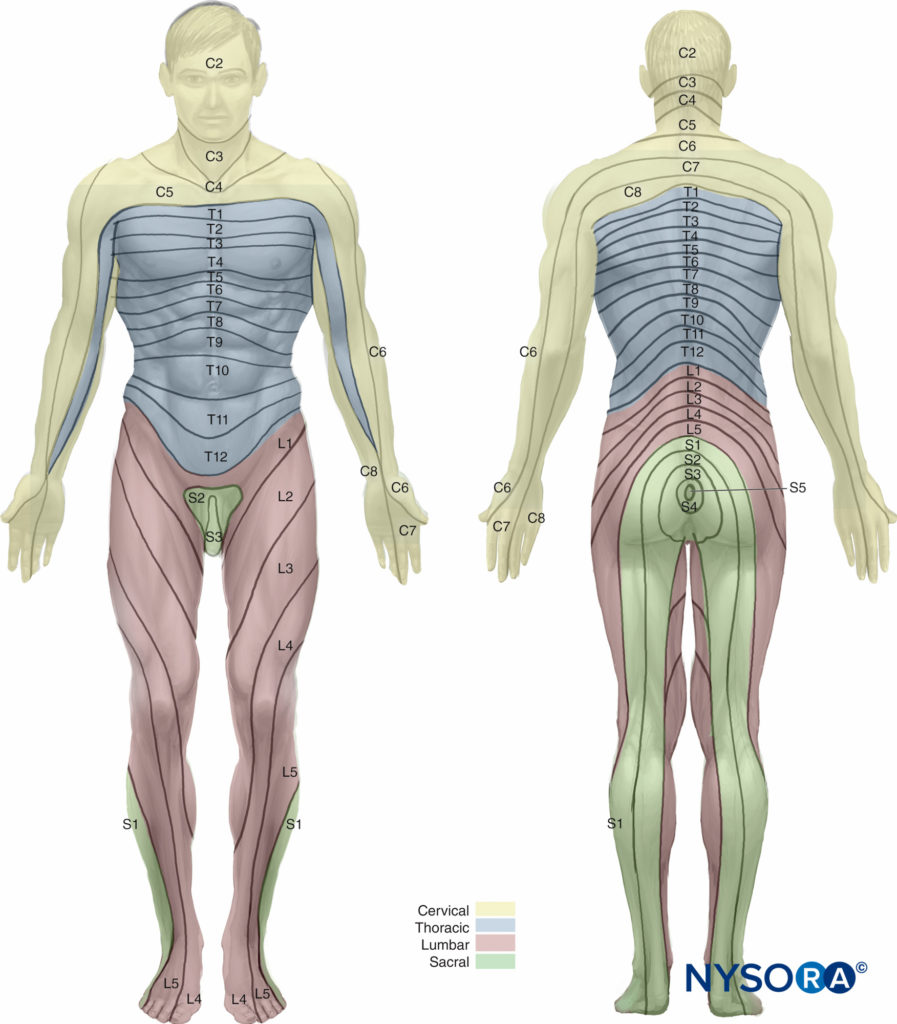
Parturients comprise the single largest group to receive epidural analgesia. For adequate pain relief during the first stage of labor, coverage of the dermatomes from T10 to L1 is necessary; analgesia should extend caudally to S2–S4 (to include the pudendal nerve) during the second stage of labor. Epidural placement at the L3–L4 interspace is most common in laboring patients.
However, surface anatomic landmarks may be difficult to appreciate in obstetric patients and may not reliably identify the intended interspace in this subset of patients due to both the anterior rotation of the pelvis and exaggerated lumbar lordosis. Several other factors may affect the ease of epidural placement and spread of epidurally administered LAs in parturients, including engorgement of epidural veins, elevated hormonal levels, and excessive weight gain. Refer to “Obstetric Regional Anesthesia” for additional information on epidural techniques in laboring patients.
Miscellaneous
Several nonanesthetic applications for epidural procedures have emerged. Epidural catheter infusion techniques are being used increasingly for pain control at the end of life in both children and adults, including those with cancer-related pain. There is also an evolving interest in whether epidural anesthesia and analgesia may have a protective role in sepsis. Of particular interest is whether critically ill patients may benefit from the increased splanchnic organ perfusion and oxygenation, as well as immunomodulation, seen in healthy patients who have received epidural anesthesia. However, additional studies are needed to evaluate the risk and benefits of epidural techniques in sepsis. Another novel application for epidural LAs proposes that continuous infusions may improve placental blood flow in parturients with chronically compromised uterine perfusion and intrauterine growth restriction.
There is a growing body of literature devoted to the potential beneficial effects of epidural analgesia in patients with cancer, although the data are preliminary and at times contradictory. Surgical stress and certain anesthetic agents suppress the host’s immune function, including its ability to eliminate circulating tumor cells, and can predispose patients with cancer to postoperative infection, tumor growth, and metastasis. Recent studies have demonstrated improved perioperative immune function with the use of TEA in patients undergoing elective laparoscopic radical hysterectomy for cervical cancer. Regional adjuncts to anesthesia have also been shown to have beneficial effects against recurrence of breast and prostate cancer. These protective effects may reflect both the decreased opioid requirements and the reduced neurohumoral stress response associated with epidural block.
CONTRAINDICATIONS
Serious complications of epidural techniques are rare. However, epidural hematomas, epidural abscesses, permanent nerve injury, infection, and cardiovascular collapse, among other adverse events, have been attributed to neuraxial block. As a result, an understanding of the conditions that may predispose certain patient populations to these and other complications is essential. This section reviews the absolute, relative, and controversial contraindications to epidural placement (Table 10). Ultimately, a risk-benefit analysis with particular emphasis on patient comorbidities, airway anatomy, patient preferences, and type and duration of surgery is recommended prior to initiation of epidural block.
TABLE 10. Contraindications to epidural block.
| Absolute | Patient refusal |
| Severe coagulation abnormalities (eg, frank disseminated intravascular coagulation) |
|
| Relative and controversial | Sepsis |
| Elevated intracranial pressure |
|
| Anticoagulants |
|
| Thrombocytopenia |
|
| Other bleeding diatheses |
|
| Preexisting central nervous system disorders (eg, multiple sclerosis) |
|
| Fever/infection (eg, varicella zoster virus) |
|
| Preload dependent states (eg, aortic stenosis) |
|
| Previous back surgery, preexisting neurologic injury, back pain |
|
| Placement in anesthetized adults |
|
| Needle placement through tattoo |
Absolute Contraindications
Although the contraindications to epidural block have been classified historically as absolute, relative, and controversial, opinions regarding absolute contraindications have evolved with advances in equipment, techniques, and practitioner experience. Currently, patient refusal may be considered the only absolute contraindication to epidural block. Although coagulopathy is considered a relative contraindication, initiating neuraxial block in the presence of severe coagulation abnormalities, such as frank disseminated intravascular coagulation (DIC), is contraindicated. Most other pathologic conditions comprise relative or controversial contraindications and require careful risk-benefit analysis prior to initiation of epidural block.
Relative and Controversial Contraindications
Sepsis
There is growing interest in using epidural anesthesia and analgesia to modulate inflammatory responses and to prevent or treat myocardial ischemia, respiratory dysfunction, and splanchnic ischemia in septic patients. However, there is insufficient evidence to determine whether epidural block is harmful or protective in sepsis. Despite the potential benefits of regional techniques in this setting, many anesthesiologists may be reluctant to initiate epidural block in septic patients due to concerns for relative hypovolemia, refractory hypotension, coagulopathy, and the introduction of blood-borne pathogens into the epidural or subarachnoid space. If regional anesthesia is selected, a slow-onset dosing technique after or with concurrent antibiotic, intravenous fluid, and vasopressor administration may be feasible.
Increased Intracranial Pressure
Accidental dural puncture (ADP) in the setting of elevated intracranial pressure (ICP) with radiologic evidence of obstructed cerebrospinal fluid (CSF) flow or mass effect with or without midline shift can place patients at risk of cerebral herniation and other neurological deterioration. Patients with increased ICP at baseline may also experience an additional increase in pressure on epidural drug injection. Consultation with a neurologic expert is strongly recommended, and localizing neurologic signs and symptoms should be ruled out by history and physical examination prior to initiation of neuraxial block in patients with new neurologic symptoms or known intracranial lesions (Table 11). A decision tree may aid in assessing whether it is safe to proceed with neuraxial techniques in the presence of intracranial space-occupying lesions (Figure 2).
TABLE 11. Signs and symptoms of elevated intracranial pressure.
| Headache |
| Drowsiness |
| Nausea and vomiting |
| New-onset seizures |
| Decreased level of consciousness |
| Papilledema |
| Pupillary changes |
| Focal neurologic signs |
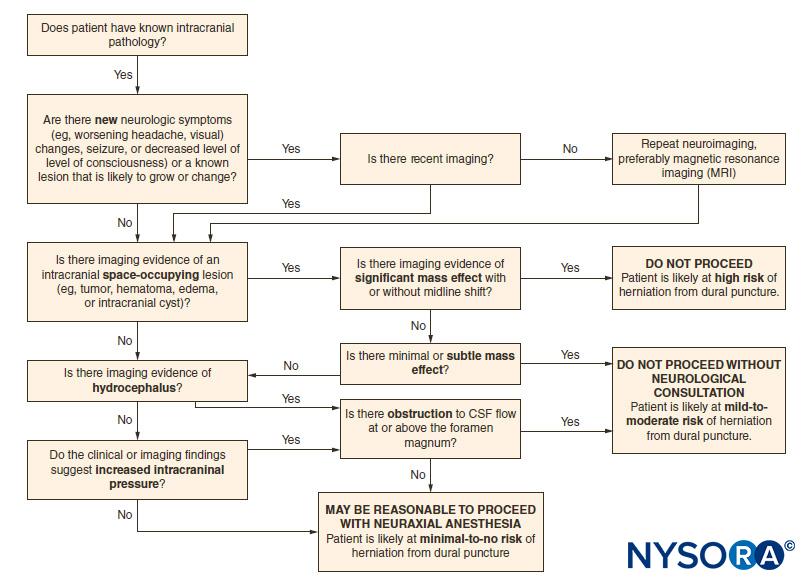
Figure 2. Safety algorithm for neuroaxial block in patients with intracranial space-occupying lesions. CSF = cerebrospinal fluid. (Reproduced with permission from Leffert LR, Schwamm LH: Neuraxial anesthesia in parturients with intracranial pathology: a comprehensive review and reassessment of risk. Anesthesiology. 2013 Sep;119(3):703-718.)
Coagulopathy
Coagulopathy is a relative contraindication to epidural placement, although thorough consideration of the etiology and severity of the coagulopathy is warranted on a case-by-case basis. Anticoagulants increase the risk of epidural hematoma and should be withheld in a timely fashion before initiation of epidural block. Precautions should also be taken before epidural catheter removal, as catheter removal may be as traumatic as catheter placement.
NYSORA Tips
• Epidural needle and catheter placement both carry a risk of epidural hematoma in patients on anticoagulants. Similar precautions should be observed during placement and removal of epidural catheters.
The American Society of Regional Anesthesia and Pain Medicine periodically updates its guidelines for the initiation of regional anesthesia in patients receiving antithrombotic or thrombolytic therapy. Briefly, neuraxial techniques in patients receiving subcutaneous unfractionated heparin (UFH) with dosing regimens of 5000 U every 12 hours are considered safe (Table 12).
TABLE 12. Epidural block in patients receiving antithrombotic therapy.
| NSAIDs (aspirin) | No contraindication |
| Clopidogrel | Wait 7 days before epidural placement |
| 5000 U subcutaneous UFH every 12 hours | No contraindication |
| >10,000 U subcutaneous UFH daily | Safety not established |
| Intravenous heparin | Wait at least 60 minutes after instrumentation before administration of heparin; consider aPTT and wait 2–4 hours prior to catheter removal |
| LMWH thromboprophylactic dose | Wait 12 hours before epidural placement |
| LMWH therapeutic dose | Wait 24 hours before epidural placement |
| Warfarin | Wait for INR to normalize before neuraxial block; remove neuraxial catheter when INR < 1.5 |
The risks and benefits of thrice-daily UFH or more than 10,000 U daily should be assessed on an individual basis; vigilance should be maintained to detect new or worsening neurodeficits in this setting. For patients receiving heparin for more than 4 days, a platelet count should be assessed before neuraxial nerve block or catheter removal due to concerns for heparin-induced thrombocytopenia (HIT). In patients who receive systemic heparinization, it is recommended to assess the activated plasma thromboplastin time (aPTT) and discontinue heparin for 2 to 4 hours prior to catheter manipulation or removal. Administration of intravenous heparin intraoperatively should be delayed for at least 1 hour after epidural placement; a delay before administration of subcutaneous heparin is not required. In cases of full heparinization for CPB, additional precautions include delaying surgery for 24 hours in the event of a traumatic tap, tightly controlling the heparin effect and reversal, and removing catheters when normal coagulation is restored.
Epidural block in patients taking aspirin and nonaspirin NSAIDs is considered safe, as the risk of epidural hematoma is low. Needle placement should be delayed for 12 hours in patients receiving low molecular weight heparin (LMWH) thromboprophylaxis and for 24 hours in those receiving therapeutic doses. It is recommended that warfarin be discontinued for several days prior to surgery and that the international normalized ratio (INR) return to baseline prior to initiation of epidural techniques. An INR below 1.5 is considered sufficient for catheter removal, although many clinicians may be comfortable manipulating catheters with higher INR values. Refer to Chapter 52 for more detailed information on these and newer agents.
Neuraxial techniques are contraindicated in the setting of DIC, which may complicate sepsis, trauma, liver failure, placental abruption, amniotic fluid embolism, and massive transfusion, among other disease processes (Table 13). If DIC develops after epidural placement, the catheter should be removed once normal clotting parameters have been restored.
TABLE 13. Conditions associated with disseminated intravascular coagulation.
| Sepsis |
| Trauma (head injury, extensive soft tissue injury, fat embolism, massive hemorrhage) |
| Massive transfusion |
| Malignancy (pancreatic carcinoma, myeloproliferative disease) |
| Peripartum (amniotic fluid embolism, placental abruption, HELLP [hemolysis, elevated liver enzymes, and low platelet count] syndrome, abnormal placentation) |
| Vascular disorders (aortic aneurysm, giant hemangioma) |
| Immunologic disorders (hemolytic transfusion reaction, transplant rejection, severe allergic reaction) |
| Liver failure |
Thrombocytopenia and Other Common Bleeding Disorders
Thrombocytopenia, which may be caused by several pathologic conditions, is a relative contraindication to neuraxial anesthesia.
While there is currently no universally accepted platelet count below which epidural placement should be avoided, many clinicians are comfortable with a platelet count above 70,000 mm3 in the absence of clinical bleeding. The cutoff may be higher or lower, however, depending on the etiology of the thrombocytopenia, the bleeding history, the trend in platelet number, individual patient characteristics (eg, a known or suspected difficult airway), and provider expertise and comfort level. In general, platelet function is normal in conditions such as gestational thrombocytopenia and immune thrombocytopenic purpura (ITP).
NYSORA Tips
• The etiology of thrombocytopenia, the patient’s bleeding history, and the trend in platelet count must be taken into account when determining the safety of initiation of epidural block in thrombocytopenic patients. Certain conditions, such as ITP and gestational thrombocytopenia, are associated with functioning platelets despite a low platelet count.
A platelet count below 50,000 mm3 in the setting of ITP may respond to corticosteroids or intravenous immunoglobulin (IVIG), when necessary. Functional platelet defects may be present in several less-common conditions, such as HELLP syndrome (hemolysis, elevated liver enzymes, and low platelet count); thrombotic thrombocytopenic purpura (TTP); and hemolytic uremic syndrome (HUS). Other conditions such as systemic lupus erythematous (SLE), antiphospholipid syndrome, type 2B von Willebrand disease (vWD), HIT, and DIC are associated with thrombocytopenia of varying degrees (Table 14).
TABLE 14. Causes of thrombocytopenia.
| Autoimmune | Idiopathic thrombocytopenic purpura |
| Thrombotic thrombocytopenic purpura |
|
| Antiphospholipid syndrome |
|
| Systemic lupus erythematosus | |
| Peripartum | Gestational thrombocytopenia |
| Preeclampsia (HELLP [hemolysis, elevated liver enzymes, and low platelet count] syndrome) |
|
| von Willebrand disease | Type 2B |
| Drug related | Heparin-induced thrombocytopenia |
| Methyldopa |
|
| Sulfamethoxazole | |
| Lymphoproliferative disorders | |
| Hemolytic uremic syndrome |
A standard platelet count has not been established for catheter removal. While some sources suggest 60,000 mm3 is appropriate, catheter removal without adverse sequelae has been reported at counts below that cutoff. If platelet number or function is impaired after an epidural catheter has been placed, such as in the case of intraoperative DIC, the catheter should remain in situ until the coagulopathy has resolved. Other common bleeding diatheses that comprise relative contraindications to the initiation of epidural block include hemophilia, vWD, and disorders related to lupus anticoagulants and anticardiolipin antibodies. Hemophilia A and B are X-linked diseases characterized by deficiencies in factors VIII and IX, respectively. Although specific guidelines are lacking, neuraxial procedures are considered safe in carriers of the disease with normal factor levels and no bleeding complications. Neuraxial techniques have been performed without adverse sequelae in homozygous patients after factor replacement therapy once factor leve ls and the aPTT have normalized. Patients with lupus anticoagulants and anticardiolipin antibodies are predisposed to platelet aggregation, thrombocytopenia, and, because of interactions between antibodies and platelet membranes, thrombosis. As a result, many of these patients are anticoagulated with heparin in the peripartum or perioperative period. Heparin levels should be monitored with a blood heparin assay, thrombin time, or activated clotting test prior to performing neuraxial block. Of note, the aPTT is elevated at baseline in these patients and is likely to remain elevated after discontinuation of heparin due to interactions between the circulating antibodies and the coagulation tests.
Von Willebrand disease is the most common inherited bleeding disorder. It is characterized by either a quantitative (type 1 and type 3) or qualitative (type 2) deficiency in von Willebrand factor (vWF), a plasma glycoprotein that binds to and stabilizes factor VIII and mediates platelet adhesion at sites of vascular injury. The clinical presentation of vWD varies: Patients with type 1, the most common type, experience mucocutaneous bleeding, easy bruising, and menorrhagia; patients with type 2 vWD may experience moderate-to-severe bleeding and, in the case of type 2B, thrombocytopenia; type 3, which is rare, presents with severe bleeding, including hemarthroses (Table 15).
TABLE 15. Classification of von Willebrand disease.
| Type | Underlying disorder | Clinical Presentation/Characteristics |
|---|---|---|
| 1 | Deficient quantity of vWF | Mucocutaneous bleeding, epistaxis, easy bruising, menorrhagia |
| 2A | Defect in quality of vWF | Moderate bleeding |
| 2B | Abnormal vWF | Moderate bleeding; thrombocytopenia; risk of thrombosis |
| 2M | Abnormal vWF binding | Rare; significant bleeding |
| 2N | Inactive vWF binding sites | May see low factor VIII and normal vWF levels |
| 3 | Severe deficiency of vWF | Severe bleeding, hemarthroses, muscle hematomas |
Both treatment options and the decision to proceed with neuraxial block also vary with the different disease presentations. Type I responds to desmopressin (DDAVP), which promotes secretion of stored vWF from endothelial cells and results in a rapid rise in both plasma vWF and factor VIII. Factor VIII concentrates and cryoprecipitate are treatment options for type 2 and type 3 vWD. Specialized laboratory tests may help confirm the diagnosis and type of vWD but are not widely available; standard coagulation tests may serve to rule out other bleeding disorders. In addition to a thorough history and physical examination, collaboration with a hematologist and other team members, and a review of any pertinent laboratory results, a risk-benefit analysis should be performed prior to initiation of epidural procedures in patients with vWD.
Preexisting Central Nervous System Disorders
Historically, the administration of neuraxial block has been contraindicated in patients with preexisting central nervous system (CNS) disease, including multiple sclerosis (MS), postpolio syndrome (PPS), and Guillain-Barré syndrome (GBS). In the case of MS, demyelinated nerves were thought to be more vulnerable to LA-induced neurotoxicity. An early study by Bader and colleagues suggested an association between MS relapse and higher concentrations of epidural LA among parturients, although a subsequent study in the same patient population failed to demonstrate an adverse effect of epidural anesthesia on either the rate of relapse or the progression of disease. A more recent retrospective study by Hebl and colleagues found no evidence of MS relapse after spinal or epidural anesthesia in 35 patients, 18 of whom received epidural block. While it is unlikely that epidural anesthesia and analgesia cause MS exacerbations, definitive studies on pharmacological properties of LAs in MS, optimal dosing regimens, and whether LAs interact directly with MS lesions are lacking. Until further data are available, it is reasonable to use low-concentration LAs and perform a thorough assessment and documentation of disease severity and neurologic status prior to initiation of central neuraxial block in patients with MS. These patients should also be informed of possible aggravation of symptoms, irrespective of anesthetic technique.
The decision to perform epidural anesthesia in patients with PPS, the most prevalent motor neuron disease in North America, requires careful analysis of the potential risks and benefits on a case-by-case basis. PPS is a late-onset manifestation of acute poliomyelitis infection that presents with fatigue, joint pain, and muscle atrophy in previously affected muscle groups. Epidural techniques in this patient population can be complicated by difficult puncture related to abnormal spinal anatomy, potential worsening of symptoms, and transient respiratory weakness. Alternatively, GA presents challenges related to sensitivity to muscle relaxants and sedatives and risks of respiratory compromise and aspiration. Although data are limited, there is no evidence that epidural techniques contribute to worsening of neurologic symptoms in patients with PPS.
Evidence linking epidural techniques to either activation or recurrence of GBS is also lacking. GBS presents with progressive motor weakness, ascending paralysis, and areflexia, most likely attributable to a postinfection inflammatory response. Older age at onset and severe initial disease are among the risk factors for prolonged neurologic dysfunction. Epidural anesthesia has been used successfully in patients with GBS, most commonly in obstetric patients, although exaggerated hemodynamic responses (hypotension and bradycardia), higher-than-normal spread of LAs, and worsening of neurologic symptoms have been reported. As always, a risk-benefit analysis is warranted prior to performance of epidural block in patients with GBS, as are assessment and documentation of neurologic examination of the patient and a thorough discussion of the risks of anesthesia. It is reasonable to avoid regional techniques during periods of acute neuronal inflammation.
Patients with spina bifida may also present a unique challenge to anesthesiologists. Spina bifida occulta occurs when the neural arch fails to close without herniation of the meninges or neural tissues. It is most commonly limited to one vertebra, although a small percentage of affected individuals have involvement of two or more vertebrae with associated neurologic abnormalities, underlying cord abnormalities, and scoliosis. In general, the use of epidural techniques is not contraindicated in patients with spina bifida occulta, although placement at the level of the occulta lesion, most commonly at L5 to S1, may have an increased risk of dural puncture and patchy or higher-than-normal response to LAs. In contrast, epidural placement in patients with spina bifida cystica has several potential risks, including risk of direct injury to the cord due to a low-lying conus medullaris, unpredictable or higher-than-expected spread of LAs, and increased risk of dural puncture.
Fever or Infection
Controversy exists regarding the administration of neuraxial anesthesia in febrile patients and in individuals infected with human immunodeficiency virus (HIV), herpes simplex virus type 2 (HSV-2), and varicella zoster virus (VZV). The use of regional anesthesia in the presence of a low-grade fever of infectious origin is controversial due to concerns of spreading the infectious agent to the epidural or subarachnoid space, with subsequent meningitis or epidural abscess formation. Fortunately, infectious complications of regional anesthesia are rare, and studies to date have failed to demonstrate a causal relationship between neuraxial procedures, with or without dural puncture, and subsequent neurologic complications. While no universal guidelines exist, available data suggest that fever does not preclude the safe administration of epidural anesthesia and analgesia. The anesthetic management of febrile patients should be based on an individual risk-benefit analysis. Whether general or regional anesthesia is chosen, antibiotic therapy should be either completed prior to or underway during initiation of the anesthetic. Adherence to strict aseptic techniques and postprocedure monitoring to detect and treat any complications are essential.
Historically, there have been concerns about the safety of neuraxial procedures in individuals infected with HIV due to both the theoretical risk of inoculation of the virus into the CNS and the possibility that neurologic manifestations of HIV may be attributed to the anesthetic technique. However, the CNS is infected early in the course of HIV infection, and there is no evidence that neuraxial instrumentation, including an epidural blood patch (EBP) for the treatment of PDPH, confers additional risk of viral spread to the CNS. There also is no evidence that the introduction of HIV-infected blood into the CSF might exacerbate a preexisting CNS infection, such as meningitis. Concerns that neurologic sequelae of HIV might be attributed to the neuraxial technique also appear to be unsubstantiated, as a temporal relationship between the epidural placement and the onset of neurologic deficits is unlikely. Nonetheless, thorough documentation of any preexisting neurologic deficit is recommended, given that neurologic complications of HIV are not uncommon and that HIV-positive individuals are at high risk for other sexually transmitted diseases that affect the CNS. Potential risks should be discussed in advance, and, as always, strict aseptic technique to protect both the patient and the anesthesiology provider must be maintained.
Areas of concern regarding the use of regional anesthesia in patients with HSV-2 include the risk of introducing the virus into the CNS during administration of neuraxial anesthesia; the possibility that a disseminated infection that develops after a regional anesthetic might be ascribed to the anesthetic itself, despite the lack of a causal relationship; and the safety of neuraxial techniques in primary HSV-2 outbreaks, which may be silent and difficult to distinguish from secondary outbreaks, but more commonly present with viremia, constitutional symptoms, genital lesions, and, in a small percentage of patients, aseptic meningitis. There are no documented cases of septic or neurologic complications following neuraxial procedures in patients with secondary (ie, recurrent) HSV infection; however, the safety of regional anesthesia in patients with primary infection has not been established. Crosby and colleagues conducted a 6-year retrospective analysis of 89 patients with secondary HSV infection who received epidural anesthesia for cesarean delivery and reported that no patients suffered septic or neurologic complications.
Similarly, in their retrospective survey of 164 parturients with secondary HSV infection who received spinal, epidural, or GA for cesarean delivery, Bader et al reported no adverse outcomes related to the anesthetic. Based on the findings in these and other reported series, it appears safe to use spinal or epidural anesthesia in patients with secondary HSV infection. Pending more conclusive data, however, it seems prudent to avoid neuraxial block in patients with HSV-2 viremia. Concerns also exist regarding the use of regional anesthesia in adults with either primary or recurrent VZV infections, such as herpes zoster (ie, shingles) and postherpetic neuralgia (PHN). However, neuraxial procedures, including epidural steroid injections, are not uncommonly used to treat acute herpes zoster, prevent PHN, and treat the pain associated with PHN, often in conjunction with antiviral therapy. The presence of active lesions at the site of injection is considered a contraindication to these and other neuraxial techniques. For the small subset of patients who are infected with primary VZV as adults, severe complications such as aseptic meningitis, encephalitis, and varicella pneumonia may result. The performance of regional anesthesia in this setting is more controversial but may be preferable to GA in some cases, primarily due to concerns for pneumonia. Ultimately, a careful risk-benefit analysis, in addition to assessment and documentation of any preexisting neurologic deficits, is recommended prior to initiation of neuraxial block in these patients.
Localized skin infection at the site of intended needle puncture is another relative contraindication to neuraxial block, primarily due to concerns that spinal epidural abscess (SEA) or meningitis may result. Hematogenous spread of a localized infection has been implicated in SEA, although a causal relationship is not clearly established in the reported cases. Maintenance of strict sterile precautions and a low index of suspicion in the presence of neurologic signs may minimize the risk. Needle insertion should be attempted after appropriate antibiotic administration, and a site remote from the localized infection is recommended.
Previous Back Surgery, Preexisting Neurologic Injury, and Back Pain
Traditionally, a history of previous back surgery was considered a relative contraindication to neuraxial block due to concerns for infection, exacerbation of preexisting neurologic deficits, and an increased likelihood of difficult or unsuccessful nerve block. Technical difficulties may be related to degenerative changes above or below the level of fusion, adhesions in the epidural space, epidural space obliteration, dense scar tissue at the point of intended needle entry on the skin surface, the presence of graft material, and the presence of extensive rods that preclude identification of or access to midline. Despite these concerns, one large retrospective study of patients with a history of spinal stenosis, peripheral neuropathy, or lumbar radiculopathy found that previous spinal surgery did not affect the success rate or frequency of technical complications. In patients with metal rods (eg, Harrington rods), anteroposterior and lateral radiographs or a copy of the operative report may help to identify the extent of instrumentation, as well as the presence of additional anatomic abnormalities. Ultrasound may aid in the identification of midline in challenging epidural cases. Potential complications, such as irregular, limited, or excessive cranial spread of LAs and an increased risk of PDPH if multiple attempts at placement are required, should be discussed with the patient during the informed consent process. Of note, similar technical difficulties encountered during the original technique can be expected during an EBP procedure. Because of these and other concerns, spinal anesthesia may be preferred, when appropriate, over epidural block.
Back pain is a ubiquitous problem that should not be considered a contraindication to neuraxial block and, rather, is a relatively common indication for epidural steroid and LA injections. One recent study found a higher than previously reported rate of new neurologic deficits and worsening of preexisting symptoms in patients with compressive radiculopathy or multiple neurologic disorders (spinal stenosis or lumbar disk disease) who received neuraxial anesthesia. However, a causal relationship was not clearly established. Many of the concerns regarding neuraxial procedures in patients with back pain can be addressed prior to initiation of neuraxial anesthesia with a thorough history and physical examination; not uncommonly, the cause of back pain is not neurologic in origin. In these cases, regional techniques are not associated with new-onset back pain and are unlikely to exacerbate the preexisting condition. Because patients with preexisting neurologic conditions may be at increased risk of postoperative neurologic complications after neuraxial techniques, a careful risk-benefit analysis is warranted on a case-by-case basis. Preexisting neurologic deficits or symptoms and their severity should be documented.
Preload-Dependent States
Traditionally, neuraxial block has been considered contraindicated in patients with severe aortic stenosis (AS) and other preload-dependent conditions, such as hypertrophic obstructive cardiomyopathy (asymmetric septal hypertrophy, ASH), due to the risk of acute decompensation in response to decreased systemic vascular resistance (SVR). The later stages of AS are associated with decreased diastolic compliance, impaired relaxation, increased myocardial oxygen demand, and decreased perfusion of the endocardium. Decreased SVR in the setting of either GA or neuraxial block leads to decreased coronary perfusion and contractility, with a further reduction in cardiac output (CO) and worsening hypotension. Bradycardia, tachycardia, and other dysrhythmias are also poorly tolerated. The current evidence regarding regional anesthesia in patients with AS is based on case reports and lacks the scientific validity provided by randomized controlled trials. However, it appears that carefully titrated CSE and continuous epidural and spinal techniques, most commonly with invasive monitoring, may be acceptable options for patients with AS. Single-shot spinal anesthetics are generally contraindicated, as gradual onset of sympathetic block is essential.
Anesthetic goals for patients with ASH are similar, with emphasis on maintaining preload, afterload, euvolemia, and vascular resistance, while avoiding tachycardia and enhanced contractility. Invasive monitoring and, if necessary, intermittent transthoracic echocardiography may help guide fluid and vasopressor requirements, as well as guide management in the event of acute decompensation.
Epidural Placement in Anesthetized Patients
Initiation of epidural block in adults under GA is controversial due to concerns that these patients cannot respond to pain and may therefore be at increased risk for neurologic complications. Indeed, paresthesias during nerve block performance and pain on LA injection have been identified as risk factors for serious neurologic deficits after regional techniques. Consequently, some experts consider close communication with the patient an essential component of safe epidural performance. Current data support the practice of epidural insertion in awake or minimally sedated patients, but needle and catheter placement in anesthetized adults may be an acceptable alternative in selected cases. Studies of lumbar epidural insertion while patients are undergoing GA have demonstrated that the risk of neurologic complications is small. Overall, the relative risk of administration of epidural block in anesthetized patients, compared with epidural placement in awake patients, is unknown due to the low overall incidence of serious neurologic complications associated with regional anesthesia.
Needle Insertion Through a Tattoo
Concerns that puncturing a tattoo during epidural placement may have adverse sequelae appear unsubstantiated in the literature. Theoretical risks are related primarily to the introduction of a potentially toxic or carcinogenic pigment into the epidural, subdural, or subarachnoid space. However, to date no significant complications related to inserting a needle through a tattoo have been reported in the literature, although potential long-term consequences cannot be dismissed.
ANATOMY
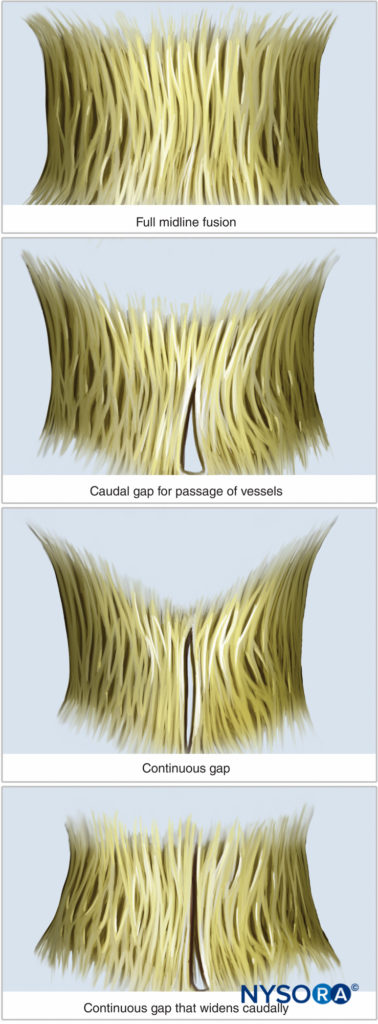
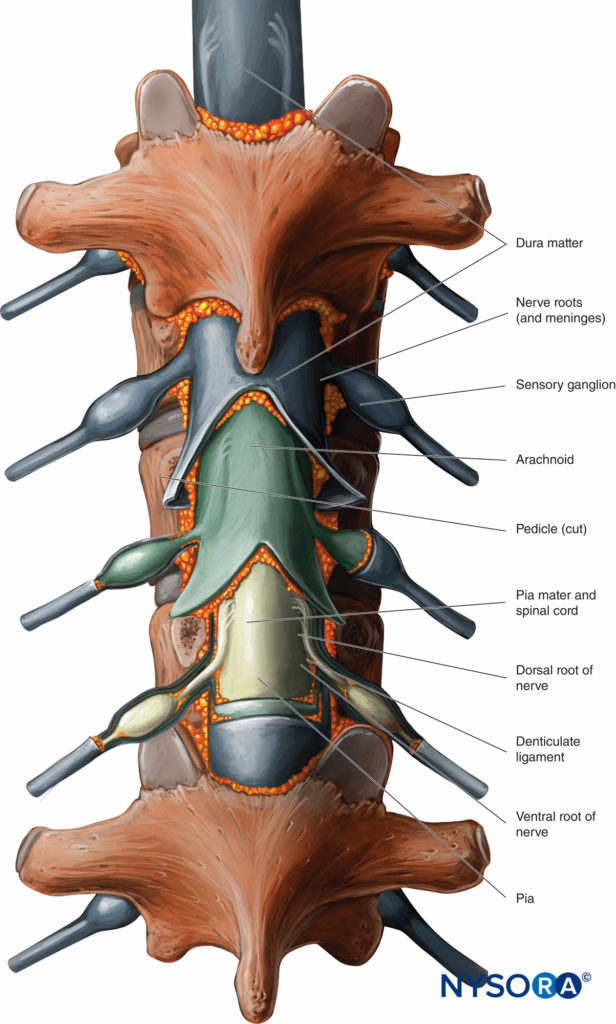
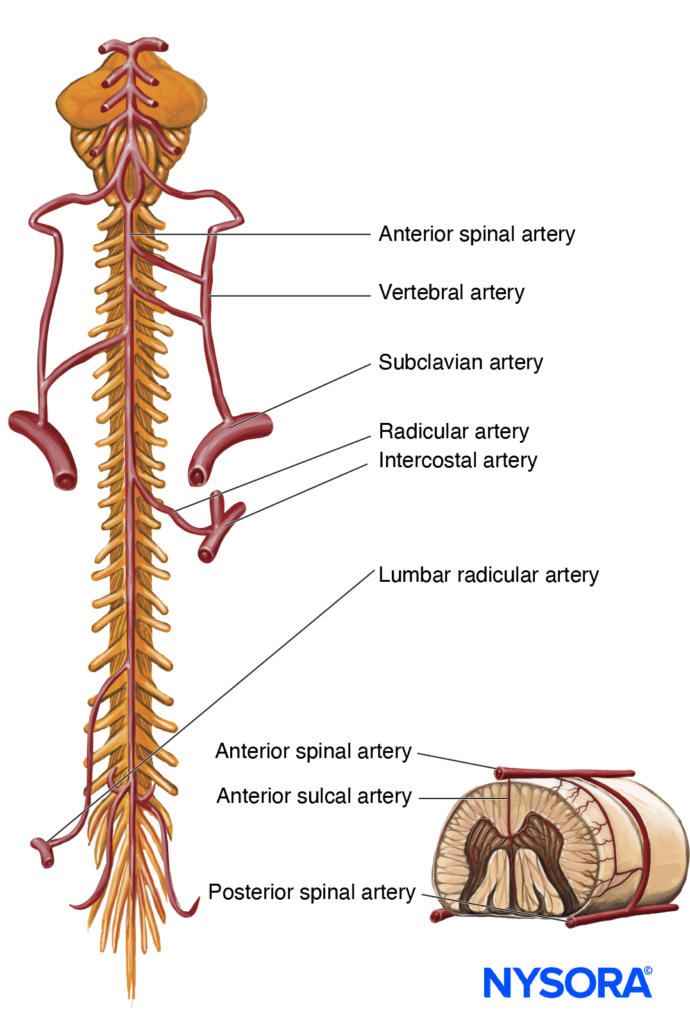
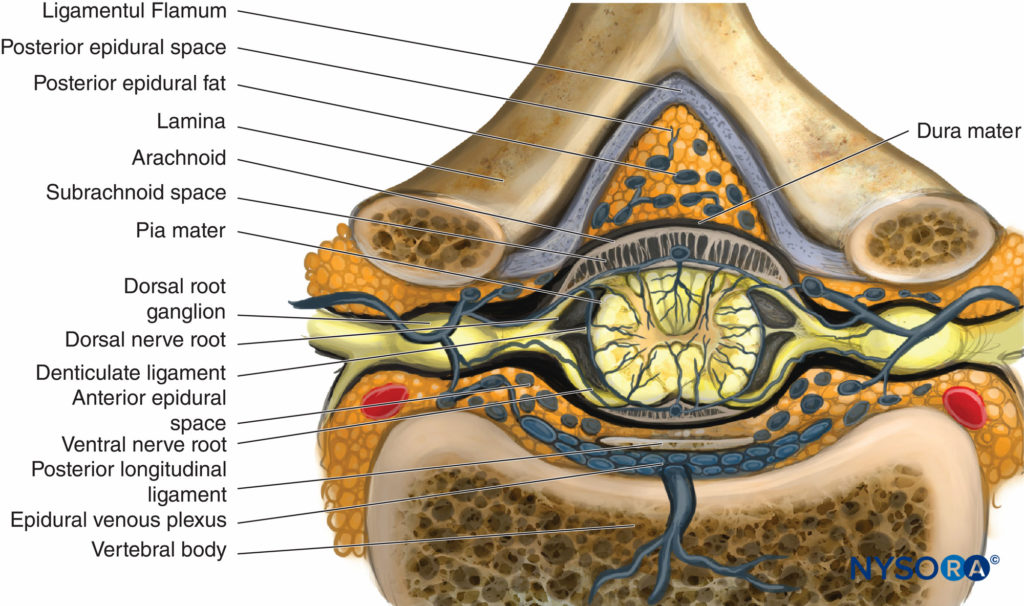
An understanding of the anatomy of the vertebral column, spinal canal, epidural space and its contents, and commonly encountered anatomic variations among individuals is essential for the safe and effective initiation of epidural block. A three-dimensional mental image of vertebral column anatomy also aids in troubleshooting when identification of the epidural space is equivocal or when complications of epidural catheterization, such as unilateral block, intravascular cannulation, or catheter migration, occur. This section presents the basic anatomic considerations for successful epidural anesthesia and analgesia and reviews several controversies in the field of applied anatomy, including the accuracy of anatomic landmarks to estimate the spinous process level, the existence (or lack thereof) of a subdural compartment, and the contents of the epidural space.
Vertebral Column
General Appearance
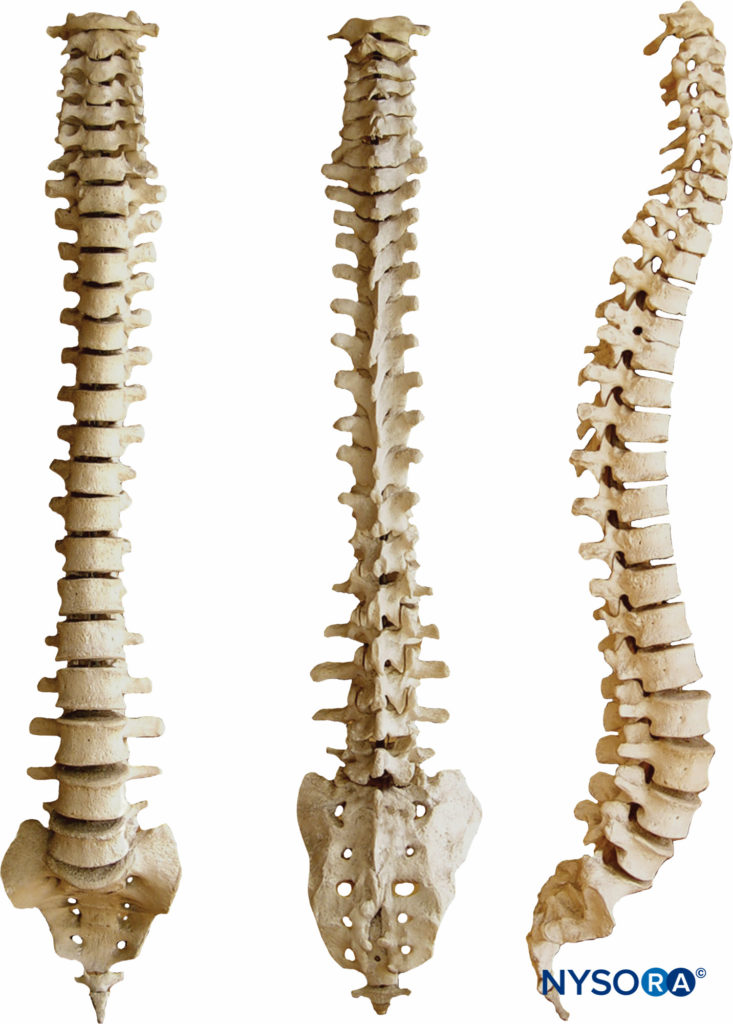
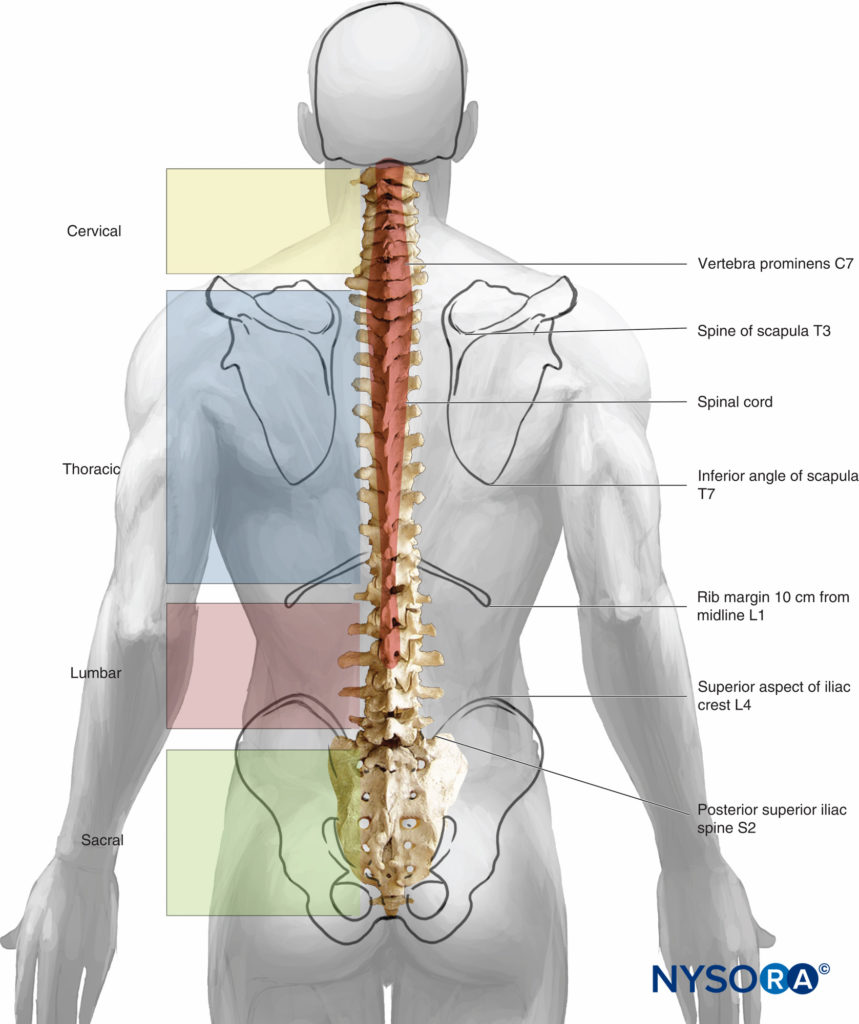
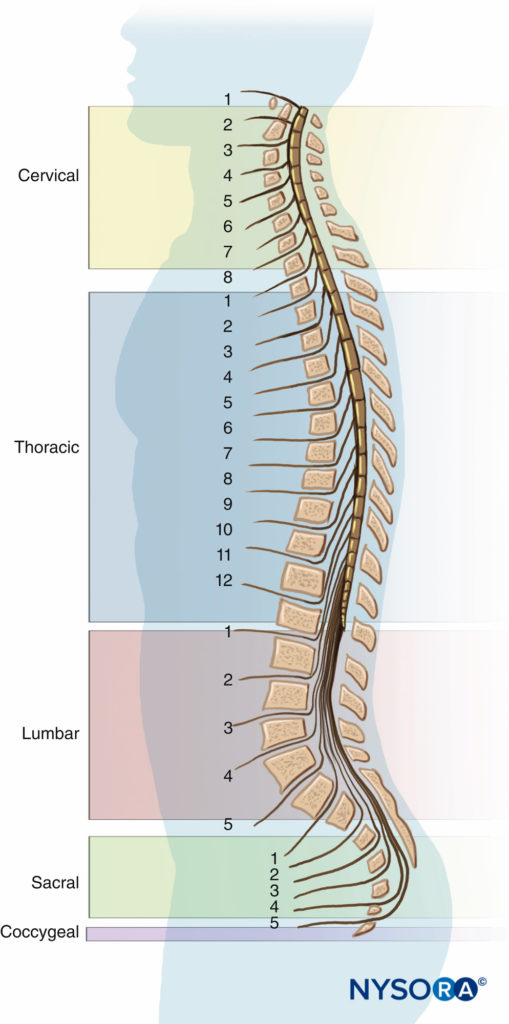
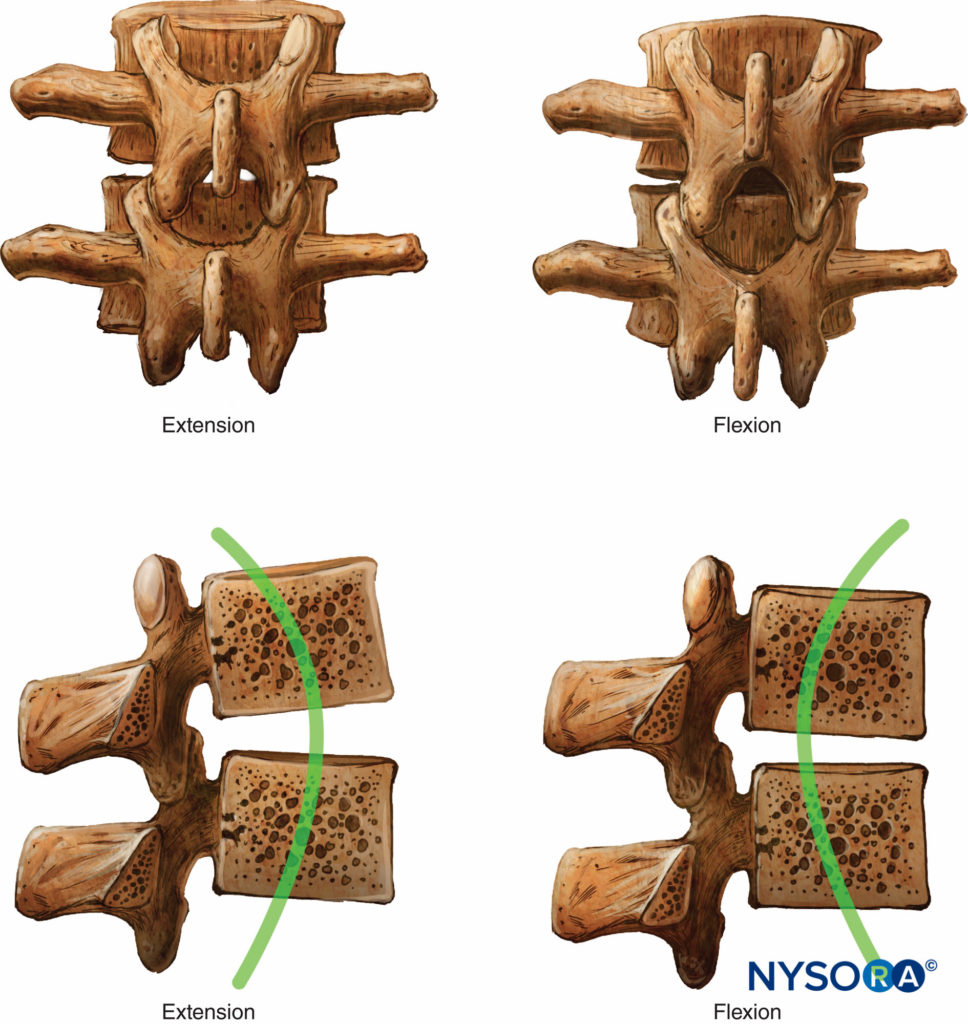
Seven cervical, 12 thoracic, 5 lumbar, 5 fused sacral, and 3 to 5 (most commonly 4) fused coccygeal vertebrae comprise the vertebral column. The vertebral column is straight when viewed dorsally or ventrally. When viewed from the side, the cervical and lumbar regions are concave posteriorly (lordosis), and the thoracic and sacral regions are concave anteriorly (kyphosis) (Figure 3).
The four physiologic spinal curves are fully developed by 10 years of age and become more pronounced during pregnancy and with aging. In the supine position, C5 and L3 are positioned at the highest points of the lordosis; the peaks of kyphosis occur at T5 to T7 and at S2.
NYSORA Tips
C5 and L3 comprise the highest points of lordosis in the supine position; the highest points of kyphosis are T5 to T7 and S2.
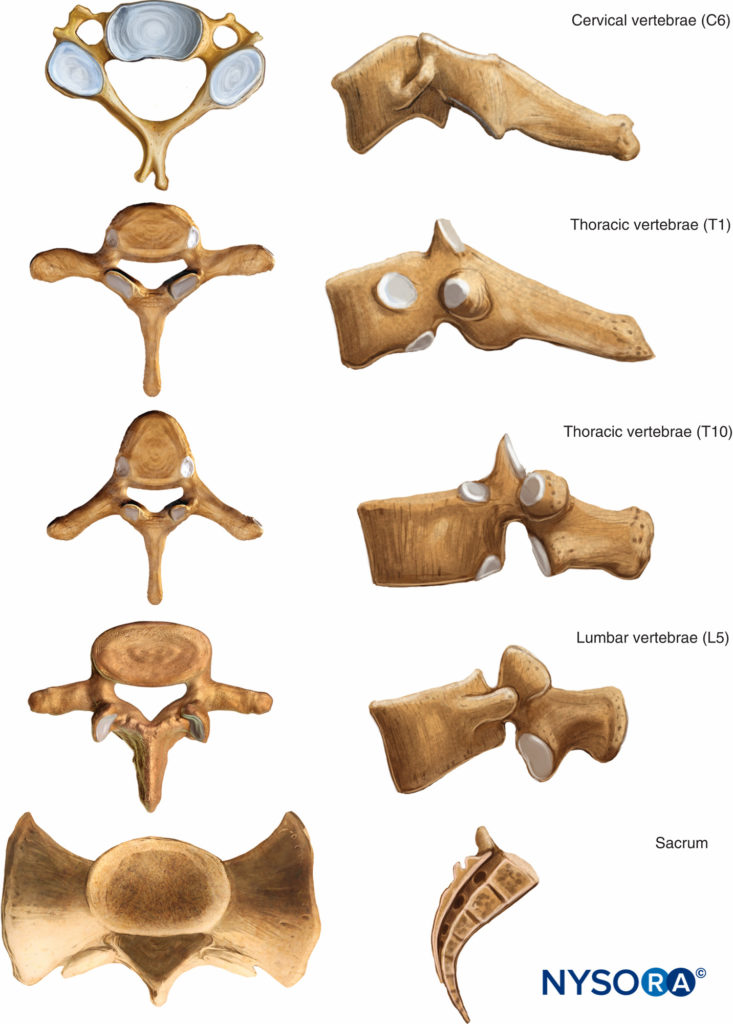
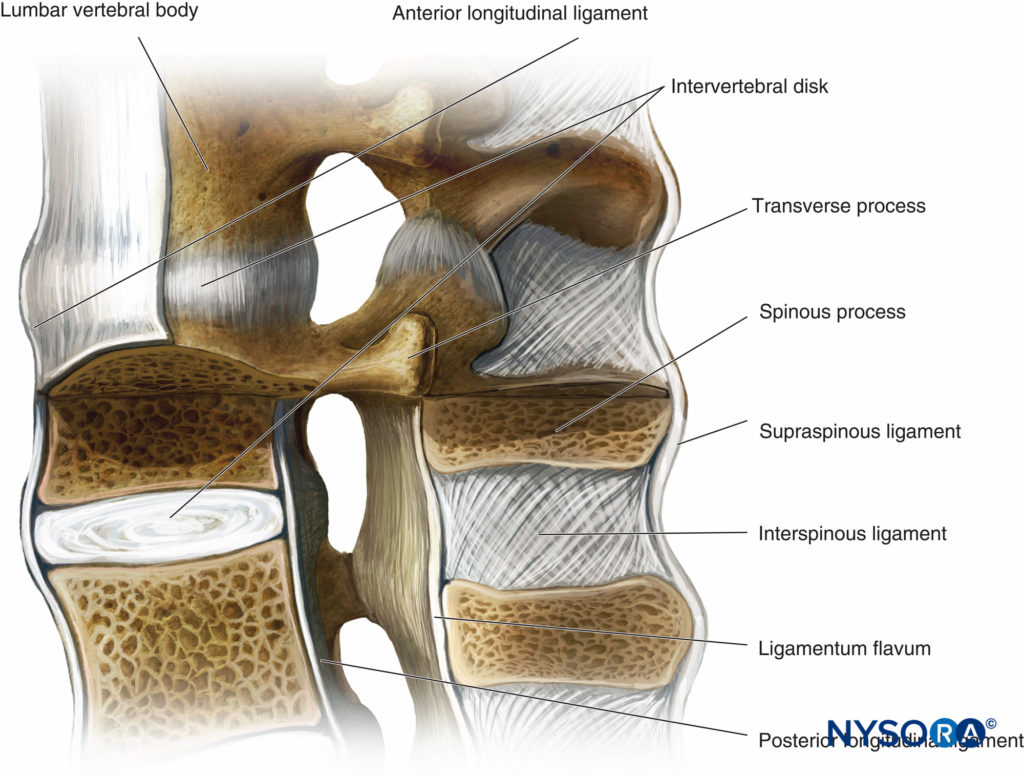
Structure of Vertebrae
With the exceptions of C1 and C2 and the fused sacral and coccygeal regions, the general structure of each vertebra consists of an anterior vertebral body (corpus, centrum) and a posterior bony arch. The arch is formed by the laminae; the pedicles, which extend from the posterolateral margins of the vertebral body; and the posterior surface of the vertebral body itself. In addition to the spinous processes, which are formed by the fusion of the laminae at midline, the vertebral arch supports three pairs of processes that emerge from the point where the laminae and pedicles join: two transverse processes, two superior articular processes, and two inferior articular processes. Adjacent vertebral arches enclose the vertebral canal and surround portions of the longitudinal spinal cord. The spinal canal communicates with the paravertebral space by way of gaps between the pedicles of successive vertebrae. These intervertebral foramina serve as passageways for the segmental nerves, arteries, and veins.
There is substantial variation in the size and shape of the vertebral bodies, the spinous processes, and the spinal canal at different levels of the vertebral column (Figure 4). C3 through C7 have the smallest vertebral bodies, while the spinal canal at this level is wide, measuring 25 mm. These cervical vertebrae, with the exception of C7, have short, bifurcated spinous processes. C7, the vertebra prominens, has a long, slender, and easily palpable horizontal spinous process protruding at the base of the neck that often serves as a surface landmark during epidural procedures. However, the first thoracic spinous process may be equally or more prominent than C7 in up to one-third of male individuals, as well as in thin patients and in patients with scoliosis and degenerative diseases. The vertebra prominens may also be difficult to distinguish from C6 in up to half of individuals, most commonly females.
The thoracic vertebral bodies are larger than the cervical vertebral bodies and are wider in the posterior than anterior dimension, contributing to the characteristic thoracic curvature. The long and slender thoracic spinous processes, with tips that point caudally, are most sharply angled between T4 and T9, making insertion of the epidural needle in the midline more difficult in the midthoracic region. Beyond T10, they increasingly resemble those in the lumbar region. Each thoracic vertebra articulates with ribs along the dorsolateral border of its body, a feature that may help distinguish the lower thoracic and upper lumbar regions. The inferior angle of the scapula and the 12th rib are widely used in clinical practice to estimate the level of the cross the L1 spinous process (Table 16).
TABLE 16. Anatomic landmarks to identify spinous processes of T7 and T12, respectively. The imaginary line connecting the caudal-most margin of the 12th ribs is often presumed to
vertebral levels.
| Vertebra prominens | C7 |
| Root of spine of scapula | T3 |
| Inferior angle of scapula | T7 |
| Rib margin | L1 |
| Superior aspect of iliac crest | L3, L4 |
| Posterior superior iliac spine | S2 |
The lumbar vertebrae are the largest movable segments, with thicker anterior than posterior dimensions that contribute to the characteristic lumbar curvature. The spinous processes in this region are blunt and large, with tips that point posteriorly.
Anatomic variations in the lumbosacral region that may have clinical implications are not uncommon. Sacralization of the last lumbar vertebra, marked by fusion of L5 to the sacral bone, and lumbarization of S1 and S2, in which fusion is incomplete, may make numbering and identification of the correct lumbar level difficult. Although probably not of clinical significance, patients with sacralization have also been found to have a higher position of the conus medullaris, which demarcates the cone-shaped terminus of the spinal cord, than those with lumbarization or without lumbosacral transitional vertebrae. In the absence of these transitional vertebrae, the largest and most easily palpable interspace corresponds to L5 to S1.
Surface Anatomic Landmarks to Identify the Spinal Level
Surface landmarks are often used to identify the intended spinal level during initiation of epidural anesthesia (Figure 5).
However, palpation and inspection of surface anatomical landmarks may fail to help localize the correct intervertebral space, particularly when considering individual variations in the vertebral level of these landmarks, the varying termination of the conus medullaris between the middle third of T12 and the upper third of L3, and anesthesiologists’ poor record of identifying the correct interspace.
Common pitfalls to using skeletal landmarks to identify the level of puncture include the following: The vertebra prominens is commonly confused with C6 and T1; the scapula may be difficult to identify during TEA placement in obese patients; tracing the vertebra attached to the 12th rib can be misleading, particularly in obese patients; and the line connecting the posterior superior iliac spines, often used to identify S2, commonly crosses the midline at variable levels between L5 and S1. Several studies have demonstrated that Tuffier’s line (also known as Jacoby’s line or the intercristal line), which joins the superior aspect of the iliac crests, may cross midline at least one, and perhaps two, levels higher than the predicted L4–L5 interspace, particularly in pregnant, elderly, and obese patients. Anesthesiologists have a poor record of estimating the correct interspace based on external landmarks. Van Gessel and colleagues found that the level of lumbar puncture is misidentified up to 59% of the time. In a more recent study, Broadbent and coworkers found that practitioners identify the correct lumbar level in only 29% of cases; the space is misidentified by two spinal levels, with the actual level higher than that predicted, in 14% of cases. Lirk et al confirmed the tendency of trained anesthetists to place the epidural needle more cranially than intended, most often within one interspace of the predicted level, also in the cervical and thoracic spinal column. Overall, given the importance of selecting the correct site of puncture, caution is advised when using surface anatomic landmarks to identify intervertebral spaces. The increasing reliance on ultrasound determination of the spinal level may decrease the incidence of complications related to misidentification of the intended interspace.
Joints and Ligaments of the Vertebral Column
General
Adjacent vertebrae of the cervical, thoracic, and lumbar regions, excluding C1 and C2, are separated and cushioned by fibrocartilaginous intervertebral disks. The soft, elastic core of each disk, the nucleus pulposus, is composed primarily of water, as well as scattered elastic and reticular fibers. The fibrocartilaginous annulus fibrosis surrounds the nucleus pulposus and attaches the disks to the bodies of adjacent vertebrae. The disks, which account for up to one-quarter of the length of an adult vertebral column, lose their water content as we age, contributing to the shortening of the vertebral column, reducing their effectiveness as cushions, and rendering them more prone to injury, particularly in the lumbar region.
The articular processes arise at the junction between the pedicles and laminae. Superior and inferior articular processes project cranially and caudally, respectively, on both sides of each vertebra. The vertebral arches are connected by facet joints, which link the inferior articular processes of one vertebra with the superior articular processes of the more caudal vertebra. The facet joints are heavily innervated by the medial branch of the dorsal ramus of the spinal nerves. This innervation serves to direct contraction of muscle that moves the vertebral column.
The Longitudinal Ligaments
The anterior and posterior longitudinal ligaments support the vertebral column, binding the vertebral bodies and intervertebral disks together (Figure 6). The posterior longitudinal ligament, which forms the anterior wall of the vertebral canal, is less broad than its anterior counterpart and weakens with age and other degenerative processes. Clinically, disk herniation occurs primarily in the paramedian portion of the posterior disk, at weak points in the posterior longitudinal ligament. This area comprises the anterior epidural space, as opposed to the more clinically relevant posterior epidural space, and should not interfere with epidural needle placement.
NYSORA Tips
• Disk herniation occurs primarily at weak points in the posterior longitudinal ligament in an area that comprises the anterior epidural space, as opposed to the more clinically relevant posterior epidural space.
Nonetheless, thorough documentation of preexisting pain and neurologic deficits in patients with known disk herniation is recommended prior to initiation of epidural anesthesia. Also of clinical relevance, a membranous lateral extension of the posterior longitudinal ligament may serve as a barrier to the spread of epidural solutions and appears to cordon the veins anterior to the dura away from the rest of the epidural space.
NYSORA Tips
• A membranous lateral extension of the posterior longitudinal ligament appears to cordon off the veins in the anterolateral epidural space, where epidural vein puncture and catheter cannulation are more likely to occur.
The Supraspinous and Interspinous Ligaments
Several other ligaments that support the vertebral column serve as key anatomic landmarks during epidural needle placement. The supraspinous ligament connects the tips of the spinous processes from C7 to L5; above C7 and extending to the base of the skull, it is called the ligamentum nuchae. This relatively superficial, inextensible ligament is most prominent in the upper thoracic region and becomes thinner and less conspicuous toward the lower lumbar region. The interspinous ligament, directly anterior to the supraspinous ligament, traverses the space between adjacent spinous processes in a posterocranial direction. It is less developed in the cervical region, which may contribute to a false LOR during cervical epidural procedures.
On histological examination, the interspinous ligament appears to have intermittent midline cavities filled with fat. Both the supra- and interspinous ligaments are composed of collagenous fibers that make a characteristic “crunching” sound or distinct tactile sensation as the epidural needle advances. During initiation of epidural placement via the midline approach, these ligaments serve as appropriate sites to engage the needle, although some practitioners may engage the needle closer to the epidural space, in the ligamentum flavum. A “floppy” epidural needle that angles laterally prior to attachment of the LOR syringe may indicate an off-midline approach, away from the supra- or interspinous ligaments.
The Ligamentum Flavum
The ligamentum flavum connects the lamina of adjacent vertebrae from the inferior border of C2 to the superior border of S1. Laterally, it extends into the intervertebral foramina, where it joins the capsule of the articular process.
Anteriorly, it limits the vertebral canal and forms the posterior border of the epidural space. At each spinal level, the right and left ligamentum flava join discontinuously in an acute angle with the opening oriented in the ventral direction, occasionally forming midline gaps filled with epidural fat. In contrast to the collagenous inter- and supraspinous ligaments, the ligamentum flavum comprises primarily thick, elastic fibers arranged longitudinally in a tight network.
Areas of ossification of the ligamentum flavum occur at different levels of the vertebral canal and appear to be a normal variant. These bony spurs, which may contribute to preexisting neurological symptoms and could potentially impede epidural needle advancement, are most commonly encountered in the lower thoracic region, between T9 and T11, and diminish in both frequency and size in the caudal and cranial directions.
The ligamentum flavum has variable characteristics, many of which are disputed in the literature, at different vertebral levels. First, its thickness varies at different levels and, possibly, in different physiologic states, with a range of 1.5–3.0 mm in the cervical segment, 3.0–5.0 mm in the thoracic segment, 5.0–6.0 mm in the lumbar segment, and 2.0–6.0 mm in the caudal region (Table 17). In isolated pregnant patients, the ligamentum flavum has been reported to be as thick as 10 mm, presumably due to edema. Also of note, the flavum’s thickness varies within the interspace itself, with the caudal region being significantly thicker than the rostral.
TABLE 17. Thickness of the ligamentum flavum at different vertebral levels.
| Vertebral Level | Thickness (mm) |
|---|---|
| Cervical | 1.5–3.0 |
| Thoracic | 3.0–5.0 |
| Lumbar | 5.0–6.0 |
| Caudal | 2.0–6.0 |
NYSORA Tips
• The ligamentum flavum varies in thickness at different spinal levels and is thickest in the lumbar region. Its thickness also varies within each interspace.
Clinically, these varying degrees of thickness may influence the risk of inadvertent dural puncture or determine whether injection of an anesthetic solution into the epidural space is possible with the skin infiltration needle.
Another controversy concerns the incidence and location of gaps formed by the incomplete fusion of the right and left ligamentum flava. In their study of 52 human cadavers, Lirk and colleagues found that up to 74% of the flava in the cervical region are discontinuous at midline. These gaps vary in location, with some occupying the entire height of the ligamentum flavum between successive vertebral arches and others occupying the caudal third portion only (Figure 7).
Veins connecting the posterior external and internal vertebral venous plexuses not uncommonly traverse the caudal portion of the gaps. In another cadaveric study, Lirk et al determined that thoracic midline gaps were less frequent than cervical gaps but more frequent than those in the lumbar region, with an incidence as high as 35.2% at T10 to T11. In cadaveric studies of the lumbar ligamentum flavum, gaps were found most commonly at L1 and L2 (22.2%) and decreased caudally (11.4% at L2 to L4; 9.3% at L4 to L5; 0% at L5 to S1). Clinically, these gaps may contribute to failure to identify the epidural space using the LOR technique at midline. The characteristic “pop” sound and tactile sensation conferred by penetration of the elastic fibers of the ligamentum flavum may be absent in the setting of a discontinuous ligamentous arch. The depth to the epidural space at midline may also be affected.
NYSORA Tips
• Ligamentum flavum midline gaps represent incomplete fusion of the right and left ligamentum flava. They are common in the cervical spine and decrease in frequency in the thoracic and lumbar regions. The variable thickness of the ligamentum flavum and the presence of midline gaps may contribute to failure to identify the epidural space.
The Spinal Canal General
The vertebrae serve primarily to support the weight of the head, neck, and trunk; transfer that weight to the lower limbs; and protect the contents of the spinal canal, including the spinal cord. An extension of the medulla oblongata, the spinal cord serves as the conduit between the CNS and the peripheral nerves via 31 pairs of spinal nerves (8 cervical, 12 thoracic, 5 lumbar, 5 sacral, and 1 coccygeal) (Figure 8). The adult cord measures approximately 45 cm or 18 inches and has two regions of enlarged diameter at C2–T2 and at T9–L2, areas that correspond with the origin of the nerve supplies to the upper and lower extremities. However, its level of termination varies with age, as well as among individuals of similar age groups. As a result of a discrepancy in the pace of growth of the spinal cord and vertebral column during development, the spinal cord at birth ends at approximately L3. By 6–12 months of age, the level of termination parallels that of adults, most commonly at L1. Below the conus medullaris, the long dorsal and ventral roots of all the spinal nerves below L1 form a bundle known as the cauda equina, or horse’s tail. A collection of strands of neuron-free fibrous tissue enveloped in pia mater comprises the filum terminale and extends from the inferior tip of the conus medullaris to the second or third sacral vertebra.
Spinal Nerves
Spinal nerves are classified as mixed nerves because they contain both a sensory and a motor component and, in many cases, autonomic fibers. Each nerve forms from the fusion of dorsal (sensory) and ventral (somatic and visceral motor) nerve roots as they exit the vertebral canal distal to the dorsal root ganglia, which contain the cell bodies of sensory neurons on either side of the spinal cord and lie between the pedicles of adjacent vertebrae.
In general, dorsal roots are larger and more easily blocked than ventral roots, a phenomenon that may be explained in part by the larger surface area for exposure to LAs provided by the bundled dorsal roots.
At the cervical level, the first pair of spinal nerves exits between the skull and C1. Subsequent cervical nerves continue to exit above the corresponding vertebra, assuming the name of the vertebra immediately following them. However, a transition occurs between the seventh cervical and first thoracic vertebrae, where an eighth pair of cervical nerves exits; thereafter, the spinal nerves exit below the corresponding vertebra and take the name of the vertebra immediately above.
The spinal nerves divide into the anterior and posterior primary rami soon after they exit the intervertebral foramina. The anterior (ventral) rami supply the ventrolateral side of the trunk, structures of the body wall, and the limbs. The posterior (dorsal) primary rami innervate specific regions of the skin that resemble horizontal bands extending from the origin of each pair of spinal nerves, called dermatomes, and the muscles of the back. Clinically, knowledge of dermatomes is essential when planning anesthetics to specific cutaneous regions (Figure 9), although anesthesia may not be conferred reliably to the underlying viscera due to a separate innervation, and there is significant overlap in spinal nerve innervation of adjacent dermatomes (Table 18).
TABLE 18. Surface landmark correlation to dermatomal level.
| Level of block | Anatomic Landmark |
|---|---|
| C6 | Thumb |
| C8 | Fifth finger |
| T1 | Inner aspect of arm |
| T4 | Nipple |
| T6 | Xiphoid process |
| T10 | Umbilicus |
| T12 | Inguinal ligament |
| S1 | Lateral aspect of foot |
| S2-S4 | Perineum |
An intricate relationship exists between the spinal nerves and the autonomic nervous system (Figure 10). Preganglionic sympathetic nerve fibers originate in the spinal cord from T1 to L2 and are blocked to varying degrees during epidural anesthesia.
They exit the spinal cord with spinal nerves and form the sympathetic chain, which extends the entire length of the spinal column on the anterolateral aspects of the vertebral bodies. The chain gives rise to the stellate ganglion, splanchnic nerves, and the celiac plexus, among other things. There are potential benefits and marked drawbacks to epidural block of the sympathetic nervous system. TEA appears to increase GI mobility by blocking the sympathetic supply to the inferior mesenteric ganglia, thereby reducing the incidence of postoperative ileus. Epidural anesthesia may also nerve block the systemic stress response to surgery, in part by block of the sympathetic nervous system. However, mid- to low-thoracic sympathetic block may be associated with dilation of the splanchnic vascular beds, a marked increase in venous capacitance, a decrease in preload to the right heart, and many of the other undesirable effects (see Physiologic Effects of Epidural block).
Cranial and sacral components comprise the parasympathetic nervous system. The vagus nerve, in particular, provides parasympathetic innervation to a broad area, including the head, neck, the thoracic organs and parts of the digestive tract. Parasympathetic innervation of the bladder, the descending large intestine, and the rectum originate at spinal cord levels S2 to S4.
Spinal Meninges
Spinal meninges cover the cord and nerve roots and are continuous with the cranial meninges that surround and protect the brain (Figure 11). The tough, predominantly collagenous outermost layer, the dura mater, encloses the CNS and provides localized points of attachment to the skull, sacrum, and vertebrae to anchor the spinal cord within the vertebral canal. Cranially, the spinal dura mater fuses with periosteum at the level of the foramen magnum; caudally, it fuses with elements of the filum terminale and contributes to formation of the coccygeal ligament; laterally, the dura mater surrounds nerve roots as they exit the intervertebral foramina. The dura mater touches the spinal canal in areas, but does not adhere to it except in pathologic conditions. It also confers both permeability and mechanical resistance to the dural sac, which terminates at S1 to S2 in adults and S3 to S4 in babies. The spinal nerve root cuffs, which have been postulated to play a role in the uptake of epidurally administered LAs, are lateral projections of both the dura mater and the underlying arachnoid lamina.
The flexible arachnoid mater, the middle meningeal layer, is loosely attached to the inner aspect of the dura and encloses the spinal cord and surrounding CSF within the subarachnoid space. It is composed of layers of epithelial-like cells connected by tight and occluding junctions, which impart its low permeability.
The cell layers of the arachnoid mater are oriented parallel to the long axis of the spinal cord (cephalocaudad), a finding that has led some investigators to claim that the architecture of the arachnoid mater, rather than the dura mater, accounts for the difference in headache rates between perpendicular and parallel insertions of beveled spinal needles. By virtue of its flexibility, the arachnoid mater may “tent” and resist puncture by an advancing needle during initiation of spinal or CSE anesthesia. A discontinuous subarachnoid septum (septum posticum) that stretches from the posterior spinal cord to the arachnoid may contribute to irregular spread of LAs in the subarachnoid space.
The innermost meningeal layer, the pia mater, closely invests the underlying spinal cord and its blood vessels, as well as nerve roots and blood vessels in the subarachnoid space, and appears to have fenestrated areas that may influence the transfer of LAs during subarachnoid nerve blocks. Caudally, the pia mater continues
from the inferior tip of the conus medullaris as the filum terminale and fuses into the sacrococcygeal ligament.
It is possible that a cavity can be created at the arachnoid-dura interface that may explain patchy or failed epidural nerve blocks with higher-than-expected cephalad spread (so-called subdural nerve blocks). Early research suggested that the subdural extra-arachnoid space comprised a true potential space, with serous fluid
that permitted movement of the dura and arachnoid layers alongside each other. Blomberg used spinaloscopy in cadaver studies to demonstrate its existence in up to 66% of humans.
However, recent evidence suggests that, unlike a potential space, this arachnoid-dura interface is an area prone to mechanical stress that shears open only after direct trauma, such as air or fluid injection. It is also possible that these clefts may actually occur between layers of arachnoid instead of between dural border cells at the arachnoid-dura interface. More information on spinal meninges and related structures are detailed in “Ultrastructural Anatomy of the Spinal Meninges and Related Structures“.
NYSORA Tips
Clefts may form at the arachnoid-dura interface as a result of mechanical stress and direct trauma. Injection of a large volume of LA intended for the epidural space in this area may result in a subdural nerve block.
Blood Supply
Vertebral and segmental arteries supply the spinal cord. A single anterior spinal artery and two posterior spinal arteries, and their offshoots, arise from the vertebral arteries and supply the anterior two-thirds of the spinal cord and the remainder of the cord, respectively (Figure 12). The anterior artery is thin at the midthoracic level of the spinal cord, an area that also has limited collateral blood supply. Segmental arteries, which emerge from branches of the cervical and iliac arteries, among others, spread along the entire length of the spinal cord and anastomose with the anterior and posterior arteries. The artery of Adamkiewicz is among the largest segmental arteries and is most commonly unilateral, arising from the left side of the aorta between T8 and L1. With regard to the venous system, anterior and posterior spinal veins, which anastomose with the internal vertebral plexus in the epidural space, drain into the azygos, the hemiazygos, and internal iliac veins, among other segmental veins, via intervertebral veins. The internal vertebral venous plexus consists of two anterior and two posterior longitudinal vessels with a variable distribution and is postulated to be involved in bloody or traumatic epidural needle and catheter placements.
Epidural Space
The epidural space surrounds the dura mater circumferentially and extends from the foramen magnum to the sacrococcygeal ligament. The space is bound posteriorly by the ligamentum flavum, laterally by the pedicles and the intervertebral foramina, and anteriorly by the posterior longitudinal ligament. Of the three epidural space compartments (posterior, lateral, and anterior), the posterior epidural space is most relevant clinically. The epidural space in general contains adipose tissue, blood vessels, nerve roots, and loose connective tissue in a nonuniform distribution. The veins in the space are continuous with the iliac vessels in the pelvis and the azygos system in the abdominal and thoracic body walls. Because the plexus is valveless, blood from any of the connected systems can flow into the epidural vessels.
In contrast to traditional dogma, these vessels are located primarily in the anterior epidural space, where they are largely confined by the membranous extension of the posterior longitudinal ligament106 (Figure 13). This area is probably a common site of epidural catheter blood vessel puncture. Also of clinical significance, the subatmospheric pressure of the epidural space diminishes significantly in the lumbar region, potentially affecting both the hanging-drop and the epidural pressure waveform techniques of identification of the epidural space.
The contents of the epidural space and their clinical implications have been debated extensively in the literature. The amount of adipose tissue in the epidural space appears to affect the spread of LA, but it remains unclear whether epidural fat prolongs nerve block duration by serving as a reservoir or decreases the amount of available drug, thereby slowing onset, or both. The reduction of adipose tissue with age is speculated to account in part for the higher levels and faster onset of epidural anesthesia in the elderly.
Similarly, the increase in adipose tissue in the lower lumbar area where the dural sac tapers may contribute to the variable effects of LA injections below L4–L5. Finally, adipose tissue in the midline gap, where the ligamentum flava fuse, may alter the tactile sensation that is normally appreciated during the LOR technique.
Another anatomic controversy of the epidural space concerns whether septae, alternately described as sparse strands and as a continuous membrane that attaches the dura to the ligamentum flavum, obstruct catheter advancement, affect the spread and onset of LAs, and contribute to unilateral nerve blocks and unintentional dural punctures. However, these septae have more recently been identified as an artifact of the midline posterior epidural fat pad. These fatty midline attachments do not appear to have a clinically significant effect on the spread of LAs. Rather, Hogan has postulated that the distribution of solution is nonuniform and directed among paths between structures in the epidural space according to differential pressures.
Distance From Skin to Epidural Space
The distance from the skin to the epidural space varies at different levels of the vertebral column. In the cervical region, Han and colleagues found that the average skin-to-epidural space depth (via the midline approach) was shallowest at C5 and C6 and increased in the caudal direction. Fujinaka et al noted that it is difficult to predict the actual depth of the cervical epidural space based on clinical characteristics. In contrast, Aldrete and coworkers, using magnetic resonance imaging (MRI) to measure the depth from the skin to the inner ligamentum flavum, noted the greatest depth at the C6-to-T1 levels, with a mean of 5.7 cm, possibly due to the presence of fatty tissue (the so-called hump pad) in the area. The depth to space in the midthoracic region from midline is influenced primarily by the sharp caudal angle of the spinous processes. As a result of the steep angle and bony impediments in this region, the paramedian approach is often preferred for midthoracic epidural placement. Several studies have sought to measure the depth to the epidural space at the lumbar level. Studies of parturients show a range of depth from skin to space of 2 to 9 cm, with 89% in the range of 3.5–7.5 cm. In their search for a multivariate model to predict the distance in an obstetric population, Segal and colleagues confirmed previously reported associations between increased weight and increased depth, as well as between oriental race and shallower spaces, with no independent association between race and depth after controlling for weight. In an earlier study, Sutton and Linter recorded that the skin to extradural space in 3011 parturients was 4 to 6 cm in 76% of the study participants. Patients with a shallow depth of 2 to 4 cm, comprising 16% of the study population, were found to be at a threefold higher risk of unintentional dural puncture. Of note, the shallow depth falls within the range of length of the LA infiltration needle. Overall, estimates of the depth to epidural space cannot be applied to the population at large, as independent variables, such as degree of flexion, patient positioning, dimpling and edema at the skin and subcutaneous tissue, and the angle of needle insertion, among other things, are difficult to quantitate and control. In the near future, routine ultrasound determination of depth to space on an individual basis prior to or during epidural needle placement might provide the most reliable means of diminishing the risk of inadvertent dural puncture and other complications of epidural anesthesia. Fluoroscopy is most appropriate in the cervical region, where spinal cord injury, total spinal anesthesia, and intra-arterial injection are among the possible complications.
The variable depth of the posterior epidural space is another clinically relevant measure that may influence the incidence of inadvertent dural puncture. The posterior epidural space viewed in the midline sagittal plane has been described as sawtoothed, characterizing its segmented shape. While studies are conflicting, at each segmental level, the depth of the posterior epidural space appears shallower at the caudal end. These variations notwithstanding, the distance between the ligamentum flavum and the dura is typically estimated as 7 mm, with a broad range from 2 mm to 2.5 cm. This anterior-posterior distance is largest in the lumbar region, at L3–L4, decreases in the thoracic region, and is absent in the cervical region.
PHYSIOLOGIC EFFECTS OF EPIDURAL block
Epidural block provides surgical anesthesia, intraoperative muscle relaxation, and intrapartum and postoperative pain relief with widespread direct and indirect effects on several physiologic systems. The extent of these physiologic effects depends on the level of placement and the number of spinal segments blocked. In general, high thoracic epidural nerve blocks (ie, above T5) and extensive epidural nerve blocks are associated with more profound physiologic changes than nerve blocks with low sensory levels (ie, below T10). This section reviews the physiologic alterations related to epidural anesthesia and analgesia.
Differential block
Differential block occurs when sensory, motor, and sympathetic nerve functions are obtunded at different rates and to different degrees. It may be observed at both onset and regression of the nerve block. In general, sympathetic block, which is not uncommonly incomplete, extends two to six dermatomes higher than sensory block, which in turn is higher than the motor block. Sensory block also occurs with a lower concentration or total dose of LA and develops faster than motor block. Among sensory functions, temperature is blocked first, followed by pinprick and, finally, touch.
Although the mechanism of differential block has not been fully elucidated, it may be attributed to anatomic features of blocked nerves (eg, diameter and presence or absence of myelin), the length of blocked nervous tissue (a minimal length of blocked nerve is required for effective neuronal block), differences in nerve lipid membrane and ion channel composition, concurrent axonal activity during nerve block onset, and LA type and concentration. These and several other mechanisms may collectively contribute to differential block.
Central Nervous System Effects
Cerebral blood flow (CBF) is autoregulated and is not affected by epidural block unless the patient experiences pronounced hypotension. However, neuraxial anesthesia does appear to have a sedative effect and to reduce anesthetic requirements for several agents, including midazolam, propofol, thiopental, fentanyl, and volatile agents. The degree of sedation and minimum alveolar concentration (MAC) sparing effect appear to correlate with the height and level of the sensory nerve block; block of the middle thoracic dermatomes is associated with greater sedative effects than block of the lower lumbar segments. Although data are conflicting, higher-concentration LAs may contribute to a greater MAC-sparing effect. The addition of opioid adjuvants, such as morphine, to the epidural LA solution does not appear to reduce volatile agent requirements any further, although it does contribute to better postoperative pain scores. Overall, decreased anesthetic requirements have most commonly been attributed to decreased afferent input induced by the neuraxial nerve block rather than to systemic effects of LAs, altered pharmacokinetics, or direct action of LAs on the brain.
Several studies have demonstrated reduced hypnotic and anesthetic requirements after central neuraxial block. In an early study of 53 American Society of Anesthesiologists (ASA) physical status I and II adult males, Tverskoy and colleagues determined that subarachnoid bupivacaine block decreased hypnotic requirements for both midazolam and thiopental. A study that followed, also in ASA physical status I and II patients, determined that epidural bupivacaine profoundly decreased midazolam hypnotic requirements. Similarly, in a small prospective, randomized, double-blind, placebo-controlled trial, Hodgson and colleagues found that lidocaine epidural anesthesia reduced the MAC of sevoflurane by up to 50%. More recently, epidural bupivacaine administered via the caudal route has been shown to have a sparing effect on both intravenous fentanyl and sevoflurane requirements during orthopedic surgery in children.
Cardiovascular and Hemodynamic Effects
Cardiovascular changes associated with epidural anesthesia and analgesia result primarily from block of sympathetic nerve fiber conduction. These changes include venous and arterial vasodilation, reduced SVR, changes in chronotropy and inotropy, and associated alterations in blood pressure and CO. The type and intensity of these changes are related to the level of nerve block, the total number of dermatomes blocked, and, relatedly, the type and dose of LA administered. In general, lumbar epidural or low thoracic nerve blocks are not associated with significant hemodynamic changes, while higher thoracic nerve blocks (particularly those involving the T1–T4 sympathetic fibers) can cause more marked changes, not all of which are detrimental. However, factors such as pregnancy, age, comorbidities, patient positioning, and hypovolemia can complicate the clinical scenario and the anticipated cardiovascular effects.
Hypotension
Hypotension associated with neuraxial block results primarily from vasodilation and increased vascular bed capacitance. Both direct inhibition of the sympathetic outflow to the nerves innervating the blood vessels and a decrease in endogenous catecholamine release from the adrenal glands contribute to arterial and venous vasodilation. In general, arteriolar smooth muscle maintains autonomous tone, even in the setting of complete sympathectomy, while veins and venules dilate maximally. However, a degree of arteriolar vasodilation does occur. The venodilatory effect also predominates because of the large amount of blood in the venous system compared to the arterial system.
The degree of hypotension associated with epidural block correlates with the sensory level. For example, a more marked increase in venous capacitance occurs with block of the sympathetic outflow to the splanchnic veins (T6 to L1) due to dilation of the extensive splanchnic bed. With low epidural nerve blocks, vasoconstriction of unblocked areas and release of catecholamines from the adrenal medullary system partially compensate for venous and arteriolar pooling and reductions in mean arterial pressure. Overall, healthy, normovolemic patients experience a nominal decrease in peripheral resistance and blood pressure during initiation and maintenance of epidural block. Risk factors for appreciable hypotension during neuraxial anesthesia include sensory level above T5, low baseline pressure, increasing age, and combined general-neuraxial anesthesia.
Severely hypovolemic patients and cardiac-compromised patients are also more likely to experience significant hypotension requiring vasopressor and inotropic support. Hypotension occurs more commonly with spinals than with epidurals, despite equivalent degrees of sympathetic block.
Heart Rate and Cardiac Function
In general, changes in heart rate and ventricular function vary with level of block, with more pronounced changes as the level increases. When the cardiac sympathetic fibers from T1 to T4 are blocked, decreased cardiac contractility and bradycardia ensue, resulting in decreased CO. Bradycardia also results from the decreased atrial stretch receptor activity attributed to decreased right atrial pressure. Venous pooling also contributes to the reduction in CO, particularly with higher nerve blocks. Missant et al studied the effects of epidural anesthesia on left and right ventricular function in a pig model and found that lumbar epidural anesthesia reduced SVR without affecting left or right ventricular function. However, TEA reduced left ventricular contractility and minimally reduced SVR, while preserving right ventricular function.
Neuraxial block appears to have certain beneficial effects on the cardiovascular system, such as improved myocardial blood flow and myocardial oxygen balance. Tissue oxygenation has been observed to improve with high TEA under certain circumstances, particularly with intravenous fluid administration.
TEA also appears to have antianginal effects improve coronary perfusion, and improve recovery from reversible myocardial ischemia. Whether this results in improved perioperative cardiac outcome following major cardiac or thoracic surgery, however, is the subject of ongoing debate. Several authors have hypothesized that TEA may also protect against postoperative arrhythmias and atrial fibrillation after major cardiac and thoracic surgeries. However, data are conflicting. Svircevic et al performed a meta-analysis comparing GA and TEA for cardiac surgery and noted fewer postoperative supraventricular arrhythmias However, Gu et al, in another recent metaanalysis, could not support such an effect.
Pulmonary Effects
The motor and sympathetic changes associated with epidural anesthesia may affect lung function, depending on the level of block. In general, tidal volume remains unchanged even during high neuraxial nerve blocks, while vital capacity may be reduced due to the decrease in expiratory reserve volume that occurs as accessory muscles involved in expiration are blocked.
The ability to cough and clear respiratory secretions may also be impaired, particularly in patients with severely compromised respiratory function at baseline. However, inspiratory muscle function is unaffected and should remain sufficient to provide adequate ventilatory function.
Higher sensory levels may result in more marked changes in lung function. In a sentinel study, Freund et al inserted a lumbar epidural catheter and administered a mean volume of 20 mL of 2% lidocaine. An extensive nerve block to T4 was achieved, but the decrease in vital capacity was minimal. However, catheter insertion at higher levels, with concomitant higher spread of LA, results in more pronounced pulmonary derangement.
In contrast, when TEA is used postoperatively, a net positive effect on lung function can be observed, most likely because the enhanced pain relief prevents splinting. In a recent review article, Lirk and Hollmann determined the role of TEA and confirmed the benefits in major abdominal and thoracic surgery.
The rare occurrence of respiratory arrest after high epidural or spinal block can be attributed to hypoperfusion of the respiratory center in the brainstem rather than to direct LA effects on either the phrenic nerve or the CNS.
Gastrointestinal Effects
The sympathetic outflow to the GI tract arises from T5 to T12, while parasympathetic innervation is supplied by the vagus nerve. Sympathectomy associated with epidural block in the mid- to low-thoracic levels results in unopposed vagal tone, which manifests clinically with increased peristalsis, relaxed sphincters, an increase in GI secretions, and, likely, more rapid restoration of GI motility in the postoperative phase. Nausea and vomiting commonly accompany hyperperistalsis and can be treated effectively with intravenous atropine. Theoretically, increased intestinal motility could contribute to breakdown of surgical anastomoses, but this has not been demonstrated in the literature. Rather, TEA may decrease the risk of anastomotic leakage and improve perioperative intestinal perfusion, although the data are somewhat conflicting. Numerous experimental and clinical studies have demonstrated that TEA protects against splanchnic hypoperfusion and reduces postoperative ileus. However, similar benefits are not seen with lumbar epidural anesthesia.
Renal/Genitourinary Effects
Because renal blood flow (RBF) is maintained through autoregulation, epidural anesthesia has little effect on renal function in healthy individuals. Compensatory and feedback mechanisms (afferent arteriolar dilation and efferent arteriolar vasoconstriction) ensure constant RBF over a broad range of pressures (50–150 mHg). During transient periods of hypotension below 50 mm Hg, oxygen delivery to the kidneys is adequately maintained.
Neuraxial block at the lumbar level has been postulated to impair control of bladder function secondary to block of the S2–S4 nerve roots, which carry the sympathetic and parasympathetic nerves that innervate the bladder. Urinary retention may occur until the nerve block wears off. The clinician should avoid administering an excessive volume of intravenous fluids if a urinary catheter is not in place.
Neuroendocrine Effects
Surgical stress produces a variety of changes in the host’s humoral and immune response. Increased protein catabolism and oxygen consumption are common. Increased plasma concentrations of catecholamines, vasopressin, growth hormone, renin, angiotensin, cortisol, glucose, antidiuretic hormone, and thyroid-stimulating hormone have been documented after sympathetic stimulation associated with both minimally invasive and major open surgery. Perioperative manifestations of the surgical stress response may include HTN, tachycardia, hyperglycemia, suppressed immune function, and altered renal function. Increased catecholamine levels can also cause increased left ventricular afterload and, in combination with other pathologic responses to stress (eg, proinflammatory responses that may lead to plaque instability via activation of matrix metalloproteinase; raised corticotropin-releasing hormone levels that reduce cardiac nitric oxide release, increase endothelin production, and aggravate coronary endothelial dysfunction), trigger acute coronary syndromes and myocardial infarctions in patients with coexisting cardiac disease. Afferent sensory information from the surgical site is thought to play a pivotal role in this response.
The surgical stress response can be influenced by sympathetic block during epidural anesthesia and analgesia. The mechanisms involved are unresolved but most likely include both direct block of afferent and efferent signals during surgical stress and direct effects of LA agents. Brodner et al demonstrated that TEA combined with GA resulted in a reduced surgical stress response when compared to GA alone.
The most critical effect of neuroendocrine activation in the perioperative period is the increase in plasma norepinephrine, which peaks roughly 18 hours after the surgical stimulus is initiated. The increase in plasma norepinephrine is associated with activation of nitric oxide in the endothelium of patients with atherosclerotic disease, producing paradoxical vasospasm. Thus, in patients with significant atherosclerotic disease, the combination of vasospasm and a hypercoagulable state may be the factors modulated by the cardioprotective effects of TEA. Indeed, studies indicated that coronary artery blood flow is improved with TEA.
Thermoregulation
Hypothermia has significant side effects, such as increased cardiac morbidity, impaired coagulation, increased blood loss, and increased risk for infection. The rate and severity of hypothermia associated with epidural anesthesia is similar to that observed during cases under GA. Hypothermia associated with neuraxial anesthesia is primarily due to peripheral vasodilation resulting in heat redistribution from the core to the periphery. In addition, reduced heat production (due to reduced metabolic activity) results in a negative heat balance due to unchanged heat loss. Finally, thermoregulatory control is impaired. Of note, rewarming with forced air warming devices occurs more rapidly with neuraxial anesthesia as compared to GA due to peripheral vasodilation.
Coagulation System
The postoperative period is a marked hypercoagulable state. Neuraxial block is associated with a decreased risk of DVT and pulmonary embolism, as well as a decreased risk of arterial and venous thrombosis.
PHARMACOLOGY OF EPIDURAL block
An understanding of the physiology of nerve conduction and the pharmacology of LAs is essential for successful epidural block. Potency and duration of LAs, preferential block of sensory and motor fibers, and the anticipated duration of surgery or need for postoperative analgesia are factors that should be considered before initiating epidural block. This section covers several practical aspects of attaining effective epidural anesthesia and analgesia.
Epidural solutions may contain an LA with or without an adjuvant drug. Dose, volume, and concentration, as well as site of injection, of the LA solution vary, resulting in different pharmacodynamic effects. A, B, and C nerve fibers vary in size and in the presence of a myelin sheath. A-delta and C fibers are responsible for temperature and pain transmission. B fibers are autonomic fibers. The larger A fibers (especially A-alpha fibers) are motor fibers. C fibers are unmyelinated and smallest in size. Because they lack a protective myelin sheath and diffusion barrier, they are blocked rapidly. A and B fibers are myelinated and larger in size than C fibers. B fibers are responsible for autonomic nervous system transmission. They are smaller in size than A-delta fibers, but larger than C fibers. It is widely accepted that autonomic fibers are more susceptible to LA nerve block than sensory fibers. Epidurally administered LA preferentially nerve blocks sympathetic neural function; this explains the more extensive sympathetic dermatomal block when compared with sensory and motor nerve blocks. However, Ginosar et al recently suggested that sensory function was more susceptible to block than sympathetic function. Several other studies concurred. The dose and concentration of LA used may account for the different findings in these studies. Because of their thick myelin sheath, motor fibers require much more LA and much more time before an adequate nerve block is achieved.
Local anesthetics produce reversible nerve block by blocking sodium passage through the nerve membrane. When LA is injected into the epidural space, several things occur. Most of the injected LA is absorbed into the venous blood, and a large part is retained in epidural fatty tissue. The primary sites of action of an epidurally administered LA are the ventral and dorsal nerve roots that pass through the epidural space. However, based on studies using labeled LAs, LAs can cross the dura and penetrate the spinal cord, but to a lesser extent than their penetration into the spinal nerve roots. The segmental nerve roots are mixed sensory, motor, and sympathetic nerve fibers. Hence, all three types of fibers will be affected (to varying degrees).
Choice of Local Anesthetics
Drugs used for epidural block can be categorized into short-, intermediate-, and long-acting LAs. Onset of epidural block in the dermatomes immediately surrounding the site of injection can usually be detected within 5 or 10 minutes, if not sooner. The time to peak effect varies with the type of LA and the dose/volume administered (Table 19).
TABLE 19. Commonly used local anesthetics for epidural anesthesia and analgesia.
| Drug | Concentration (%) | Onset Time (min) | Duration (min) |
|---|---|---|---|
| 2-Chloroprocaine | 3 | 5–15 | 30–90 |
| Lidocaine | 2 | 10–20 | 60–120 |
| Bupivacaine | 0.0625–0.5 | 15–20 | 160–220 |
| Ropivacaine | 0.1–0.75 | 15–20 | 140–220 |
| Levobupivacaine | 0.0625–0.5 | 15–20 | 150–225 |
The shortest-acting LA for neuraxial block is chloroprocaine, an ester. In the past, chloroprocaine was associated with adhesive arachnoiditis when large volumes were accidentally administered into the subarachnoid space. In addition, severe back pain was not uncommonly reported when large volumes were administered in the epidural space, most likely due to the ethylenediaminetetraacetic acid (EDTA) and bisulfite preservatives in the solution. Since 1996, preservative-free chloroprocaine has been available and has not been associated with either neurotoxic effects or back pain. In ambulatory settings and for emergency cesarean deliveries with in situ epidurals, chloroprocaine can provide excellent surgical anesthesia quickly, without delaying recovery room discharge.
Delivered via the epidural route, 2% lidocaine is an intermediate-acting LA commonly used for surgical anesthesia. When epinephrine is added to the solution (1:200,000), it prolongs the duration of action by up to 60%.
Long-acting LAs used for epidural block are bupivacaine, levobupivacaine (no longer available in the United States), and ropivacaine. Dilute concentrations (eg, 0.1% to 0.25%) can be used for analgesia, while higher concentrations (eg, 0.5%) may be more appropriate for surgical anesthesia. The addition of epinephrine to these solutions can prolong the duration of action, although this effect is less reliable with long- versus intermediateacting agents. Severe cardiotoxic reactions (hypotension, atrioventricular nerve block, ventricular fibrillation, and torsades de pointes) refractory to usual resuscitation methods can result from accidental intravascular injection of bupivacaine. The rationale for the resistance to resuscitative measures lies in its high degree of protein binding and more pronounced effect on cardiac sodium channel block. Levobupivacaine, the S-enantiomer of bupivacaine, has a similar profile to bupivacaine but with lesspronounced cardiotoxic effects. Ropivacaine, a mepivacaine analogue, has a similar profile of action to bupivacaine. In most studies, ropivacaine has demonstrated a slightly shorter duration of action than bupivacaine, potentially with a less-dense motor nerve block at equipotent doses. A deterrent to the broader use of ropivacaine in clinical practice is its higher cost.
Onset and Duration of Local Anesthetics
Alkalinization of the LAs, which are marketed in a water-soluble, ionized state, hastens onset. By increasing the concentration of the nonionized form, more lipid-soluble LA is available to penetrate the neural sheath and nerve membrane. Adding sodium bicarbonate immediately before injection of lidocaine, mepivacaine, or chloroprocaine produces a clinically significant faster onset of anesthesia and may also contribute to a denser nerve block. However, ropivacaine and bupivacaine will precipitate with the addition of bicarbonate unless a very low concentration is used. Combining short- and long-acting drugs for rapid onset and a prolonged sensory nerve block has not been proven to be effective. For example, mixing 2-chloroprocaine with bupivacaine for the rapid onset of the former and long duration of the latter results in shortening the duration and effectiveness of the bupivacaine.160 Continuous drug administration and the use of additives obviate the need for mixing LAs.
NYSORA Tips
Combining short- and intermediate- or long-acting LAs for rapid onset with prolonged duration of action has not been proven to be effective. Continuous drug
administration and the use of additives obviate the need for mixing LAs.
Adding epinephrine to certain LAs can increase the duration of action, most likely by decreasing vascular absorption. The effect is greatest with 2-chloroprocaine, lidocaine, and mepivacaine and is less effective with the longer-acting agents. Other vasoconstrictors, such as phenylephrine, have not been proven to be as effective in reducing the peak blood levels of LAs as epinephrine.
Adjuvants to Local Anesthetics in the Epidural Space
A variety of other classes of drugs have been studied more recently to try to improve the quality of neuraxial block. In addition to several opioids (eg, fentanyl, sufentanil, and preparations of morphine); α-adrenergic agonists; cholinesterase inhibitors; semisynthetic opioid agonist-antagonists; ketamine; and midazolam have been studied, with mixed results. The administration of clonidine in the epidural space has been studied extensively. An α2-adrenergic agonist, clonidine appears to prolong the duration of action of LAs, although the mechanism remains unclear. Animal studies have shown that clonidine reduces regional spinal cord blood flow, therefore slowing the rate of drug elimination. Kroin and colleagues demonstrated that the mechanism by which clonidine prolongs the duration of a nerve block when mixed with LAs is not mediated by α-adrenoreceptors; rather, it is more likely related to the hyperpolarization-activated cation current Ih.
Some of the potential benefits of the administration of clonidine in the epidural space may include the following:
1. Prolongation and enhancement of the effects of epidural LAs without an additional risk of hypotension
2. Reduction in LA dose requirements for labor epidural analgesia
3. Effective analgesia without motor impairment
4. Synergistic effect with opioids and opioid agonist-antagonists
5. Modulation of the stress response to thoracic surgery
6. Preservation of lung function after thoracotomy
7. Possible reduction in cytokine response, further reducing pain sensitivity
Side effects that are commonly associated with epidural clonidine include dose-independent hypotension, bradycardia, sedation, and dry mouth. Combining clonidine with other agents, such as opioids, anticholinergics, opioid agonist-antagonists, and ketamine, may enhance the beneficial effects of these drugs while minimizing adverse side effects.
Neostigmine, a cholinesterase inhibitor, is a more recent addition to the list of epidural additives for selective analgesia. The mechanism of action for its analgesic effect appears to be the inhibition of the breakdown of acetylcholine and the indirect stimulation of muscarinic and nicotinic receptors in the spinal cord. Although experience with epidural neostigmine is limited, it has been reported to provide postoperative pain relief without inducing respiratory depression, motor impairment, or hypotension. When combined with other opioids, clonidine, and LAs, it may provide benefits similar to clonidine without the side-effect profile of any of these drugs given alone. Observations in patients with cancer pain showed promise that its use might be associated with less nausea and vomiting than the intrathecal application. In an investigation randomizing 48 patients to receive 0, 1, 2, or 4 μg/kg of epidural neostigmine in addition to a bupivacaine spinal anesthetic for minor knee surgery, no case of intraoperative nausea or vomiting was observed, and postoperative nausea scores did not differ between groups. These results need to be corroborated by further studies before epidural neostigmine can be recommended for daily practice.
Other agents, such as ketamine, tramadol, droperidol, and midazolam, have been considered for epidural administration, with mixed results. Considerable controversy surrounds the use of midazolam intrathecally. Despite multiple publications recommending its use, recent studies have demonstrated that even a single dose of intrathecal midazolam may have neurotoxic effects. Until its safety profile can be ensured in human subjects, it is not recommended for neuraxial use at this time.
One agent that shows promise is the extended-release formulation of one of the oldest opioids, morphine. DepoDur, the brand name for extended-release epidural morphine, uses a drug-release delivery system called DepoFoam. DepoFoam is composed of microscopic lipid-based particles with internal vesicles that contain the active drug and slowly release it. Recent studies have demonstrated effective pain relief with relatively minor side effects for up to 48 hours when appropriately dosed. However, concerns about delayed respiratory depression have limited its clinical use in this early stage of its clinical use.
Other Factors Affecting Epidural block Injection Site
The epidural block is most effective when the nerve block or the catheter is inserted in a location that corresponds to the dermatomes covered by the surgical incision. The most rapid onset and the densest nerve block occur at the site of injection. By inserting the catheter closer to the dermatomal distribution of the surgical site, a lower dose of drug can be given, thereby reducing side effects. This concept is especially important when thoracic epidural analgesia is used for postoperative analgesia.
After lumbar epidural injection, the analgesic and anesthetic effects spread to a greater degree cranially then caudally. Of note, there is a delay in onset of anesthesia at the L5–S1 segments secondary to the larger size of these nerve roots. With thoracic injection, the LA spreads evenly from the site of injection, but meets resistance to block in the lumbar region because of the larger nerve roots. By controlling the dose in the thoracic region, a true segmental block affecting only the thoracic region can be established. Lumbar and sacral regions will be spared, thereby avoiding more extensive sympathetic block and subsequent associated hypotension and bladder dysfunction, as well as lower limb motor block.
Dose, Volume, and Concentration
The dose of LAs necessary for epidural anesthesia or analgesia is a function of the concentration of the solution and the volume injected. Concentration of the drug affects the density of the nerve block; the higher the concentration, the more profound the motor and sensory nerve block. Lower concentrations can selectively produce a sensory nerve block.
Volume and total LA dose are the variables that affect the degree of spread of the nerve block. A larger volume of the same concentration of LA will nerve block a greater number of segments. However, if the total dose of LA is unchanged but the concentration is doubled, the volume can be halved to achieve similar spread of LA. A generally accepted guideline for dosing epidural anesthesia in adults is 1–2 mL per segment to be blocked. This guideline should be adjusted for shorter patients and for very tall patients. For example, to achieve a T10 sensory level from an L3–L4 injection, approximately 8 mL of LA should be administered. Below concentrations of the equivalent of 1% lidocaine, motor nerve block is minimal, regardless of the volume of the LA injected, unless doses are given at repeating intervals.
Time to repeat a dose of LAs depends on the duration of the drug. Doses should be administered before the nerve block regresses to the point the patient experiences pain, commonly referred to as “time to two-segment regression.” This is defined as the time it takes for the sensory nerve block to regress by two dermatome levels. When two-segment regression has occurred, one-third to one-half of the initial loading dose can safely be administered to maintain the nerve block. For example, the time to two-segment regression of lidocaine is 60–140 minutes (Table 20).
TABLE 20. Redosing local anesthetics.
| Drug | Concentration (%) | Time to Two-Segment Regression (min) | Recommended Time for “Top-Up” Dose From Initial Dose (min) |
|---|---|---|---|
| 2-Chloroprocaine | 3 | 45–75 | 45 |
| Lidocaine | 2 | 60–140 | 60 |
| Bupivacaine | 0.10 | 180–260 | 120 |
| Ropivacaine | 0.10 | 180–260 | 120 |
Patient Positioning
Patient positioning during initiation of epidural block does not appear to affect the resultant spread of analgesia or anesthesia. The patient may be placed in either the lateral or sitting position. The midline of the spine is easier to palpate when the patient is sitting, especially in the obese patient, therefore making the nerve block technically easier. Whether the patient is in the sitting or the lateral position, there is no significant difference in nerve block height. It has been suggested in a study by Seow and associates that there is slightly faster onset time, duration, and density of motor nerve block on the dependent side when the epidural is placed with the patient in the lateral position.
Patient Characteristics: Age, Weight, Height, and Pregnancy
With advancing age, the LA dose required to attain a specific nerve block is reduced. Some studies have observed a nonclinically significant difference in nerve block height (between one and four segments higher) with a fixed volume and concentration of LA in patients older than age 50. Greater spread in the elderly may be related to the reduced size of the intervertebral foramina, which theoretically limits the egress of LAs from the epidural space. Decreased epidural fat, which allows more of the drug to bathe the nerves, and changes in the compliance of the epidural space, which may lead to enhanced cephalad spread, have also been proposed.
There is little correlation between the spread of analgesia and the weight of the patient. However, in morbidly obese patients, there may be compression of the epidural space related to increased intra-abdominal pressure; a higher nerve block may be attained with a given dose of LA.
Height appears to play little role in LA requirements. For short patients (≤5 ft 2 in.), the common practice has been to reduce the dose to 1 mL per segment to be blocked (instead of 2 mL per segment). Bromage suggested a more precise dosing regimen of increasing the dose of LA by 0.1 mL per segment for every 2 in. above 5 ft of height. The safest practice is to use incremental dosing and monitor the effect to avoid excessively high anesthetic levels.
Pregnancy causes an increased sensitivity to both LAs and general anesthetics, although the studies regarding the causes are conflicting. Elevated levels of progesterone and endogenous endorphins may contribute. Conflicting evidence regarding the spread of LA in pregnant versus nonpregnant individuals has been published.
Intermittent Versus Continuous Epidural Nerve Block
The decision whether to use intermittent dosing after the initial loading dose, a continuous infusion, or patient-controlled or programmed intermittent bolus dosing may be influenced by the nature of the surgery or procedure, staffing, and equipment.
All of these options can provide safe and effective epidural analgesia or anesthesia. Advantages of continuous infusion include greater cardiovascular stability, fewer labor requirements, decreased incidence of tachyphylaxis, decreased frequency and severity of side effects related to bolus injections, less rostral spread, decreased risk of the potential for contamination, and the ability to achieve a steady state of anesthesia. Intermittent manual bolus dosing, on the other hand, is simple and does not require additional equipment (eg, infusion devices).
EPIDURAL TECHNIQUE
Several factors influence the success of epidural block, including the clinician’s experience and knowledge of anatomy, patient preparation and positioning, the level of epidural catheter insertion, and the technique used to initiate the procedure.
This section reviews factors that contribute to successful epidural placement, starting with patient selection and preparation, equipment requirements, and current recommendations for the prevention of infectious complications associated with neuraxial techniques. It then presents technical aspects of cervical, thoracic, and lumbar epidural placement and addresses various controversies related to the technique of neuraxial block, such as the optimal method to identify the epidural space and the efficacy of the epidural test dose.
Patient Evaluation
As in the case with any anesthetic, the risks and benefits of epidural placement should be discussed with the patient in a manner consistent with informed consent. Any concerns and questions should be addressed prior to the administration of premedication. When a language barrier exists, trained interpreters or telephone translation services should be utilized. The patient’s medical history and active medication list should be reviewed prior to the initiation of epidural block, with particular emphasis on the presence of conditions that may predispose the patient to serious complications. Drug therapy that influences the patient’s clotting function or physiologic response to block of the sympathetic preganglionic fibers should be taken into consideration, including when the last dose was administered. The patient’s last oral intake should also be documented. For those patients receiving epidural block as the sole anesthetic or as an adjuvant to GA for elective surgical procedures, the ASA guidelines for nothing by mouth should be enforced. Patients with medical conditions that worsen with reduced afterload or preload (eg, severe AS, mitral stenosis, hypertrophic cardiomyopathy) and patients who may experience worsening shortness of breath, such as those with restrictive lung disease or severe COPD, may require additional testing. Clinical conditions that predispose patients to neuraxial infections, such as immunosuppression, DM, pancreatitis, and alcohol or drug abuse, may require further evaluation or laboratory studies. Preexisting neurologic deficits or CNS disorders should be assessed and documented. History of sensitivity or adverse reaction to opioids or LAs and complications related to prior epidural procedures require further investigation.
Physical examination should include an evaluation of the spine for evidence of scoliosis or prior back surgery, focal infection, severely limited range of motion, or other findings that may make epidural placement more challenging or impossible. Obesity, especially central obesity, may obscure surface landmarks.
Routine laboratory studies are not required for epidural placement in healthy patients for routine procedures. Many clinicians may choose to obtain a complete blood cell count (CBC), particularly when appreciable blood loss is expected or when the patient is known to be anemic. Baseline assessment of the patient’s coagulation status or platelet count should be obtained in patients with known or suspected coagulation disorders, bleeding diatheses, and thrombocytopenia, as well as in patients receiving antithrombotic or thrombolytic therapy or any medications known to affect platelet quality or function (besides routine NSAIDs).
NYSORA Tips
• Routine laboratory studies are not required for initiation of epidural block in healthy patients for routine procedures.
• Patients with known or suspected bleeding disorders and those receiving antithrombotic or thrombolytic therapy require assessment of baseline coagulation status or platelet count (and possibly platelet function).
• Patients undergoing surgeries with anticipated blood loss or hemodynamic changes may require additional workup, including a CBC.
Preparation
A large-bore intravenous catheter for fluid or emergency drug administration must be secured prior to initiation of epidural block. Fluid preloading is not required and may be harmful in certain subsets of patients with decreased serum colloid oncotic pressure (eg, those with burns, preeclamptic patients).
However, reversible conditions, such as severe hypovolemia, should be managed prior to nerve block placement and dosing.
Appropriate monitoring during performance of epidural block depends on the purpose of the epidural nerve block and when and where the epidural is to be dosed. Epidural nerve blocks for analgesia, such as for labor analgesia, require intermittent blood pressure monitoring during placement and for the duration of the epidural infusion, as well as continuous pulse oximetry with heart rate monitoring during placement and nerve block initiation. Electrocardiogram (ECG) monitoring should be available. In laboring patients, fetal heart rate monitoring before and after placement is recommended if continuous monitoring is not feasible.
Sedatives or analgesics are not uncommonly administered to alleviate patient stress and discomfort during epidural placement and may require additional monitors and equipment, such as a nasal cannula. If premedications are administered, medical personnel who can provide continuous monitoring should be present. Of note, excessive sedation should be avoided to ensure patient cooperation during positioning, to detect the presence of paresthesias during placement, and to evaluate the level of sensory block and the effect of the test dose (if administered). Standard ASA monitors are required for initiation and intraoperative management of epidural anesthesia. Emergency drugs and equipment must be readily available during initiation of all central neuraxial procedures (Table 21).
TABLE 21. Emergency equipment and drugs for initiation of neuraxial block.
| Airway equipment | Ambu bag with mask |
| Oxygen source |
|
| Oral and nasal airways |
|
| Laryngoscope handles and blades |
|
| Endotracheal tubes |
|
| Eschmann stylet/bougie |
|
| Syringes and needles | |
| Emergency drugs | Ephedrine |
| Phenylephrine |
|
| Epinephrine |
|
| Atropine |
|
| Sedative/hypnotic |
|
| 20% lipid emulsion |
|
| Succinylcholine |
Communication With Surgical Staff
A discussion with the surgical staff regarding the operative approach, the desired positioning of the patient, the estimated length of the surgical procedure, the anesthetic or analgesic goals of the block, and postoperative analgesic requirements can help to determine whether a continuous epidural, a single-shot epidural, or a CSE is preferable. The surgical staff can also share information about the patient that is not readily available in the chart or immediately apparent during the preoperative interview.
When feasible, to minimize unnecessary delays the nerve block can be initiated in the preoperative area or in the operating room while the nursing staff is setting up the surgical equipment.
Wherever the nerve block is performed, sufficient space for the anesthesiologist and, optimally, an assistant, as well as adequate lighting, monitoring, and esuscitation equipment are essential.
Equipment
Commercially prepared, sterile, disposable epidural trays are available from several manufacturers. A standard kit typically includes the following: a sterile drape; prep swabs; 4 × 4 gauze sponges; a paper towel; povidone-iodine solution; an ampoule of 0.9% preservative-free sodium chloride; a 5-mL ampoule of 1.5% lidocaine with epinephrine 1:200,000; a 5-mL ampoule of 1% lidocaine for skin infiltration; a filtering device (needle or straw); a bacterial filter; needles and syringes of various sizes; a styletted epidural needle with cm markings; a 5- or 10-mL glass or plastic LOR syringe (either Luer lock or Luer slip); a catheter connector securing device; an epidural catheter with centimeter gradations and a connector/adapter; a thread assist device (TAD); a needle guard for sharps disposal; and labels.
In an adult epidural kit, the epidural needle is typically 17 or 18 gauge and 9 cm (roughly 3.5 in.) in length, with surface markings at 1-cm intervals. Longer needles up to 15 cm (6 in.) in length are available for obese patients. The Tuohy needle, which is commonly supplied in noncustom kits, has a curved tip with a blunt bevel designed to permit easier identification of tissue as the needle advances and facilitate passage of the epidural catheter. Wings at the junction of the needle shaft and hub may allow for better control as the needle is passed through tissue, particularly when using the “hanging drop” technique for epidural space identification, although some practitioners may prefer epidural needles without wings or with attachable wings (Figure 14).
Epidural needles with a back-eye opening for exit of a spinal needle (for CSEs) and double-lumen needles with separate openings for the spinal needle and catheter are also available.
Epidural catheters vary in diameter, materials, and tip design. In commercially prepared kits, 19-gauge catheters are usually paired with 17-gauge epidural needles; 20-gauge catheters are paired with 18-gauge needles. Many currently available epidural catheters are nylon blends with varying degrees of stiffness to facilitate threading. Some stiff nylon catheters have specially designed flexible tips intended to veer away from veins, nerves, and other obstacles encountered in the epidural space. Wire-reinforced catheters embedded in either a polyurethane or nylon-blend catheter represent a more recent technological advance and are becoming increasingly popular (Figure 15). Adult versions are 19 gauge in diameter and designed for use with a 17-gauge epidural needle; pediatric versions are available from some manufacturers.

Figure 15. Single end-hole wire-reinforced catheter. (Used with permission from Epimed International.)
Many commercially available nylon and wire-reinforced catheters are manufactured in both single end-hole and multiorifice versions (Figure 16). A lack of robust data precludes a full assessment of whether clinical outcomes, such as the incidence of paresthesias, epidural vein cannulation, intrathecal migration, and adequate analgesia, are improved with the uniport or multiport design. However, a 2009 prospective, single-blind, randomized controlled trial by Spiegel et al investigated the success of labor analgesia, the number of episodes of breakthrough pain requiring supplemental medicine, and the occurrence of complications, such as paresthesias and intravascular and intrathecal catheter placement, in 493 parturients who received either a single end-hole, wire-reinforced polyurethane catheter or a multiorifice, wire-reinforced nylon catheter. The authors found no statistically significant difference in outcomes between the two groups and postulated that the flexibility afforded by the wire coil may eliminate any of the potential advantages of the multiport design.

Figure 16. Multi-orifice wire-reinforced catheter. (Used with permission from Epimed International.)
NYSORA Tips
• The use of wire-reinforced epidural catheters appears to reduce the incidence of complications associated with epidural techniques, including epidural vein cannulation, paresthesias, and inadequate analgesia.
• Current data suggest that clinical outcomes are similar with the use of uniport and multiport spring-wound catheters; the flexibility afforded by the stainless steel coil appears to negate any potential benefits of a multiport design.
Additional equipment that may be needed for initiation of epidural procedures includes 0.5% chlorhexidine with ethanol (Hydrex®) or 2% chlorhexidine with 70% isopropyl alcohol (ChloraPrep®), which is not supplied in epidural trays; a transparent sterile, occlusive dressing for the puncture site; and tape to secure the catheter. To minimize the remote risk of chemical arachnoiditis, the skin disinfection solution should not make contact with the epidural drugs or equipment and should be given adequate time to dry. Usually a large clear dressing (eg, Tegaderm”) and adhesive tape are sufficient to prevent catheter dislodgement and to keep the epidural insertion site visible and clean. A sterile pen to label medications and a 25- or 27-gauge spinal needle (for CSEs) can be dropped onto the sterile field.
NYSORA Tips
• A clear sterile occlusive dressing is recommended to prevent catheter dislodgement.
• The catheter and its centimeter markings should be visible to the anesthesia provider to ensure that the catheter remains at the original insertion site and that CSF and heme return are absent prior to dosing.
Analgesia and Sedation During Nerve Block Initiation
Analgesia or sedation can be provided to improve patient comfort during neuraxial block. However, there is emerging evidence that intravenous sedatives may increase pain perception in an agent-type- and pain-type-specific manner. Light sedation with a benzodiazepine (most commonly midazolam) or a short-acting opioid prior to epidural placement is usually sufficient. This may also be appropriate for obstetric patients. In a small, double-blind randomized study, Frölich and colleagues found that maternal analgesia and sedation with fentanyl and midazolam prior to spinal placement was not associated with adverse neonatal effects. Importantly, mothers in both the group that received premedication and the control group showed no difference in their ability to recall the births of their babies.
For those who prefer to be “asleep” during epidural placement, a propofol infusion can be titrated to maintain sedation without respiratory impairment in selected clinical settings.
However, it is preferable to have adult patients awake and cooperative enough to alert the anesthesia provider to the presence of paresthesias during initiation of neuraxial block and to participate in assessment of the sensory level. In clinical scenarios in which the administration of premedication prior to epidural placement may not be appropriate, there appears to be a placebo effect from the use of gentler, more reassuring words during lidocaine skin wheal administration, which is often considered the most painful part of the procedure. Studies suggest that the following tips may also serve to reduce pain on injection of LA: Chloroprocaine (with or without sodium bicarbonate) may be less painful than lidocaine for skin infiltration; adjusting the pH of lidocaine to approximate physiologic pH reduces pain on injection; and cryoanalgesia (skin cooling) may be as effective as buffering the LA solution with sodium bicarbonate.
NYSORA Tips
The following tips may serve to reduce pain on injection of LA for skin infiltration:
• Communication with the patient during the procedure and verbal reassurance
• Adjusting the pH of lidocaine with the addition of sodium bicarbonate to more closely approximate physiologic pH
• Skin cooling (cryoanalgesia) or topical anesthetic before skin puncture
Patient Positioning
Optimal patient positioning is essential for successful epidural placement. Depending on the patient’s medical status (eg, body habitus and ability to cooperate), the planned procedure, the anesthesia provider’s experience, the baricity of the intrathecal solution (for CSE placement), and several other factors, the sitting, lateral decubitus, jackknife, or prone position can be used.
Each position has advantages and disadvantages. Regardless of which position is selected for the initiation of neuraxial procedures, it is useful to have an assistant to help maintain the position until the procedure is complete. Overall, while epidural nerve block can be initiated with the patient in any position that permits access to the back, improper positioning can turn an otherwise-easy epidural placement into a needlessly challenging one. Several positioning devices are available commercially to facilitate patient positioning without the aid of nursing personnel.
Sitting Position
In general, it is technically easier to identify the midline in the sitting position, particularly in obese and scoliotic patients. Anesthesia providers may also be more experienced and more comfortable performing neuraxial procedures in the sitting position. The sitting position has also been observed to provide the most direct route to the epidural space, with shorter distance from skin to space and, in the case of CSEs with dextrose-free LA and hypobaric intrathecal opioids, greater cephalad spread of the sensory nerve block. However, elderly patients, parturients in advanced stages of labor, patients with hip fractures, heavily sedated patients, and uncooperative patients may not be able to assume or maintain the sitting position (Table 22).
TABLE 22. Advantages of sitting position for
initiation of neuraxial block.
| Easier to identify midline, particularly in obese and scoliotic patients |
| Practitioners more experienced in sitting position |
| Shorter procedure time |
| Shorter distance from skin to epidural space |
| Greater cephalad spread of hypobaric solutions |
If the sitting position is chosen, the patient should be assisted to sit on the operating room table or bed with the backs of the knees touching the edge of the bed and the feet resting on a stool or hanging over the bed. The patient should relax the shoulders and curve the back out toward the clinician, assuming a “slouched” or “mad-cat” position. It is useful to have an assistant stand in front of the patient and help the patient attain maximal spinal flexion (Figure 17). Flexing the neck should help to flex the lower spine and open the vertebral spaces (Figure 18). Asking the patient to hug a pillow may also help with positioning.
Figure 17. A, B: Epidural placement in the sitting position with assistant helping to position the patient.
Lateral Decubitus Position
The lateral decubitus position may be more appropriate for patients who cannot comfortably assume the sitting position. Additional benefits include the following: Sedation can be used more liberally; vagal reflexes can be minimized; hemodynamic changes may be better tolerated; there may be less need for a well-trained assistant to help maintain positioning; and there appears to be a reduced incidence of unintentional epidural vein cannulation and dural puncture (Table 23). Finally, in the case of CSEs with hyperbaric LAs, unilateral nerve blocks for certain orthopedic procedures may be more easily attained in the lateral position.
TABLE 23.
Sedation can be used more liberally
Reduced patient movement
Increased patient comfort
Improved patient cooperation
Improved patient satisfaction
Reduced catheter displacement
Decreased incidence of epidural vein cannulation
Attenuation of vagal reflexes
Hemodynamic changes better tolerated
Bedside assistance may not be required
Intentional unilateral block for surgical procedures feasible
In the lateral decubitus position, the patient’s back should be fully aligned with the edge of the table or bed (Figure 19).
The left lateral recumbent position may be preferable for righthanded physicians and may provide improved hemodynamic stability for parturients. The coronal plane of the patient should be perpendicular to the floor, with the tips of the spinous processes pointing toward the wall. The thighs should be flexed toward the abdomen and the knees drawn to the chest; the neck should be in a neutral position or flexed so that the chin rests on the chest. Asking the patient to “assume the fetal position” may help maximally flex the spine. The hips should be aligned one above the other, and the nondependent arm should extend toward and rest on the nondependent hip. The patient’s head may need to be elevated with a pillow to avoid rotation of the spine. Obese patients or those with larger hips may require additional pillows to maintain proper alignment. Directing the needle toward an imaginary line that extends cephalad and caudad from the umbilicus may optimize chances of midline insertion, which is particularly important during initiation of CSEs (Table 24). The bevel of the epidural needle is directed toward the patient’s head.
TABLE 24. Tips for attaining optimal lateral position.
Align patient’s back with edge of table or bed
Align coronal plane perpendicular to floor
Flex thighs toward abdomen
Neck should be neutral or flexed
Align hips one above the other
Rest nondependent arm on nondependent hip
Elevate head with pillow to avoid rotation of the spine
Infection Control
Adherence to strict aseptic techniques is essential during initiation and maintenance of neuraxial block. The ASA Task Force on Infectious Complications Associated With Neuraxial Techniques advises the following measures: remove jewelry on fingers and wrists; use careful hand washing before gloving; use caps, masks (changed with each new patient encounter), and sterile gloves; use chlorhexidine with alcohol for skin preparation; drape the patient under sterile conditions; and cover the catheter insertion site with a sterile occlusive dressing. A single application of chlorhexidine with alcohol appears as efficacious as two applications for skin disinfection. When chlorhexidine, which is not supplied in epidural kits, is not available, the use of povidone-iodine with alcohol is preferable to povidone-iodine alone. All antiseptic solutions are considered neurotoxic if they come into direct contact with the meninges; care should be taken to keep needles and drugs in the epidural tray separate from the skin disinfectants. Bacterial filters may be helpful for patients with chronic or extended continuous epidural infusions, but there are no data to support that they decrease the incidence of catheter-related infections. The catheter should
remain in place no longer than necessary, and catheter disconnects and filter changes should be kept to a minimum. The data are insufficient to support wearing a surgical gown during initiation of epidural procedures (Table 25).
TABLE 25. Aseptic technique during epidural block initiation and maintenance.
| Remove jewelry on fingers and wrists |
| Perform careful hand washing before gloving |
| Use cap, mask, and sterile gloves |
| Use chlorhexidine with alcohol for skin preparation |
| Drape the patient under sterile conditions |
| Cover catheter insertion site with sterile, occlusive dressing |
| Keep catheter in place no longer than necessary |
| Keep catheter disconnection and filter changes to a minimum |
Techniques to Identify the Epidural Space
Three techniques can be used to identify the epidural space: LOR, hanging drop, and ultrasonography. Despite increasing interest in ultrasound-assisted neuraxial procedures, the LOR technique, which relies on the different tissue densities as the needle passes through ligaments into the epidural space, is the most commonly used technique. Both LOR to fluid, with or without an air bubble, and air are recognized as acceptable means of identifying the epidural space. Randomized trials comparing saline to air for LOR have suggested that saline is superior. However, these trials may overstate the difference between the two media by forcing the anesthesia provider to use a less-preferred technique. Regardless of which technique is used during routine epidural placement, it is important to bear in mind that LOR to air is not recommended for EBP procedures.
Evidence in the literature is lacking concerning the best method to identify the epidural space in children, who are commonly anesthetized during placement. Recently, the use of ultrasound has been advocated. However, the technique can be cumbersome and requires experience with ultrasound imaging. With refinements in technology and expanding practitioner experience, ultrasound guidance may facilitate placement of epidural catheters in this patient population.
Loss of Resistance to Air
The LOR to air technique is associated with several complications (Table 26). Air is compressible and may result in a false LOR and, relatedly, an increased incidence of ADP. ADP in the setting of LOR to air may result in pneumocephalus, a severe headache that develops immediately after injection of air into the subarachnoid space. Pneumocephalus, in turn, can result in serious neurologic injury, such as hemiparesis and generalized convulsions, as well as nausea and vomiting and delayed recovery from GA.
TABLE 26. Complications associated with loss of resistance to air.
| Pneumocephalus |
| Increased risk of accidental dural puncture |
| Faster onset of signs and symptoms of PDPH |
| Higher incidence of PDPH |
| Incomplete/patchy block |
| Spinal cord or nerve root compression by air |
| Venous air embolism |
| Subcutaneous emphysema |
| Increased incidence of epidural vein cannulation |
| Difficult catheter insertion |
The incidence of PDPH and the onset of symptoms may also be higher when the LOR to air technique is used to identify the epidural space. In addition, the LOR to air technique has been associated with a higher incidence of unblocked segments or patchy pain relief and neurologic deficits related to compression of nerve roots or the spinal cord by air bubbles. Venous air embolism (VAE) in the presence of tears in the epidural venous plexus or if the pressure from the air source is higher than the venous pressure has been reported. Finally, both an increased incidence of epidural venous cannulation and difficult catheter insertion have been associated with LOR to air, particularly in the absence of fluid predistension of the epidural space, although the data are conflicting.
Advocates of LOR to air feel that it is easier to detect ADP if air alone is used, as any fluid return is undeniably CSF in the absence of saline injection. In the event of an equivocal ADP, CSF can be distinguished from saline with the use of a urine reagent strip to check for glucose and protein; if positive, the diagnosis of CSF can be made. CSF can also be distinguished from saline or LA by the temperature differential; CSF is expected to be body temperature. Advocates of the LOR to air approach also point to the inadequate sensory nerve block and delay in nerve block onset that may occur if large volumes of saline are injected into the epidural space, presumably due to a dilutional effect. This, however, can be avoided by limiting the amount of saline injected. For practitioners who routinely perform CSEs, an argument could also be made that injecting saline complicates the identification of CSF prior to the administration of intrathecal medications.
To identify the epidural space with the LOR to air technique, advance the needle slowly, exerting either continuous or intermittent pressure on the LOR syringe. As the needle enters the ligamentum flavum, there is usually a distinct sensation of increased resistance followed by a subtle “give” when light pressure is exerted on the plunger. Avoid injecting air on identifying the epidural space due to concerns for pneumocephalus (in the event of ADP) and patchy, inadequate analgesia.
Loss of Resistance to Saline With or Without an Air Bubble
The syringe is filled with 2–3 mL of saline or saline with a clearly visible air bubble. The bubble provides a gauge of the appropriate pressure to be applied on the LOR syringe; it will compress and provide some resistance if the epidural needle tip is engaged in ligament but will dissipate effortlessly with only light pressure once the needle enters the epidural space. The saline can be injected directly for fluid predistension. The small air bubble should not result in complications associated with LOR to air if it also is injected. Omit the air bubble when performing an EBP due to the remote possibility that the air could be introduced into the subarachnoid space via the meningeal breach.
With LOR to saline with or without air, the needle is advanced in the same fashion as with air. Continuous or intermittent pressure can be exerted on the plunger of the needle.
The Hanging Drop Technique
The hanging drop technique relies on the subatmospheric pressure of the epidural space, which is more pronounced and reliable in the cervical and thoracic regions than in the lumbar segments. Dural tenting from the advancing epidural needle also contributes to the pressure that appears to “suck in” the drop of fluid. To identify the epidural space with this approach, an epidural needle with wings is required. A drop of saline is placed at the hub of the needle once the needle is engaged in ligament. The needle is advanced continuously with the thumb and index fingers firmly grasping the wings and the third through fifth fingers of both hands positioned against the patient’s back. Entry into the epidural space is signaled by entry of the drop into the hub of the needle.
The hanging drop technique is most effective in the thoracic region, where the subatmospheric pressure is more notable. However, this technique carries a higher risk of meningeal tear, in part because of the epidural needle’s proximity to the dura. Also, patients with severe obstructive lung disease may have attenuated subatmospheric pressure, even in the thoracic region; the hanging drop technique may not be appropriate in this setting.
Ultrasonography
Ultrasound technology is being used increasingly to aid in the identification of the epidural space. Studies suggest that the use of ultrasonography to identify the anticipated depth to space, particularly in obese parturients, and to identify the midline prior to placement reduces the number of attempts, minimizes the risk of complications, and facilitates the procedure without significantly prolonging the procedure. Ultrasound guidance also serves to help identify the correct interspace, which may be difficult based on anatomic landmarks alone. See Chapter 40 for more detailed information about ultrasound-guided neuraxial techniques.
Technique of Epidural block
There are four common approaches to the epidural space: midline, paramedian, Taylor (modified paramedian), and caudal. Clinical expertise in each of these techniques gives the anesthesiologist more flexibility when performing epidural block. For all approaches, monitors should be in place and the skin should be prepped and draped in a sterile fashion before initiation of the procedure. Emergency equipment and medication must be immediately available. Sedation may be used, as appropriate. In general, the epidural needle bevel should be facing cephalad regardless of the approach used to access the epidural space unless an intentional unilateral nerve block is desired (eg, for lower extremity orthopedic procedures performed under CSE).
Midline Approach
This approach is most commonly used for epidural placement in the sitting position and for epidural procedures in the lumbar, low thoracic, and cervical spine region.
1. An epidural tray can be placed to the anesthesiologist’s right for right-handed and left for left-handed clinicians.
2. Identify the desired interspace by surface anatomic landmarks and palpation or with ultrasonography. The needle used to anesthetize the skin can also be used as a “finder needle” to help identify bony landmarks, particularly in obese patients.
3. Infiltrate the skin and subcutaneous tissue with LA (most commonly 1% lidocaine) along the intended path of the epidural needle between adjacent spinous processes. A large skin wheal with a smaller volume of LA in the subcutaneous tissue will serve to anesthetize the skin adequately without
obscuring landmarks.
4. Insert the styletted epidural needle along the same track with the bevel oriented cephalad. During needle insertion, the dorsum of the anesthesiologist’s noninjecting hand can rest on the patient’s back with the thumb and index finger holding the hub of the epidural needle. A modified approach is to advance with the dominant hand firmly wrapped around the hub of the epidural needle while the index finger and thumb of the nondominant hand grasp and guide the needle shaft. Placing the tips of the middle fingers on the patient’s back and grasping the needle wings with both thumbs and forefingers is an alternative method to engage the needle.
To engage the epidural needle properly, advance through the skin, subcutaneous tissue, fatty tissue, supraspinous ligament, interspinous ligament, and, possibly, into the ligamentum flavum; at that point, the needle should sit firmly in the midline (Figure 20). If the needle shaft wobbles or deviates sideways, it is not properly anchored in ligament. The epidural needle can be engaged in the interspinous ligament or the ligamentum flavum.
NYSORA Tips
• The epidural needle advances through skin, subcutaneous tissue, supraspinous ligament, interspinous ligament, and ligamentum flavum before reaching the epidural space.
• The needle can be engaged in either the interspinous ligament or the ligamentum flavum during initiation of epidural block.
• Lateral deviation or a “wobbly” needle indicates that the needle is not properly engaged in ligament, necessitating withdrawal and re-direction toward midline.
Determining which ligaments are traversed is an acquired skill. The interspinous ligament may feel “gritty” against the advancing needle, while the ligamentum flavum offers more resistance. However, midline gaps in the ligamentum flavum are not uncommon, and obstetric patients may have softer ligaments. The depth from the skin to the ligamentum flavum generally ranges from 4 to 6 cm in normal-size adults, although there is a great deal of variability. After the ligaments are penetrated, it is no longer advisable to change the direction of the needle tip without withdrawing the needle several centimeters or to the skin level. The stylet should be placed in the epidural needle while redirecting to avoid the accumulation of bony debris or soft tissue plugs that may hinder the flow of CSF in the event of an ADP.
5. Remove the stylet from the epidural needle and attach the LOR syringe with air or saline (with or without an air bubble) firmly to the hub of the needle. Glass or lowresistance plastic LOR syringes are appropriate. Care should be taken to ensure that glass syringes are not “sticky.”
Multiple hand positions are appropriate to advance the epidural needle into the epidural space: The back of the nondominant hand can rest firmly on the patient’s back, with the thumb and forefinger grasping the needle shaft, while pressure is exerted on the LOR plunger either continuously or intermittently by the thumb of the dominant hand. The nondominant hand can rest on the patient’s back with the thumb and forefinger extending to and stabilizing the needle hub, while the thumb of the dominant hand applies pressure (Figure 21). Or, the middle through fourth or fifth fingers of the nondominant hands can rest on the back, with both thumbs and forefingers grasping the wings of the epidural needle and the dominant hand intermittently releasing its position and exerting pressure on the LOR syringe plunger (Figure 22).
Figure 21. Advancing epidural needle: nondominant hand on patient’s back with thumb and forefinger on needle hub.
As the needle enters the epidural space, the plunger of the LOR syringe suddenly “gives.” Avoid injecting the full contents of the syringe, particularly with LOR to air, if possible. For continuous epidurals, a small volume of saline can be injected into the epidural space to dilate the space, thereby reducing the risk of epidural vein cannulation and facilitating catheter insertion. Note the depth of the needle at the skin. The marking on the needle at the skin represents the depth from the skin to the epidural space. Because the centimeter markings are not numbered, it may help to count the number of centimeter markings between the skin and the epidural needle hub and subtract that number from the length of the needle. For example, if 4 markings remain visible between the skin and the needle hub, subtract 4 from 9 (the common length of an epidural needle) to determine that the depth to epidural space is 5 cm.
Insert the catheter with the assistance of the insertion device that fits into the epidural needle hub until the 15-cm mark is visualized entering the needle hub; then, remove the needle without dislodging the catheter (Figure 23). The catheter should be threaded no more than 5–6 cm into the epidural space; 2–3 cm is appropriate for short surgical procedures. To determine where the catheter should be secured at the skin, add 2–6 cm, depending on the distance the catheter is to be threaded, to the previously calculated depth to epidural space. For example, if the needle entered the epidural space at 7 cm, the catheter should be secured at the 12-cm mark at the skin to ensure that 5 cm of the catheter rests in the epidural space.
NYSORA Tips
• An easy way to measure depth to epidural space when using the LOR technique is to count the number of centimeter markings that are visible between the skin and the needle hub. Subtract that number from the length of the needle. For example, most epidural needles are 9 cm in length. If 4 centimeter markings are visible after epidural space identification, subtract 4 from 9 to conclude that the depth to space is 5 cm. The epidural catheter should be inserted no more than 5 or 6 cm beyond that distance (ie, taped at 10–11 cm at the skin).
For the less-common single-shot epidural technique, the LA may be administered directly through the needle in divided doses over several minutes. This technique, however, requires that the patient remain immobile during dosing and may result in painful pressure with large volumes. For the continuous catheter technique, administering LA through the needle is not recommended, as correct catheter placement cannot be verified.
A clear occlusive dressing should be applied over the insertion site to allow inspection of the catheter. The catheter should be secured to the patient’s back with the connector at the patient’s shoulder. Using clear tape has the advantage of permitting the practitioner to visualize the proximal and distal “flashback” windows of the catheter prior to administering boluses of LA.
Paramedian Approach
The paramedian approach offers a larger opening into the epidural space than the midline approach and is particularly useful for patients who cannot be positioned easily or who cannot flex the spine during epidural placement; for patients with calcified ligaments or spinal deformities (eg, kyphoscoliosis, prior lumbar surgery); and for epidural techniques in the low- to midthoracic area. The spinous processes from T4–T9 are sharply angled and have tips that point caudally, making midline insertion of the epidural needle more difficult.
The “feel” of the paramedian approach is different from that of the midline approach because different tissues are penetrated. The supraspinous and interspinous ligaments are midline structures that are not traversed in the paramedian approach.
Instead, the epidural needle penetrates paraspinous tissue with little resistance before entering the ligamentum flavum. Several approaches to the paramedian technique have been described. Essentially, needle entry is directed caudal and lateral to the inferior aspect of the superior spinous process of the desired interspace and walked off the lamina in a cephalad direction (Figure 24).
1. Identify the intended interspace with surface landmarks, palpation, or ultrasound guidance. Raise a skin wheal roughly 1 cm lateral and 1 cm caudad to the inferior aspect of the superior spinous process of the desired spinal level.
2. The epidural needle is inserted 15° off the sagittal plane, angled toward midline with a cephalad tilt.
3. If bone (most likely lamina, if depth and angle of approach are appropriate) is encountered, the needle is redirected in a cephalad and medial direction (See Part VI, Pediatric Anesthesia). If the lateral aspect of the spinous process is encountered, the needle should be redirected laterally and cephalad.
Taylor Approach
The Taylor approach is a modified paramedian approach utilizing the large L5–S1 interspace. It is an excellent approach for hip surgery or for any lower extremity surgery in trauma patients who cannot tolerate the sitting position. This approach may provide the only available access to the epidural space in patients with ossified ligaments.
1. With the patient in the sitting or lateral position, a skin wheal is placed 1 cm medial and 1 cm caudad to the posterior superior iliac spine.
2. The epidural needle is inserted into this site in a medial and cephalad direction at a 45° to 55° angle.
3. As in the classic paramedian approach, the first resistance felt before entry to the epidural space is on entry into the ligamentum flavum.
4. If the needle contacts bone (usually the sacrum), the needle should be walked off the bone into the ligament and then into the epidural space in progressively more medial and cephalad directions.
Caudal Approach
The caudal approach is commonly used in pediatrics for single-shot or continuous epidural catheter placement for intraoperative and postoperative analgesia. In adults, it is usually reserved for procedures requiring block of the sacral and lumbar nerves (eg, anal and vaginal surgeries, inguinal herniorrhaphy, cystoscopy); epidurography; and lysis of adhesions in patients with low-back pain with radiculopathy after spinal surgery.
The sacrum is a triangular-shaped bone formed by the fusion of the sacral vertebrae. Nonfusion of the fifth sacral vertebral arch creates the structure known as the sacral hiatus, which is covered by the sacrococcygeal ligament (an extension of the ligamentum flavum) and bordered by bony prominences known as the sacral cornua. The sacral hiatus is the point of access into the sacral epidural space. It is usually identified as a groove above the coccyx (Figure 25).
If fluoroscopy is not used, there are two methods to identify the hiatus:(1) The sacral hiatus lies at the apex of an equilateral triangle connecting the posterior superior iliac spines and pointing caudad. (2) The bony protuberances (the sacral cornua) that surround the sacral hiatus can be palpated by applying firm pressure with the index finger as it moves cephalad from the coccyx.
1. Place the patient in a lateral or prone position (with pillow under pelvis and hips internally rotated, if prone). In the lateral position, the dependent leg is flexed slightly, while the nondependent leg is flexed to a greater degree (until the knee contacts the bed).
2. Advance the needle at a 45° tilt (relative to the skin surface).
3. A distinct “pop” or “snap” is felt when the needle pierces the sacrococcygeal membrane.
4. If the dorsal aspect of the ventral plate of the sacrum is encountered, withdraw the needle slightly, decrease the angle of insertion, and advance again. The needle angle is lowered until it is almost flat against the skin (ie, parallel to the coronal plane) for male patients. Female patients may require a 15° tilt.
5. After LOR is encountered, advance the needle slightly into the caudal canal. Advancing too far may lead to ADP or unintended intravascular injection or epidural vein cannulation during catheter placement. If LOR is equivocal, several milliliters of saline can be injected through the caudal needle while palpating the skin overlying the sacrum. The needle is likely positioned correctly if a skin bulge does not develop.
6. Aspirate for blood or CSF before injecting LA.
7. An epidural catheter can be inserted through the needle and advanced to the desired level.
Cervical Epidural block
Single-shot or continuous cervical epidural techniques are used for a variety of surgical and pain procedures, including carotid endarterectomy, thyroidectomy, and chronic neck pain conditions. Both the midline and paramedian approaches are used to perform cervical procedures, although fluoroscopic guidance is becoming increasingly common. Cervical epidural block can be initiated in the prone, lateral, or sitting position. The prone position is used most commonly for fluoroscopic-assisted procedures, although the sitting position can be used. Whichever position is used, flexion of the neck serves to increase the distance from the ligamentum flavum to the dura mater, increasing the margin of safety for these procedures, and to expand the interlaminar space.
As in the case of lumbar and thoracic epidural procedures, both the LOR and the hanging drop techniques are suitable methods to identify the epidural space. Either air (preferably a small volume) or saline (with or without an air bubble) can be used for LOR. However, the ligamentum flavum is discontinuous at midline in the cervical region in a large percentage of patients, contributing to a false LOR. Also, it is important to bear in mind that the ligamentum flavum is thinner at this level (1.5–3 mm) than at the lumbar and thoracic levels.
In the cervical region, the C7–T1 interspace is widest and easiest to access. In addition, the depth from the skin to the epidural space is larger at this interspace, and the distance from the epidural space to the dural sac is greater than at other cervical levels. However, using palpation and surface landmarks to identify the C7–T1 level is not always reliable; the vertebra prominens (presumed to be C7) is not uncommonly confused with C6 and T1 in certain patient populations. Single-shot injections at this level should be administered slowly. For continuous catheter techniques, the catheter is usually threaded no more than 2–3 cm.
Initiation and Management of Epidural block
Test Dose
Before administering medications through the epidural catheter, subarachnoid, intravascular, and subdural placement should be ruled out. Although rare, catheter migration may occur after initial confirmation that the catheter is in the epidural space; each bolus should be preceded by confirmation of proper catheter location.
Although the efficacy of LA with epinephrine in detecting misplaced catheters has been questioned, many clinicians still routinely rely on the pharmacologic test dose. The classical dose combines 3 mL of 1.5% lidocaine with 15 μg of epinephrine.
The intrathecal injection of 45 mg of lidocaine should produce a significant motor nerve block if the catheter is in the subarachnoid space, although recent evidence suggests that this is not always reliable. A change in heart rate of 20% or greater (or, alternatively, an increase in heart rate of 10 to 25 beats per minute) within 1 minute suggests that the catheter has been placed in (or has migrated into) a vessel and should be replaced. If the heart rate does not increase by 20% or greater or if a significant motor nerve block does not develop within 5 minutes, the test dose is considered negative. Exceptions to this rule have been observed in laboring patients, anesthetized patients, and patients receiving β-adrenergic blocking agents.
Relying on ECG changes after the test dose and the use of nerve stimulators have been advocated as alternative methods to confirm epidural placement, although these methods also have shortcomings.
The safety and efficacy of the traditional test dose is debated in the literature. In laboring patients, a change in heart rate attributed to the epinephrine may in fact be due to a painful contraction, contributing to a false-positive interpretation. Alternatively, a true-positive test result in this patient population can result in an epinephrine-induced decrease in uterine blood flow. Patients with HTN, including women with preeclampsia, can experience a severe increase in blood pressure that may not be well tolerated after an intravenous dose of 15 μg of epinephrine. Volatile general anesthetics may interfere with the response to epinephrine and contribute to a high percentage of false-negative results in children, who are most commonly anesthetized during epidural placement. In patients on β-adrenergic blocking agents, heart rate changes may not be reliable. An increase in systolic blood pressure greater than 20 mm Hg has been used as an indicator of intravascular injection in this patient population.
Additional studies are needed to determine the optimal strategy to detect intrathecal and intravascular catheter placement. Fortunately, with the widespread use of low-concentration LA infusions for epidural analgesia, the risk of systemic LA toxicity is vastly reduced; the utility of a traditional test dose to evaluate for intravascular cannulation in this setting is limited. In addition, epidural catheter design innovations over the past several decades, particularly the introduction of flexible catheters, have contributed to a marked decrease in the incidence of both intrathecal catheter migration and epidural venous cannulation or migration. Nonetheless, incremental dosing of LA (ie, 3- to 5-mL aliquots), with simultaneous aspiration for blood and CSF and careful observation, is required when dosing an epidural. In the future, new methods of detecting misplaced catheters, such as acoustic signal guidance, nerve stimulation, and ultrasound-guided insertion, may replace the classical test dose.
Dosing Regimen
After the epidural catheter has been aspirated to check for blood or CSF or after a negative test dose, the catheter can be dosed to provide analgesia or anesthesia. As mentioned, LA concentration determines the density of nerve block, while the volume and total dose of LA determine the spread. As a general guideline, the initial loading dose can be determined as follows: 1–2 mL of LA per segment to be blocked in a lumbar epidural, 0.7 mL per segment for a thoracic epidural, and 3 mL per segment for a caudal epidural. The loading dose should be administered through the catheter in 3- to 5-mL aliquots at 3- to 5-minute intervals, permitting time to assess the patient’s response to dosing and to avoid systemic toxicity. Appropriate loading doses for postoperative analgesia include 10 mL of 0.2%–0.25% bupivacaine, levobupivacaine, or ropivacaine with or without adjuvants; however, patients may experience varying degrees of motor nerve block. Recent evidence suggests that higher volumes of lower-concentration LAs may provide better spread and improved analgesia. Up to 20 mL of 0.0625%–0.125% bupivacaine or the equipotent dose of ropivacaine may be administered incrementally as a loading dose. Higher-concentration LAs are required for surgical anesthesia. Up to 20 mL of 2% lidocaine, with or without epinephrine 1:200,000 and sodium bicarbonate, or 15 mL of 0.5% bupivacaine or ropivacaine may be used to initiate epidural anesthesia in the lumbar region.
Maintenance of the desired level of anesthesia can be accomplished through intermittent or continuous dosing after the initial loading dose. With manual boluses, one-quarter to one-third of the initial amount can be administered at timed intervals, depending on duration of action of the initial LA (ie, short, intermediate, or long acting), although several maintenance regimens are appropriate. Manual boluses are usually given during prolonged surgery; a continuous infusion, however, can be started after the initial bolus to maintain surgical anesthesia. Continuous infusions require the same diligent attention to the patient as any other anesthetic. The usual infusion rate is between 4 and 15 mL/h. The wide range is usually dependent on the age, weight, extent of sensory or motor block desired in a particular patient; site of catheter insertion; and the type and dose of LA. Thus, individualization is necessary, and a fixed rule cannot be applied for this purpose.
Patient-controlled epidural analgesia (PCEA), most commonly with infusions of low-concentration LAs and opioid adjuncts, are increasingly used for postoperative analgesia and for laboring patients. A demand bolus at timed intervals, with or without a loading dose and a background infusion, can be programmed to optimize patient comfort with less LA consumption. Pumps that deliver automated mandatory boluses at timed intervals, with or without a basal infusion, have been developed, although they are not yet widely available.
For thoracic epidural block, several dosing regimens can be used to minimize hemodynamic changes and respiratory impairment (in awake patients). An initial dose of 3 to 6 mL of dilute bupivacaine 0.125%–0.25% or 0.1%–0.2% ropivacaine with or without fentanyl, hydromorphone, or preservative-free morphine can be followed by 3 mL of 0.25%–0.5% bupivacaine every 30 min. An alternative regimen is as follows: Administer a loading dose with 3–6 mL of 0.125% bupivacaine or 0.1%–0.2% ropivacaine with an opioid (fentanyl 2 μg/mL or hydromorphone 20 μg/mL) at least 30 minutes before the end of the case, as tolerated. Start an infusion of bupivacaine 0.0625% or 0.1% ropivacaine with fentanyl or hydromorphone at 3–5 mL/h before the patient leaves the operating room.
The level and duration of epidural anesthesia depends primarily on the injection site and the volume and concentration of the drug. Other factors such as age, pregnancy, and sex are less important factors but need to be considered. The addition of fresh epinephrine and 8.4% sodium bicarbonate to lidocaine, mepivacaine, and chloroprocaine will decrease the latency, improve the quality, and prolong the duration of the nerve block. Epinephrine is less effective with the long-acting LAs. Adding bicarbonate to ropivacaine and bupivacaine can cause precipitation. The addition of opioids (eg, fentanyl) has been shown to improve the quality of the nerve block without any effect on duration.
Top-Up Dosing
Repeat doses, commonly referred to as “top-ups,” should be administered before the level of the nerve block has receded more than two dermatomes. One-quarter to one-third or more of the original loading dose of LA can be administered for each repeat dose, although different top-up doses may be required for different clinical scenarios. For example, if the patient is comfortable but the sensory level is not adequate, a high-volume, low-concentration LA top-up may be appropriate. This may also be the case if the block is unilateral or patchy but the patient desires to maintain motor strength. However, if the patient requires a denser nerve block for surgical anesthesia or for the second stage of labor, for example, less volume of a higher-concentration LA may be a better choice. Overall, the anesthesiologist must have a working knowledge of the characteristics of the LA used to properly implement a redosing protocol.
Problem Solving
Epidural placement presents unique challenges that are directly related to practitioner experience, the clinical scenario, and patient characteristics, among other things. Most of these problems can be overcome if the clinician recognizes the problem, is familiar with vertebral column anatomy, and knows how to make adjustments in technique (Table 27).
TABLE 27. Problem solving during initiation of epidural block.
| Problem | Possible Explanation | Action |
|---|---|---|
| Needle floppy; needle angles laterally | Entry off midline; missed supraspinous ligament | Reassess midline; redirect needle |
| Bone contact at < 2 cm | Contacted spinous process; spinal flexion inadequate | Reidentify interspace; place needle in caudal region of interspace |
| Bone contact at ≥ 4 cm | Needle entry too lateral; contacted lamina | Redirect needle toward midline |
| Bony resistance throughout | Ossified ligaments; arthritic spine | Consider paramedian approach |
| Inability to advance catheter | False loss of resistance; narrow epidural space; needle too close to dura mater; obstructed needle orifice | Fluid predistention; rotate needle bevel; use stiffer catheter; advance epidural needle slightly; attempt new placement at different interspace; withdraw needle to ligamentum flavum and readvance |
| Heme in catheter | Epidural vein cannulation; needle entry too lateral; engorged epidural veins | Withdraw catheter 1–2 cm and flush with saline; perform new placement if heme persists; consider initiating epidural procedure in lateral position |
| Warm, clear fluid return in needle or catheter | Accidental dural puncture; intrathecal placement | Distinguish cerebrospinal fluid from saline or local anesthetic; if cerebrospinal fluid, consider continuous spinal or new placement at different interspace |
| Pain/paresthesia on catheter insertion | Catheter advanced > 6 cm into epidural space; catheter near nerve root | Withdraw catheter to < 6 cm in epidural space (2–3 cm for short surgical procedures); perform new placement if pain persists |
| Inability to palpate spinous processes | Obesity; severe arthritis; patient with previous back surgery | Optimize patient position; consider midline approach for obese patients; use long finder needle to help identify bony landmarks; consider placement in lateral position if patient unable to flex spine; use ultrasonography |
| Inability to flex spine | Elderly; arthritis; patient with previous spinal instrumentation | Consider paramedian approach; consider placement in lateral position |
| Curvature of spine | Scoliosis | Use ultrasonography; if possible, perform procedure below level of curvature (otherwise direct needle into curve) |
Difficulty Identifying the Epidural Space
Several troubleshooting measures may help if equivocal LOR occurs while trying to identify the epidural space. First, ensure that the LOR syringe is tightly connected to the epidural needle. If using the LOR to air technique, next put 2–3 mL of saline into the LOR syringe and push gently (ie, with the little finger). If using LOR to saline, omit the bubble for this step. The saline will flow easily if the tip of the epidural needle is in the epidural space but will encounter resistance if the needle tip is in soft tissue.
If resistance is met, continue injecting the saline into the soft tissue and then resume the original LOR technique. Often, the familiar feedback from the LOR syringe returns after saline has dissipated throughout the soft tissue planes.
Another method to distinguish between soft tissue and the epidural space during the LOR to saline technique is to place a small bubble in the LOR syringe. The bubble should compress to varying degrees when the epidural needle is in soft tissue or ligament but will inject effortlessly if the needle is in the epidural space.
If LOR is still equivocal, insert a 25- or 27-gauge spinal needle through the epidural needle to puncture the dura. If CSF is visible in the spinal needle, the epidural needle is properly placed. The absence of CSF indicates that the epidural space has not yet been encountered or that the epidural needle is off midline, in the lateral epidural space. If LOR remains equivocal, attempt to thread the catheter.
Many catheters, particularly flexible or wire-reinforced versions, will not advance if the epidural needle is not fully in the epidural space.
Paresthesias During Epidural Needle or Catheter Placement
Patients not uncommonly report paresthesias during epidural procedures, particularly on direct questioning by the clinician. Because a paresthesia indicates that the needle or catheter is near a nerve, the needle should be withdrawn and redirected if the sensation persists. Alternatively, the epidural procedure can be initiated at another interspace. Most often, however, the needle can simply be redirected away from the side where the paresthesia was detected. During catheter placement, fluid predistention may help to reduce the incidence of paresthesias, although the data are conflicting. Threading the catheter no more than 5–6 cm appears to reduce the risk of paresthesias. The use of flexible catheters and, in particular, wire-reinforced catheters also appears to reduce the incidence of paresthesias. If a paresthesia is transient, it is acceptable to continue advancing the needle or threading the catheter.
Accidental Dural Puncture
Accidental dural puncture complicates an estimated 1% of epidural procedures, although the reported incidence varies substantially in the literature. Management options include placing a continuous spinal catheter or withdrawing the epidural needle and repeating the epidural procedure at a different interspace. Whether a spinal catheter is placed or a new epidural placement is performed, the choice should be made quickly to avoid excessive egress of CSF through the large-bore epidural needle. A continuous spinal technique may have the slight advantage of reducing the incidence of PDPH and the need for an EBP (see discussion that follows), although the data are limited and conflicting. In cases in which identification of the epidural space was difficult or with high-risk patients (eg, obese parturients with a high likelihood of conversion to cesarean delivery and surgical patients with anticipated difficult airways), placing a continuous spinal catheter may also be advantageous. This option avoids the risk of a second dural puncture and has been reported to provide reliable analgesia and anesthesia, although the data are conflicting. Disadvantages of threading a continuous spinal catheter include the risk of an accidental injection of large doses of LA intended for the epidural space and, possibly, an increased risk of infection. Protocols should be in place to alert all providers when a spinal catheter has been placed.
If the practitioner elects to place the epidural at another interspace, he or she incurs the risk of a second ADP. Also, there is a concern for LA passage from the epidural to the subarachnoid space via the dural breach, resulting in a higher-than-anticipated nerve block. Although it may not be necessary, it is reasonable to reduce the basal rate for continuous or PCEA pumps; as always, use caution when injecting boluses of LA or epidural morphine. The evidence does not support the use of the epidural catheter for a prophylactic EBP, although more recent studies may demonstrate some benefit. More elaborate considerations of the anatomy, pathophysiology, and treatment of PDPH are discussed in Postdural Puncture Headache.
Difficulty Threading the Catheter
Difficulty threading the catheter is not uncommon, even if the needle is properly engaged in the epidural space. This problem is more common with flexible, soft-tipped catheters. Troubleshooting measures include confirming that the epidural needle is properly positioned in the epidural space (see previous discussion); injecting several milliliters of saline to “open” the epidural space; advancing the epidural needle slightly so that the entire bevel is engaged in the epidural space (the LOR syringe with saline without an air bubble should be attached during this step); rotating the bevel of the epidural needle; inserting a different, less-flexible catheter; retracting the needle to the ligament and identifying the epidural space again; and repeating the epidural procedure at a different spinal level. Occasionally, the epidural needle is plugged with tissue debris that nerve blocks the passage of the catheter. Replacing and then removing the stylet may serve to remove the obstructing debris. The subatmospheric pressure is variable in the lumbar region; asking the patient to take a deep breath is unlikely to facilitate catheter threading, particularly when with flexible, wire-reinforced catheters. Overall, performing a new placement at another interspace appears to confer less risk of ADP than rotating the needle or cautiously advancing the epidural needle. If electing to start over at another interspace, withdraw the needle and the catheter simultaneously to avoid shearing of the catheter.
Unilateral Nerve Block
After an epidural has been dosed adequately, the patient may complain that one side is densely blocked, while the opposite side has intact pain and motor function. The most common explanation for a unilateral nerve block is that the catheter has advanced too far into the epidural space, permitting the tip of the catheter to enter the intervertebral foramen or be in close proximity to a nerve. Current data suggest that there is no indication to advance a catheter (either single end hole or multiorifice) more than 6 cm into the epidural space. If a unilateral nerve block persists despite appropriate depth of insertion, consider pulling the catheter back 1–2 cm, leaving 3–4 cm (2–3 cm for short procedures) in the epidural space. If the patient remains uncomfortable despite catheter manipulation, place the patient in the lateral position with the unblocked side down and administer several milliliters of dilute LA. If these maneuvers have no effect, replace the catheter.
Blood in the Epidural Needle or Catheter
Epidural vein cannulation is not uncommon, although the incidence has declined substantially with the widespread use of flexible catheters. The epidural veins lie primarily in the anterior epidural space, cordoned off by the posterior longitudinal ligament and its fascia. A bloody tap may be an indication that needle or catheter insertion is too lateral and should be redirected toward midline. Other measures to minimize the risk of epidural vein cannulation during catheter insertion include the use of wire-reinforced catheters, the administration of fluid to open the epidural space prior to threading the catheter, and avoiding catheter insertion beyond 5–6 cm, among others (see Local Anesthetic Systemic Toxicity). If blood returns through the catheter despite these measures, the catheter can be withdrawn slightly and flushed with saline. This can be repeated until either the blood ceases to return or there is insufficient length of catheter in the epidural space, at which point the catheter must be replaced.
Pain Despite Adequate Nerve Block Height and Density
Persistent pain despite adequate nerve block height and density may be a result of incomplete block (“window” of pain), “patchy” block, or poor sacral spread. A window of pain in which a distinct, small area remains unblocked despite an otherwise dense nerve block may be difficult to troubleshoot. It is reasonable to administer top-ups and turn the patient with the window side of the catheter down. Injecting opioids into the epidural space may also help. However, performing a CSE technique that provides density from the spinal portion or replacing the epidural
at another interspace may be required. Extreme caution should be exercised when deciding to perform CSE anesthetic in case of failed epidural anesthesia as there may be a higher risk of high spinal anesthesia. Wherever possible, a continuous spinal catheter with gradual dosing of the spinal anesthetic should be considered.
A “spotty” or “patchy” nerve block may result from the injection of air when using the LOR to air technique, from individual anatomic variations that contribute to “missed” dermatomes, or from catheter migration. Administering additional LA, with or without an opioid, after a sufficient amount of time has elapsed since the initial dose is appropriate. It may also help to withdraw the catheter 1–2 cm and place the patient with the less blocked side in the dependent position. However, it is also reasonable to replace the catheter, particularly if multiple topups have been administered and if there is a high likelihood of conversion to surgical anesthesia.
In the case of poor sacral spread, the following measures may help: Raise the head of the bed and redose the catheter with a more highly concentrated LA; administer 100 μg of epidural fentanyl to improve the quality of the nerve block; or inject preservative-free neostigmine 500–750 μg or clonidine 75 μg epidurally.
Replacing a stand-alone epidural with a CSE also improves sacral analgesia, as the sacral nerve is large and occasionally difficult to nerve block with LAs administered in the epidural space.
Inadequate Analgesia Despite Fully Dosed Epidural Catheter
Most often, the best strategy is to replace the epidural catheter. To assess whether an epidural is functioning properly, feel whether both legs are warm to touch (LA-induced vasodilation should make the lower extremities warm if the epidural is properly placed and fully functioning). Assess also whether the patient has decreased temperature perception and decreased response to pinprick in the dermatomes that correspond to the expected sensory block. Consider administering a definitive dose of LA (eg, 5–10 mL of 2% lidocaine with or without epinephrine in incremental doses) to determine whether the catheter is functioning, provided that the resulting motor block is not contraindicated. Evaluate the patient’s motor strength and temperature and pain perception after each dose. Monitor vital signs for any indication of sympathectomy-induced hypotension. It is advisable not to administer more than 10 mL of LA if the catheter remains equivocal; removing the catheter and performing a spinal anesthetic incurs the risk of a high or total spinal if an excessive amount of LA has already been administered epidurally. Recent evidence suggests that the number of top-ups is a reliable indicator of whether an epidural used for analgesia can be successfully used for surgical anesthesia. If several top-ups have been administered and the degree of analgesia remains equivocal, the catheter should be removed and replaced.
Dissipating Nerve Block Requiring Larger Doses
This problem occurs for several possible reasons. Patients who received CSEs with either spinal fentanyl or a combination of opioid and LA may experience an abrupt transition from relief to an inadequate epidural nerve block, particularly if the spinal anesthetic has resolved before a sufficient volume of the epidural infusion has accumulated. Increasingly larger doses of LA per epidural may be required to compensate for the inadequate epidural loading dose and to meet high patient expectations after experiencing the comfort of the spinal portion. Alternatively, if the epidural catheter has been used for analgesia and has been dosed frequently, tachyphylaxis to the LA can occur. Another possibility is that the catheter has migrated into a vessel (see previous discussion) or become completely dislodged from the epidural space. If the catheter remains in its initial insertion site, administer a bolus of higher-concentration LA and increase the infusion rate (if continuous). Consider adding an opioid or clonidine to enhance the quality of the nerve block.
Failed Epidural Analgesia
The problem of failed epidural analgesia is often seen in obstetrics. An epidural catheter is placed and dosed, but the patient remains uncomfortable. More LA is administered, with subjective improvement. Subsequently, the patient is taken to the operating room for cesarean delivery, which requires a dense T4 sensory level, and the nerve block is inadequate. Several options exist if an epidural fails despite troubleshooting measures. In elective situations, the epidural catheter can be replaced, preferably at a different interspace, and cautiously redosed to decrease the risk of high epidural block. For more urgent procedures, a CSE can be performed. A reduced dose of spinal medication is necessary if a large volume of epidural LA was administered while troubleshooting or if the patient has a partial nerve block. With a CSE, the sensory level can be raised as necessary with supplemental epidural dosing. A reduced dose singleshot spinal may also be appropriate if speed of onset is a concern.
However, replacing a failed epidural with a spinal technique incurs the risk of high or total spinal anesthesia. Infiltrating the skin and subcutaneous tissue with LA or performing a peripheral nerve block, depending on the time remaining in the surgery and the type of surgery, may provide alternatives. Conversion to GA is appropriate if there is insufficient time to repeat the neuraxial technique or to place a peripheral nerve block or if performing another neuraxial procedure presents undue risk.
Optimally, a nonfunctioning epidural will be recognized and replaced before large doses of LA have been administered and before alternative anesthetic techniques are required. The number of boluses required to maintain adequate analgesia is a reliable indicator that an epidural used for analgesia may fail on conversion to surgical anesthesia. As a general rule, if catheter function remains equivocal during dosing, stop injecting after a predetermined volume (eg, 10 mL) to ensure that performing another regional technique does not lead to high or total spinal anesthesia or to LA systemic toxicity (LAST).
Difficulty Removing the Epidural Catheter
Occasionally, resistance is met on attempted removal of the epidural catheter. Using excessive force may lead to catheter breakage and retained catheter fragments. In the event of catheter entrapment, placing the patient in the lateral decubitus position or in the original insertion position and applying continuous, gentle traction may facilitate removal. Sometimes, positioning the patient in the identical position in which the catheter was inserted may be necessary. Taping the catheter to the skin under traction and reattempting removal later, threading a stylet, and injecting saline into a wire-reinforced catheter have also been observed to assist in removal. Reports of neurologic sequelae from retained fragments are rare, suggesting that surgical removal is unwarranted in the asymptomatic patient.
COMPLICATIONS AND COMMON SIDE EFFECTS
Complications of epidural block can be classified broadly as either drug or procedure related. Potential drug-related complications include LAST, allergy to LAs, direct LA-induced nervous tissue injury, and drug or mode of delivery errors. Procedure-related complications may be mild to moderate or transient, such as back pain, pneumocephalus, and PDPH. Potentially life-threatening complications include subdural injection of LAs, total or high spinal, infectious or aseptic meningitis, cardiac arrest, SEA, epidural hematoma formation, and permanent neurologic injuries. In contrast to complications, several known or expected side effects accompany initiation and maintenance of epidural block without adversely affecting long-term patient outcomes. This section reviews both the complications and the common side effects associated with epidural block, with emphasis on risk factors, preventive measures, and treatment. Several of the complications are covered in greater detail elsewhere in this textbook.
Local Anesthetic Systemic Toxicity
Local anesthetic systemic toxicity results from excessive plasma concentration in the blood due to unintentional intravascular injection or, less commonly, systemic absorption from the injection site. Direct intravascular injection can occur with unintentional epidural vein cannulation during catheter placement or subsequent catheter migration into a vessel. Risk factors for intravascular cannulation include trauma to the epidural vessels during nerve block initiation, the use of stiff catheters, pregnancy, and patient positioning during epidural placement, among others (Table 28).
TABLE 28. Risk factors for epidural vein cannulation.
| Trauma to epidural vessels during block initiation |
| Multiple attempts at placement |
| Stiff, nonflexible catheter |
| Engorged epidural veins (eg, pregnancy) |
| Sitting position |
The risk of epidural vein cannulation in obstetric patients may be reduced with initiation of epidural block in the lateral position, the use of wire-reinforced catheters, the use of a single end-hole (versus multiorifice) catheters, fluid predistention with normal saline prior to threading the catheter, and limiting the depth of catheter to 6 cm or less (Table 29). Limiting the number of attempts at epidural placement; avoiding the lateral epidural space, where vessel puncture is more likely; and avoiding direct administration of LAs through the epidural needle may also reduce the risk of direct intravascular injection.
TABLE 29. Strategies to avoid epidural vein cannulation.
| Placement in lateral position |
| Use of flexible, wire-reinforced catheter |
| Use of single- versus multiorifice catheter |
| Fluid predistention prior to threading the catheter |
| Limit depth of catheter insertion to < 6 cminsertion |
Although the data are inconclusive regarding the role of catheter material and tip configuration, the use of flexible catheters may reduce the risk of subsequent catheter migration into a vessel. Because of preferential efflux from the proximal port of multiport catheters during continuous infusion techniques, there remains a remote possibility that a distal port may migrate into a vessel unnoticed until a manual bolus is administered. This can be avoided with the use of a single-orifice catheter.
Dosing the epidural catheter in 3- to 5-mL increments with frequent negative aspiration for blood and CSF is recommended to detect misplaced catheters. Most commercially available epidural catheters have distal and proximal “flashback” windows to facilitate visualization of blood or CSF on aspiration. The use of a transparent dressing and tape improves visualization of these windows. Although the use of the traditional epinephrine test dose is controversial, a test dose may be used to assess whether the catheter tip is in a blood vessel.
The degree of systemic absorption is determined in part by the site of injection, the dose and concentration of LA injected, properties of the LA administered, the vascularity of the injection site, and the presence or absence of epinephrine in the solution. Certain conditions and comorbidities, such as advanced age, liver failure, low plasma protein concentration, severe cardiac dysfunction, ischemic heart disease, cardiac conduction abnormalities, and metabolic and respiratory acidosis, may also predispose patients to systemic toxicity. In general, systemic absorption from the epidural space is less likely to
occur than from areas of higher vascularity. The areas of highest plasma concentration from absorption in descending order are as follows: intercostal, caudal, paracervical, epidural, brachial plexus, and sciatic/femoral (Table 30).
TABLE 30. Relative order of peak plasma concentration of local anesthetic associated with regional anesthesia (descending order).
| Intercostal |
| Caudal |
| Paracervical |
| Epidural |
| Brachial plexus |
| Sciatic/femoral |
However, trauma to the vessels during initiation of the epidural procedure may lead to more rapid intravascular absorption from the epidural space than anticipated. The addition of epinephrine to the LA solution diminishes systemic absorption but may not be appropriate in highly vascular areas, where systemic absorption is likely, or for all patient populations. Epinephrine may also unnecessarily prolong the duration of action of LAs. Toxicity associated with systemic absorption of LAs can be reduced by careful patient selection, remaining vigilant for signs and symptoms of toxicity, limiting the total dose of LA administered, use of appropriate LA concentrations, and, possibly, by using the newer amide LAs, such as ropivacaine and levobupivacaine.
Racemic bupivacaine has been associated with greater cardiotoxicity due to enhanced binding to and slower dissociation from ion channels in the myocardium.
Early CNS signs and symptoms of LA toxicity include lightheadedness, dizziness, tinnitus, perioral numbness and tingling, slurred speech, diplopia or blurred vision, restlessness, and confusion. Muscle twitching, tremors of the facial muscles and extremities, shivering, and generalized seizures occur at higher plasma concentrations, followed by global CNS depression, as manifested by drowsiness, unconsciousness, and respiratory arrest. Acidosis, hypercarbia, and hypoxia both predispose to and exacerbate CNS toxicity. Cardiovascular manifestations at high plasma concentrations include hypotension, bradycardia and other arrhythmias, and cardiac arrest (Table 31).
When LAST is recognized or suspected, refrain from administering additional LA and call for assistance. Treatment requires immediate attention to airway support, suppression of seizure activity, and preparedness for cardiopulmonary resuscitation and, possibly, CPB. Current guidelines recommend limiting individual epinephrine doses to less than 1 μg/kg during resuscitative efforts. Lipid emulsion (20%) therapy should be commenced with an initial loading dose of 1.5 mL/kg, followed by a continuous infusion of 0.25 mL/kg/min for a minimum of 10 minutes after circulatory stability has been restored. Refer to Local Anesthetic SystemicToxicity for a more detailed discussion of LAST.
TABLE 31. Signs and symptoms of local anesthatic
systemic toxicity.
| Central Nervous System Toxicity | Cardiovascular Toxicity |
|---|---|
| Perioral tingling and numbness | Hypotension |
| Lightheadedness/dizziness | Peripheral vasodilation |
| Tinnitus | Bradycardia, conduction delays |
| Visual disturbances | Ventricular dysrhythmias |
| Restlessness, agitation | Cardiac arrest |
| Slurred speech | |
| Shivering | |
| Generalized seizures | |
| Respiratory depression/ arrest |
Allergy to Local Anesthetics
True allergic reactions to LAs can occur, but fortunately are rare. Most documented reactions are not mediated by immunoglobulin E (IgE) and can be attributed to reactions to other agents administered concomitantly (eg, additives, epinephrine, preservatives, antibiotics) or to a delayed type IV hypersensitivity reaction (ie, mild contact dermatitis). Alternatively, reported reactions may be due to anxiety, a vasovagal episode, endogenous sympathetic stimulation, or an adverse patient response to the surgical, dental, ophthalmic, or obstetric procedure itself.
Based on an extensive review of the literature, Bhole and colleagues estimated the prevalence of true IgE-mediated allergy to be less than 1%. An immune complex–mediated reaction associated with reduced or depleted serum complement levels is even rarer.
When a patient reports a history of allergy to LAs, it is important to elicit a detailed history, including which LA was implicated, the dose and route of administration, the reaction that occurred, and the clinical setting. True allergic reactions may present with a range of dermatologic, cardiac, or respiratory signs and symptoms, such as hives, pruritus, angioedema, hypotension, shock, and bronchospasm. Although the current literature suggests that allergic reactions to amide LAs are more common than reactions to ester-linkage-type LAs, this may reflect the current preferential use of the former. Historically, adverse reactions, especially contact dermatitis, have been reported more often with ester agents, such as procaine, benzocaine, tetracaine, and chloroprocaine. This may be partly attributable to the fact that ester compounds are derivatives of para-aminobenzoic acid (PABA), an additive found in many household items (eg, lotion, sunscreen, cosmetics); previous exposure to PABA has been hypothesized to sensitize individuals to ester LAs. Alternatively, methylparaben, a preservative in both amide and ester LAs that is metabolized to PABA, may account for many of the reported allergic reactions. Cross-reactivity between the amide and ester groups has been reported, but is exceedingly rare and likely attributable to a common preservative. Cross-reactivity can occur between ester LAs and, less commonly, between amide LAs.
Reliable tests to identify sensitivity to LAs are currently lacking. The presence of serum mast cell tryptase can confirm an anaphylactic reaction in the immediate aftermath, while skin prick testing, intradermal testing, and subcutaneous challenge tests may help identify the causative agent. Management of an allergic reaction to LAs includes removing the offending agent; early administration of intravenous epinephrine to treat hypotension and cardiovascular collapse; airway support, if necessary; and, possibly, intravenous administration of histamine-1 and -2 receptor blockers, bronchodilators, and corticosteroids.
Arachnoiditis
Arachnoiditis is a rare disorder marked by inflammatory changes in the arachnoid mater. Although the precise mechanism remains unclear, fibrosis develops and adhesions form between the nerve roots and the membranes that surround the brain, spinal cord, and cauda equina. In chronic, adhesive cases, collagen deposits ultimately encapsulate the nerve roots, creating nerve root atrophy as a result of an interruption to the blood supply. Trauma, surgery, infections, contaminants, disinfectants, contrast media, tumors, subarachnoid hemorrhage, and the subarachnoid administration of irritants (eg, steroids) can precipitate these inflammatory changes. Accidental intrathecal administration of large volumes of chloroprocaine containing the preservative sodium bisulfite has also been linked to arachnoiditis, although the role of the preservative has been called into question in recent studies.
A link between epidural nerve block or catheter placement and arachnoiditis has not been clearly established in the literature. No data exist on the risk of arachnoiditis with the use of the antiseptic solution chlorhexidine in humans; nonetheless, it is prudent to keep the solution away from all drugs and equipment in the spinal and epidural kits and to permit it to dry before initiating neuraxial procedures. Chlorhexidine in alcohol remains the solution of choice for skin disinfection prior to initiation of central neuraxial block.
The clinical presentation of arachnoiditis is complex, with varied symptomatology, and may be delayed for several months. The most common clinical features are back pain that radiates to the lower extremities and increases on exertion; buttocks pain; muscle spasms; decreased range of motion of the trunk; sensory abnormalities; motor weakness or paralysis below the level of injury that does not typically progress; and urinary sphincter dysfunction (Table 32).
Unfortunately, the mixed clinical presentation can lead to misdiagnosis, and arachnoiditis may be attributed incorrectly to spinal stenosis, lumbar disk disease, spinal tumors, or other compressive lesions of the spine. Diagnosis can be confirmed by myelography, computed tomography (CT), or MRI. Characteristic MRI findings show conglomerations of roots residing centrally in the dural sac, adhesions tethering the nerve roots peripherally, and soft tissue replacing the subarachnoid space.
TABLE 32. Clinical presentation of arachnoiditis.
| Back pain radiating to lower extremities, worsens with activity |
| Buttocks pain |
| Muscle spasms |
| Motor weakness/paralysis |
| Decreased range of motion of trunk |
| Urinary sphincter dysfunction |
Unfortunately, significant neurologic improvement is unlikely with current therapies, including intravenous corticosteroids, NSAIDs, and antibiotic therapy. Deficits may progress to severe and permanent disability.
Backache
Back pain is a common postoperative complaint, with an incidence that ranges from 3% to 31% after nonobstetric surgery, regardless of the anesthetic technique. Although the etiology is multifactorial, both postoperative and peripartum back pain are often attributed to neuraxial techniques when a temporal association exists.
Backache after epidural block is more common, more severe, and longer lasting than after spinal procedures. Local trauma, inflammation of the ligaments, needle puncture of an intervertebral disk, stretching of the joint capsules and ligaments beyond their physiologic range, and muscle spasm may account for some of the reported postepidural back pain. The use of larger needles, the insertion of catheters, and the increased volume of LAs, when compared with spinal techniques, may also play a role. Large epidural doses of 2-chloroprocaine containing the preservative EDTA have also been associated with back pain; similar complications have not been observed with preservative-free 2-chloroprocaine. In a recent study, Hakim and colleagues identified the following independent risk factors for persistent (ie, ≥3 months) low-back pain after nonobstetric surgery with epidural anesthesia: multiple attempts at epidural placement, higher body mass index (BMI), surgery in the lithotomy position, and surgical time exceeding 2.5 hours.
Back pain after epidural block is usually self-limiting and should resolve within 7–10 days. Patients should be encouraged to refrain from bed rest. NSAIDs, acetaminophen, or heat may provide symptomatic relief. If pain persists, progresses, or is out of proportion to what might be expected, other etiologies, such as TNS, herniated disk, spinal stenosis, arachnoiditis, sacroiliitis, musculoskeletal injury, nerve injury, epidural abscess, and epidural hematoma, should be considered. Prophylactic interventions that may help prevent back pain associated with epidural procedures include performing a field nerve block to anesthetize the recurrent spinal nerves that innervate the interspinous ligaments and muscles prior to initiating epidural block; adding NSAIDs to the LA used for skin infiltration; and administering epidural dexamethasone.
Despite the widespread association between musculoskeletal back pain and neuraxial procedures, studies of pregnant women who have received epidural analgesia for labor pain provide compelling evidence that back pain is unrelated to neuraxial techniques. Several randomized controlled trials and prospective cohort studies have shown that new, long-term postpartum back pain is not caused by intrapartum epidural analgesia.
Postdural Puncture Headache
Postdural puncture headache is a common complication of spinal anesthesia, lumbar punctures (“spinal taps”), and epidural procedures complicated by ADP or unrecognized dural tear. The incidence of ADP is generally accepted to be at or below 1%; up to 80% of patients may experience PDPH following ADP. Although the precise mechanism remains poorly understood, signs and symptoms of PDPH appear to result from CSF leakage through the dural hole. In the upright position, the brain tissue sags in the cranial vault, creating painful traction on the dura, falx cerebri, cerebral blood vessels, tentorium, cranial nerves, and nerve roots. This traction also contributes to the cranial nerve palsies that are not uncommonly seen in patients with PDPH. Compensatory cerebral vasodilation in response to the decrease in CSF also appears to play a role in the genesis and severity of PDPH.
A universally accepted definition of PDPH is lacking in the literature. According to the International Headache Society, a PDPH develops within 5 days of a lumbar puncture, is usually accompanied by neck stiffness or subjective hearing symptoms,
and resolves spontaneously within 2 weeks or after effective treatment with an EBP. Clinically, patients commonly complain of a fronto-occipital headache that is mild or absent in the supine position and intensifies when the head is elevated. The pain may extend into the neck, shoulders, and upper extremities and may be accompanied by nausea and vomiting, dizziness, diplopia, tinnitus, blurred vision, nystagmus, and hearing loss. Cranial nerve involvement should prompt expeditious evaluation and treatment. The headache develops within 48 hours in the vast majority of cases (most commonly in the first 24 hours) (Table 33). Headaches that occur during or immediately after epidural procedures are more likely due to accidental injection of air during identification of the epidural space with the LOR to air technique (pneumocephalus).
TABLE 33. Signs and symptoms of postdural puncture headache.
| Fronto-occipital headache; intensifies when head is elevated |
| Neck stiffness |
| Neck, shoulder, and/or arm pain |
| Tinnitus, hearing loss |
| Nausea, vomiting, dizziness |
| Diplopia, blurred vision, nystagmus |
PDPH typically resolves spontaneously within 1 to 2 weeks but may last months or even years; a substantial percentage of patients may develop chronic headaches after ADP with a largebore Tuohy needle.
Risk factors for PDPH include younger age, female gender, lower BMI, pregnancy, pushing during the second stage of labor, the use of cutting versus atraumatic spinal needles, and the use of larger-gauge needles (Table 34). There is lesscompelling evidence regarding the role of needle bevel orientation, the number of dural punctures, the approach used to enter the epidural space (paramedian vs. midline), patient positioning during initiation of the epidural procedure, and the technique used to identify the epidural space (LOR to air versus saline with or without an air bubble).
TABLE 34. Risk factors for postdural puncture headache.
| Younger age |
| Female gender |
| Low body mass index |
| Loss of resistance to air |
| Pushing during second stage of labor |
| Use of cutting needle |
| Use of larger-gauge needle |
Several interventions for the prevention or treatment of PDPH have been proposed. There appears to be little benefit from conservative measures, such as bed rest and aggressive fluid administration. However, symptomatic relief may be obtained with analgesics, pharmacologic agents with vasoconstricting properties (caffeine, theophylline, sumatriptan), and, possibly, corticotropin (ACTH). In a quantitative systematic review of available evidence for measures to prevent PDPH, Apfel et al found that the administration of epidural morphine prior to removal of the catheter may confer some advantage, but this conclusion was based on one small randomized controlled trial. In a recent meta-analysis, Heesen et al. suggested that insertion of an intrathecal catheter following ADP may protect against PDPH and may reduce the need for an EBP, but additional studies are warranted. The evidence to date regarding the routine use of prophylactic EBP is not conclusive. There are limited data to support epidural patching with normal saline, dextran 40, and gelatin and fibrin glue.
Epidural blood patch, preferably early in the course of the headache, remains the gold standard for treatment. Observational studies reported rapid recovery in over 90% of patients after EBP, although relief may be transient in a small percentage of these patients. A well-designed, randomized controlled trial in neurology patients demonstrated that EBP offers complete resolution of symptoms in a large percentage of patients and reduces the severity of symptoms in those who do not experience complete resolution. In addition, early treatment with an EBP decreases the length of hospital stay and emergency room visits and permits patients to resume their activities of daily living sooner than otherwise feasible with expectant management.
Prior to performing an EBP, other causes of headache, such as preeclampsia/eclampsia and meningitis, should be ruled out. In certain clinical scenarios, it may also be necessary to rule out elevated ICP. Using sterile techniques, the epidural space at or below the level of prior ADP is identified using LOR to normal saline. The air bubble is omitted due to the concern that air may enter the dural breach, leading to pneumocephalus. Up to 20 mL of the patient’s blood (drawn aseptically) is slowly injected into the space; the clinician should stop injecting blood if the patient experiences moderate-to-severe pain or pressure in the lower back or neck region. Although the optimal volume of blood remains to be determined, injection of more than 20 mL appears to confer no additional benefit. The patient typically remains supine for at least 1 hour after the EBP. Back pain and, less frequently, neck pain are commonly experienced during the procedure and, when severe, may alert the clinician to stop injecting blood. To minimize the risk of infections and related sequelae, both the acquisition of autologous blood and identification of the epidural space should be performed using strict aseptic techniques. For a more in-depth discussion of PDPH, refer to Postdural Puncture Headache.
Subdural Injection
The subdural space has been described historically as a potential space between the normally closely adherent arachnoid mater and the overlying dura mater, although it may represent a cleft along the dural border cell layer that results only from direct tissue damage. Injection of a small dose of LA into the area can have profound hemodynamic and sympatholytic effects.
Subdural injection is a relatively rare occurrence, with an estimated incidence of 0.1%–0.82% of epidural injections. Clinical features that may help to distinguish subdural from epidural or spinal anesthesia include a higher-than-expected sensory block with poor caudal spread and sacral sparing; a higher-than-anticipated level of motor block of variable density; and a speed of onset that more closely resembles epidural anesthesia (10–20 minutes). Subdural injections commonly result in bilateral block, although unilateral or patchy nerve blocks can occur, with more notable sensory and motor changes in the upper extremities and inadequate analgesia and anesthesia in the lower extremities. Patients may develop Horner syndrome (ptosis, miosis, and anhidrosis), facial and corneal anesthesia, and dyspnea. In addition, mild-to-moderate hypotension may develop (Table 35). Treatment may require cardiovascular and respiratory support, including the administration of intravenous fluid and vasopressors and, possibly, endotracheal intubation with mechanical ventilation. However, case reports have described the use of subdural catheters to attain surgical anesthesia.
TABLE 35. Clinical presentation of a subdural nerve block.
| Higher-than-expected sensory block |
| Poor caudal spread, sacral sparing |
| Higher-than-expected motor block of variable density |
| block usually bilateral, but may be unilateral or asymmetric |
| Horner syndrome (ptosis, miosis, anhidrosis) |
| Facial and corneal anesthesia |
| Dyspnea |
| Hypotension |
Total Spinal Anesthesia
Total spinal block, which complicates an estimated 1 in 1400 attempted epidural procedures may result from unrecognized ADP with unintentional injection of an epidural dose of LA, the administration of a large dose of LA into the subdural compartment, and undetected migration of the epidural catheter tip into the subarachnoid space. It has also been observed when one hole of a multiorifice catheter is lodged in the subarachnoid space; with translocation of LA through an accidental or intentional dural breach; after CSE techniques; and after a failed epidural block is replaced with a spinal technique.
Total spinal anesthesia usually develops within minutes of LA administration, although it may occur unexpectedly later with changes in patient positioning or after a previously functioning epidural catheter has migrated into the subarachnoid space. During total spinal block, the LA spreads high enough to nerve block the entire spinal cord and, occasionally, the brainstem. Ascending sensory and motor changes develop rapidly, followed by profound hypotension, bradycardia, dyspnea, and difficulty phonating and swallowing. Unconsciousness and apnea may result from direct LA action on the brainstem, respiratory muscle paralysis, and cerebral hypoperfusion. Treatment includes airway support and, if necessary, endotracheal intubation; the administration of 100% oxygen; and hemodynamic support with intravenous fluids and vasopressors. Epinephrine should be used early and in escalating doses to stabilize the heart rate and blood pressure in unstable patients. As the nerve block recedes, the patient will regain consciousness and control of breathing followed by recovery of motor and sensory function. The administration of sedation until the nerve block regresses may be appropriate once the patient is stable.
Total spinal anesthesia can usually be avoided during continuous epidural catheter techniques by careful administration of LA in small, divided doses, with frequent aspiration and, possibly, the use of an epidural test dose. Patients should be monitored during top-ups, during incremental dosing to attain surgical anesthesia, and while on PCEAs. Unusual patient complaints and unexpected hemodynamic changes may warrant immediate removal and replacement of the catheter. If unintentional dural puncture is recognized during needle placement, the needle can be removed and placed at another interspace or a spinal catheter can be inserted. If ADP is recognized after catheter insertion, either proceeding with a continuous spinal technique or repeating the epidural procedure at another interspace is appropriate. A reduced dose of LA may be required if a catheter is successfully placed at a different spinal level after a prior dural puncture. If a spinal catheter is placed, the catheter should be clearly labeled, the infusion pump should be labeled and configured at a reduced dose, and all practitioners involved should be informed. Optimally, procedures and policies should be in place regarding management of spinal catheters.
Spinal Epidural Abscess
Spinal epidural abscess is a rare disorder that affects elderly and immunocompromised patients disproportionately. Individuals with prolonged intensive care unit stays, intravenous drug users, and patients with bacteremia, DM, alcohol dependence, cancer, HIV, and end-stage renal disease are at increased risk compared with the general population (Table 36). In recent decades, the incidence of SEA has increased, in part due to the increase in spinal instrumentation, the rise of illicit drug use, and the aging population.
TABLE 36. Predisposing conditions for spinal epidural abscess.
| Elderly | Steroid injection |
| Diabetes mellitus | Alcoholism |
| HIV/AIDS | Liver disease |
| Chronic steroid use | Renal failure |
| Adrenal insufficiency | Rheumatoid arthritis |
| Chronic epidural catheter | Cellulitis |
| Prolonged urinary catheterization | Psoas abscess |
| Indwelling vascular device | Intravenous drug use |
| Recent spinal instrumentation | Osteomyelitis |
An estimated 5% of SEAs are associated with epidural procedures. Risk factors for this rare complication include extended epidural catheter infusions and localized or systemic infection at the time of initiation of the nerve block. The site of epidural placement also appears to place some patients at higher risk for SEA formation, with thoracic and lumbar catheters implicated more often than cervical catheters. Poor adherence to sterile technique and, possibly, multiple attempts at epidural placement may place patients at additional risk.
Bacteria gain access to the epidural space through either hematogenous spread (most commonly) or contiguous spread; the source of access is not identified in the remainder of the cases. Staphylococcus aureus and, increasingly, methicillin-resistant S. aureus (MRSA) account for the vast majority of the SEA cases. Pathogens that are less commonly involved include Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus epidermidis, with the last more commonly associated with neuraxial procedures, including epidural block and epidural steroid injections. The infection appears to injure the spinal cord via direct mechanical compression or thrombosis (vascular occlusion from septic thrombophlebitis) or a combination of the two, although the precise mechanism has not been elucidated.
Early diagnosis, prompt treatment, and consistent follow-up are essential to avoid irreversible neurologic damage from SEA. The most common clinical symptoms are back pain, fever, and neurologic changes, such as leg weakness or sensory deficits, but a majority of patients do not present with this triad. Instead, patients may present with bladder dysfunction, sepsis, meningitis, paraplegia or quadriplegia, urinary tract infection (UTI), mental status changes, inflammation at the catheter site, headache, neck stiffness, or nausea and vomiting. Symptoms most commonly present within 7 days but may be delayed for 60 days or more. Elevated white blood cell (WBC) count and elevated erythrocyte sedimentation rate (ESR) or C-reactive protein may also be present, but these laboratory findings are nonspecific. If SEA is suspected, gadolinium-enhanced MRI is the diagnostic tool of choice. Some investigators have proposed that MRI scanning be considered in patients who have received epidural catheters if systemic and local signs of infection (eg, pus or erythema at the epidural insertion site) develop, even in the absence of neurologic deficits.
Broad-spectrum intravenous antibiotic administration, ultimately tailored to blood or tissue cultures, without surgical drainage may be appropriate treatment for SEA in the absence of neurologic symptoms. However, prompt surgical intervention (decompressive laminectomy, debridement of infected tissue, and abscess drainage) may be required, depending on the clinical presentation. Most likely owing to a delay in diagnosis or an initial misdiagnosis, morbidity associated with SEA remains high at 33%–47%, while mortality is estimated to be 5%. Neurologic status prior to intervention is the strongest predictor of final outcome. There is also a strong association between poor outcome and age greater than 70 years, MRSA strain infection, and the presence of DM or adrenal insufficiency.
The risk and long-term sequelae of SEA can be reduced with careful patient selection, maintenance of strict sterile techniques during initiation of epidural procedures, the administration of antibiotics prior to initiation of neuraxial block in patients with fever or localized infection, removal of indwelling catheters at the earliest sign of infection at the puncture site, and maintenance of a high index of suspicion in patients with risk factors who present with nonspecific neurologic complaints or local and systemic signs of infection, possibly several weeks after an epidural procedure.
Meningitis
Bacterial meningitis following epidural anesthesia is a rare event. Microorganisms can be transmitted via syringes, catheters, needles, infusion tubing, and medications injected into the epidural space, as well as from the clinician or patient. Similar infectious complications can occur with nonanesthetic procedures, such as EBP, myelography, epidural steroid injection, and diagnostic lumbar puncture. Most cases appear to be caused by contamination of the puncture site by organisms from the naso- or oropharynx of the health care provider. Less commonly, contaminants from incompletely sterilized skin and direct or hematogenous spread from an endogenous infectious site are implicated. A dural puncture, such as in the setting of a CSE, spinal, or an ADP, is believed to place patients at higher risk by allowing transfer of blood-borne pathogens across the blood-brain barrier. However, the incidence of bacterial meningitis remains low despite the increasing use of CSE and spinal techniques. Also, diagnostic lumbar puncture in the setting of bacteremia is rarely associated with meningitis. Additional risk factors include breaches in aseptic technique, reinsertion of the stylet that has been exposed to ambient air, difficulty performing the neuraxial procedure, and, relatedly, multiple attempts at spinal or epidural placement.
Signs and symptoms of meningitis include fever, headache, lethargy, confusion, nuchal rigidity, nausea/vomiting, photophobia, and Kernig sign (Table 37).
TABLE 37. Signs and symptoms of bacterial meningitis.
| Fever |
| Mental status changes (lethargy, confusion) |
| Headache |
| Nuchal rigidity |
| Nausea, vomiting |
| Backache |
| Photophobia |
| Seizures |
| Focal neurologic deficits |
| Kernig sign |
| Brudzinski sign |
Symptoms usually present within 6 to 36 hours after the anesthetic procedure. Because the initial clinical presentation is similar to that of a PDPH, the diagnosis of meningitis can be delayed. Meningitis can be distinguished from PDPH by the presence of a fever, mental status changes (ie, lethargy and confusion), and a headache that is not positional in nature. The diagnosis is confirmed with CSF analysis and culture with or without prior head CT. The CSF is often cloudy, with leukocytosis (predominantly neutrophils), elevated protein content, and low glucose concentration. Early diagnosis is essential. Common pathogens include Streptococcus salivarius and other strains of viridans streptococci, S. aureus, P. aeruginosa, Neisseria meningitidis, and Enterococcus faecalis. In many cases, no organism is isolated. Treatment of bacterial meningitis includes immediate empiric broad-spectrum antibiotic therapy, such as vancomycin with a third-generation cephalosporin, ultimately tailored to blood or CSF culture results. Neurologic sequelae may include cranial nerve palsies, hemiparesis, quadriparesis, and aphasia. If diagnosis and treatment are delayed, death may result. Adherence to full aseptic precautions, including removal of jewelry, hand washing, appropriate skin preparation with individual packets of antiseptic solution (preferably chlorhexidine with alcohol), the use of a sterile drape and dressing, and, at a minimum, the use of caps, sterile gloves, and face masks (changed between each patient encounter), is critical to minimize the risk of bacterial meningitis associated with neuraxial instrumentation. Alternatives to neuraxial techniques should be offered to patients at high risk for infectious complications, and patients with known or suspected bacteremia should be started on antibiotic therapy prior to neuraxial instrumentation.
Spinal Cord and Nerve Root Injury
Neurologic deficits can be caused by direct trauma to the spinal cord or spinal nerves, from spinal cord ischemia, from accidental injection of neurotoxic drugs or chemicals, or from hematomas or abscesses. Fortunately, serious neurologic injury is an extremely rare complication of neuraxial anesthesia, with an estimated incidence of 0.03%–0.1%. Horlocker and colleagues evaluated the records of over 4000 patients who had received lumbar epidurals for thoracic surgery while under GA and found no cases of neurologic complications. In another extensive review of 45,000 patients undergoing epidural placement, 40 cases of neurologic injuries were reported. Of note, 22 of these patients experienced paresthesias during the epidural procedure. There have been a few case reports of myelopathy and paraplegia occurring with thoracic epidurals placed in anesthetized patients, but these complications are exceedingly rare. Most peripheral neuropathies associated with neuraxial techniques resolve spontaneously. Those that become permanent are usually limited to persistent paresthesias and limited motor weakness.
Cauda Equina Syndrome
Cauda equina syndrome (CES), a rare state of neurologic compromise due to lumbosacral root compression, is characterized by bowel and bladder dysfunction, low-back pain, perineal sensory loss and other patchy sensory deficits, unilateral or bilateral sciatica, and lower extremity motor weakness. It has been associated with trauma, infection, lithotomy position, and ischemic compression by a hematoma, abscess, tumor, prolapsed intervertebral disk, or spondylolisthesis. CES has also been linked to direct neurotoxicity from large volumes or high concentrations of hyperbaric LAs in the sacral CSF. The nerve roots of the cauda equina have a poorly developed epineurium and limited blood flow and appear to be particularly susceptible to the pooling of LAs that may accompany continuous spinal infusions with microbore catheters, accidental intrathecal injection of large doses of LA intended for epidural anesthesia, and repeat intrathecal injections after failed spinal nerve block. Cases of CES have also been reported after single-shot spinals.
Whether intrinsic neurotoxicity of LAs, microenvironmental factors, excessively large doses of LAs, the microbore catheter used for continuous spinal anesthesia, patient positioning, the surgical procedure, or a combination of these factors is primarily responsible for CES has not been fully elucidated in the literature. The US Food and Drug Administration (FDA) removed small-bore continuous spinal catheters from the market in the early 1990s after a series of reports of CES in association with their use. However, continuous spinal anesthesia remains a useful technique. The use of lower concentrations of LAs, limiting the total dose of LA, limiting the depth of insertion of the spinal catheter, and using maneuvers to increase the spread of LA if maldistribution is suspected may minimize the risk of CES. Some investigators have also advocated using alternatives to hyperbaric 5% lidocaine given that safe alternatives exist. Unfortunately, CES is a permanent disability.
Symptoms consistent with this syndrome should prompt an early neurology consult and imaging studies. High-dose corticosteroids; surgical decompression (eg, in the case of a lumbar synovial cyst, hemangioma, or metastatic tumors); and treatment of the underlying disorder with chemotherapeutic agents or antibiotics (eg, in the case of malignancy or abscess formation) have been used, but limited data are available on optimal therapies and the course of recovery.
Epidural Hematoma
Epidural hematoma is a rare occurrence that can lead to cord compression, cord ischemia, or myelopathy similar to that caused by a space-occupying tumor. The incidence of hematoma associated with epidural block is estimated at 1:150,000, somewhat higher than that of spinal anesthetics (1:220,000). However, the incidence varies dramatically with the patient population and may be significantly higher in a subset of patients with a less-compliant epidural space and a greater likelihood of coagulation disorders. Indeed, hemostatic abnormalities during either initiation of epidural block or removal of the epidural catheter are present in the majority of reported cases, although a large proportion of the documented cases also occur spontaneously, with no predisposing factors.
Complicated epidural needle or catheter placement also appears to place the patient at risk for epidural hematoma formation. Coagulation disturbances that predispose patients to the development of epidural hematoma may be iatrogenic or secondary to underlying disease. Iatrogenic disturbances that may predispose patients to epidural hematoma formation are often associated with antithrombotic or thrombolytic therapy. The most recent American Society of Regional Anesthesia and Pain Medicine guidelines can be used to assist the anesthesiologist in determining the most appropriate and safest period for initiating epidural block in anticoagulated patients (see Neuraxial Anesthesia & Peripheral Nerve Blocks in Patients on Anticoagulants).
Thrombocytopenia is a relatively common cause of coagulopathy and may be related to pregnancy (gestational thrombocytopenia, HELLP syndrome, or preeclampsia/eclampsia), immune disorders (eg, ITP), or hepatic dysfunction, among other things. Unfortunately, there is no universally accepted platelet count that can be considered safe for performance of neuraxial block, and there is no widely available bedside test to assess platelet function. The nature of the underlying disorder must be taken into account (eg, Is the process dynamic, with rapidly dropping platelets? Is platelet function intact despite a low number? Is the patient on concomitant antiplatelet therapy?
Does he or she have another disorder affecting coagulation? and so on), and patients with thrombocytopenia should be approached with caution prior to initiation of neuraxial block.
Signs and symptoms of epidural hematoma may progress rapidly from mild sensory or motor deficits to devastating paraplegia and incontinence. Early signs include back pain and pressure, with motor and sensory deficits. The back pain associated with epidural hematoma may be severe and persistent.
Bowel and bladder incontinence, radicular pain, and worsening lower extremity neurologic deficits ensue. The onset of symptoms is usually within 12 hours to 2 days of initiation of neuraxial block or removal of an epidural catheter. Unfortunately, motor and sensory deficits within this timeframe may be mistaken for residual epidural block. Recurrence of motor and sensory block after partial or total resolution or, alternatively, prolonged nerve block should raise concerns for an epidural hematoma and prompt immediate consultation with a neurologist or neurosurgeon, as well as prompt MRI scanning. A negative MRI cannot rule out a developing hematoma, which may not be recognized by an inexperienced radiologist. Surgical decompression within 8 hours is advocated to minimize the risk of permanent neurologic injury.
Anterior Spinal Artery Syndrome
Anterior spinal artery syndrome (ASAS) occurs most commonly in patients with vascular disease and concomitant decreased spinal blood flow due to obstruction, compression, or hypotension. However, it has also been described in the setting of acute thoracic disk herniation, spondylosis, arteriovenous malformation, and similar pathologic conditions that can disrupt the tenuous blood flow in the anterior spinal artery distribution. ASAS is the most common neurologic complication after abdominal aortic surgery but has also been reported after surgery on the thoracic spine. Massive blood loss and persistent hypotension induced by neuraxial anesthesia have been implicated in the intraoperative development of this potentially life-threatening syndrome. ASAS presents with immediate, painless paraplegia and loss of lower extremity sensory function. Proprioception and vibration sense are spared. Prognosis is poor, with permanent and disabling neurologic deficits. Correction of intraoperative hypotension is essential in patients at high risk for ASAS.
Cardiac Arrest
Cardiac arrest resulting in death or brain damage is a rare complication of epidural block. Causes include unintentional total spinal anesthesia, LAST, myocardial ischemia, respiratory compromise, or any of several circulatory events that do not fall within these categories, such as complete block of the preganglionic cardiac accelerator fibers or vagal predominance in the setting of sympathetic block. Although the mechanism of increased vagal tone has not been fully elucidated, block of the sympathetic efferents results in vasodilation and a decrease in venous return. Decreased preload, in turn, may enhance cardiac vagal tone. Bradycardia, reduced CO, and cardiac arrest may result and can be attributed in part to reflex activity. The paradoxical Bezold-Jarisch reflex, for example, triggers slowing of the heart rate in response to decreased ventricular volume to permit more time for complete filling of the heart.
To minimize the risk of cardiac arrest associated with vagal predominance after neuraxial procedures, Pollard and colleagues proposed maintenance of adequate preload, the use of vagolytic drugs and pressor agents, if necessary, appropriate patient selection, and caution when changing patient positioning. Early epinephrine administration is recommended if bradycardia is marked. Vasopressin may be more effective than epinephrine for cardiopulmonary resuscitation during epidural anesthesia due to its longer-lasting effect and the improved acid-base profile after multiple doses, although adherence to the most current advanced cardiovascular life support (ACLS) protocol is recommended. Patient-related risk factors for cardiac arrest after neuraxial anesthesia include male gender, ASA I physical status, a low baseline heart rate (below 60), a sensory level above T6, age below 50, the use of β-adrenergic blocking agents, and prolonged PR interval. Rapid onset of action of LAs, the use of LAs that cause a more profound sympathetic nerve block, and a broader spread of nerve block have also been associated with hypotension and bradycardia after epidural block. Ligouri and colleagues have identified the following secondary factors that may precipitate or contribute to the severity of the increased vagal tone associated with bradycardia and asystole during epidural anesthesia: opioid administration, hypoxemia, sedation, hypercarbia, chronic medication use, and coexisting medical disease.
Side Effects
Several common side effects accompany epidural procedures, including transient fever in obstetric patients, nausea and vomiting, pruritus associated with neuraxial opioids, shivering, and urinary retention. Numerous studies have found an association between epidural labor analgesia and new-onset maternal intrapartum fever, although the relationship may not be causal. The increase in maternal temperature is often subclinical and self-limited and does not appear to have an adverse effect on the fetus.
Nausea and vomiting are common after both GA and neuraxial block. In the setting of epidural anesthesia and analgesia, nausea and vomiting may be attributed to hypotension or to the epidural adjuvants, such as opioids. Lipophilic opioids, such as fentanyl and sufentanil, administered epidurally appear to confer some decreased risk of nausea and vomiting when compared to epidural morphine. The administration of intravenous ondansetron (possibly in combination with other antiemetic agents), supplemental oxygen, and anxiolytics, as well as prompt correction of hypotension and hypovolemia, is recommended in the multimodal approach to therapy. Intravenous dexamethasone also appears to be effective in reducing nausea and vomiting associated with epidural morphine. Ephedrine, which is thought to have antiemetic effects unrelated to its hemodynamic effects, antihistamines, and anticholinergics also show promise.
Pruritus, a common side effect of neuraxial opioids, is observed more often during spinals than epidurals. It is usually transient and most commonly affects the nose and other areas of the face. The pure opioid antagonist naloxone effectively reverses opioid-induced pruritus, but at the cost of reversing the analgesia. The partial agonist-antagonist nalbuphine appears to be the most effective treatment of opioid-induced pruritus. A single 5-mg dose is often sufficient; a second 5-mg dose is occasionally necessary. Antihistamines are ineffective; opioid-induced pruritus is not a histamine-mediated reaction.
Shivering is another common side effect of neuraxial analgesia and anesthesia, occurring more quickly and more intensely in spinal versus epidural anesthesia. The mechanism remains unclear but may be related in part to impairment of central thermoregulatory control and redistribution of body heat.
Hypotension, as defined by a greater than 20% reduction in systolic, diastolic, or mean blood pressure, is common following epidural block. High thoracic nerve blocks, obesity, concurrent general and neuraxial anesthesia, hypovolemia, and excessive intraoperative blood loss are among the risk factors. Patients may present with nausea, vomiting, light-headedness, mental status changes, shortness of breath, difficulty breathing, and cardiac arrhythmias. Cardiovascular collapse may accompany severe cases. Optimally, the blood pressure should be maintained within 20% of the patient’s resting baseline. Proposed methods to reduce the incidence of hypotension after neuraxial block include the judicious administration of vasopressors (most commonly, ephedrine or phenylephrine); volume expansion with a crystalloid or colloid solution at the time of initiation of the nerve block (ie, a coload); maintenance of left uterine displacement after 18 to 20 weeks’ gestation in obstetric patients; changing patient positioning (eg, from supine to sitting) gradually; placing the nerve block at a lower spinal segment, when feasible; slow titration of epidural LAs; and reducing the dose of intrathecal or epidural LA. In cases of severe hypotension, elevating the lower extremities or placing military antishock trousers will reduce venous pooling. Alternative α- and β-adrenergic pressor agents, such as norepinephrine and epinephrine, should also be considered. Positioning the patient in reverse Trendelenburg in an attempt to limit the spread of the LA should be avoided; Trendelenburg will serve to alleviate venous pooling in the lower extremities and improve blood flow to the heart and brain (Table 38). Many of these measures to reduce the incidence and severity of hypotension following neuraxial block remain under debate in the literature and are beyond the purview of this chapter.
TABLE 38. Treatment of hypotension following neuraxial block.
| Discontinue epidural infusion, if applicable |
| Volume expansion with intravenous fluid administration |
| Intravenous vasopressor administration (phenylephrine and/or ephedrine) |
| Place patient in Trendelenburg position |
| Ensure uterine displacement after 18–20 weeks’ gestation |
| Elevate lower extremities |
| Administer atropine 0.4–0.5 mg IV with concurrent bradycardia |
| Wrap legs with thromboembolic deterrent stockings/ pneumatic compression hose; place military antishock trousers |
| Norepinephrine or epinephrine administration as needed |
SUMMARY
Both the indications for neuraxial techniques and the patient population that is considered appropriate for these procedures have expanded over the past several decades. Epidural block is currently being advocated as an adjuvant to GA for cardiothoracic, major vascular, and other high-risk surgeries; as the sole anesthetic in surgeries that were previously performed exclusively under GA; and for acute and chronic pain management. Neuraxial techniques are also increasingly being used in the ambulatory setting, where the decrease in PONV and improved pain relief permit earlier discharge; for a variety of diagnostic procedures; and to alleviate pain in adults and children in the end-of-life setting.
The benefits of epidural block are well established. Epidural techniques provide optimal pain relief following major surgeries and have been associated with fewer cardiovascular, respiratory, GI, and hematologic complications when compared with GA. The incidence of perioperative thromboembolic events, the time to return of GI function, and length of intensive care stay, among other things, appear to be reduced with epidural block. In addition, epidural anesthesia has been associated with prolonged survival and decreased cancer recurrence rates in patients with breast, localized colon, prostate, and ovarian cancers. However, the potential role of epidural block in reducing mortality after major surgery is still debated in the literature.
Despite the many potential advantages of epidural block, neuraxial techniques are not without risks, although major complications are rare. A risk-benefit analysis on a case-by-case basis and informed patient consent are warranted prior to initiation of epidural block.
This text is a sample of content from the Compendium of Regional Anesthesia on the NYSORA LMS.
NYSORA’s Compendium of Regional Anesthesia is simply the most comprehensive, and practical curriculum on Regional Anesthesia from A to Z, featuring NYSORA’s Premium content. As opposed to textbooks and e-books, the Compendium is continuously updated and features NYSORA’s newest videos, animations, and visual content.
The Compendium is one of several gold-standard educational courses on NYSORA’s Learning System (the NYSORA LMS), and registration to NYSORALMS.com is free. The FULL access to the Compendium, however, is based on an annual subscription, as it requires an army of illustrators, video editors, and an educational team to continue making it the BEST tool for education on everything regional anesthesia. While you can think of the compendium as an ebook on steroids, a quick test drive will give you a real-time feel of how incredible the Compendium really is. Your subscription will transform the way you read about regional anesthesia:
- Learn visually: Everything regional, including spinal, epidural, and nerve block procedures and management protocols
- Review step-by-step techniques instructions for over 60 nerve blocks
- Access NYSORA’s fabled illustrations, animations, and videos (such as Reverse Ultrasound Anatomy)
- Access RA info on any device via the desktop platform and mobile app
- Get real-time updates
- Review infographics for exam preparation (e.g. EDRA)
- Use the Community feed with real case discussions, images and videos are posted and discussed by subscribers and world’s top experts alike.
Even if you do not wish to subscribe to the Compendium, do register to the NYSORA LMS, be the first to know what’s new in regional anesthesia, and get involved in case discussions.
Here’s what the activity feed on NYSORA LMS looks like:
We are convinced that once you experience the Compendium on the NYSORA LMS, and you’ll never go back to your old books, and your subscription will support keeping NYSORA.com free for the rest of the world.
Additional Reading
- Frölich MA, Caton D: Pioneers in epidural needle design. Anesth Analg 2001;93:215–220.
- Cortés RC: Lumbar epidural anesthesia, 1931–1936: A second debut. Rev Esp Anestesiol Reanim 2005;52:159–168.
- Dogliotti AM: Segmental peridural spinal anesthesia: A new method of nerve block. Am J Surg 1933;20:107–118.
- Curbelo MM: Continuous peridural segmental anesthesia by means of a ureteral catheter. Curr Res Anesth Analg 1949;28:13–23.
- Modig J, Borg T, Karlström G, et al: Thromboembolism after total hip replacement: Role of epidural and general anesthesia. Anesth Analg 1983;62:174–180.
- Jørgensen LN, Rasmussen LS, Nielsen P, et al: Antithrombotic efficacy of continuous extradural analgesia after knee replacement. Br J Anaesth 1991;66:8–12.
- Rodgers A, Walker N, Schug S, et al: Reduction of postoperative mortality and morbidity with epidural or spinal anaesthesia: Results from overview of randomised trials. BMJ 2000;321:1–12.
- Mauermann WJ, Shilling AM, Zuo Z: A comparison of neuraxial nerve block versus general anesthesia for elective total hip replacement: A metaanalysis. Anesth Analg 2006;103:1018–1025.
- Schindler I: Regional anesthesia in the elderly: Indications and contraindications. Acta Anaesthesiol Scand Suppl 1997;111:209–211.
- Cherng CH, Wong CS, Chang FL, et al: Epidural morphine delays the onset of tourniquet pain during epidural lidocaine anesthesia. Anesth Analg 2002;94:1614–1616.
- Christopherson R, Beattie C, Frank SM, et al: Perioperative morbidity in patients randomized to epidural or general anesthesia for lower extremity vascular surgery. Anesthesiology 1993;79:422–434.
- Sproviero M: Neuraxial anesthesia for adult genitourinary procedures. In Wong CA (ed): Spinal and Epidural Anesthesia. McGraw-Hill, 2007, pp 229–236.
- Soriano D, Ajaj S, Chuong T, et al: Lidocaine spray and outpatient hysteroscopy: Randomized placebo-controlled trial. Obstet Gynecol 2000;96:661–664.
- Goldenberg M, Cohen SB, Etchin A, et al: A randomized prospective comparative study of general versus epidural anesthesia for transcervical hysteroscopic endometrial resection. Am J Obstet Gynecol 2001;184: 273–276.
- Murphy M, Heit MH, Fouts L, et al. Effect of anesthesia on voiding function after tension-free vaginal tape procedure. Obstet Gynecol 2003; 101:666–670.
- Nerve Block BM, Liu SS, Rowlingson AJ, et al: Efficacy of postoperative epidural analgesia: A meta-analysis. JAMA 2003;290:2455–2463.
- Ballantyne JC, Carr DB, deFerranti S, et al: The comparative effects of
postoperative analgesic therapies on pulmonary outcome: Cumulative meta-analyses of randomized, controlled trials. Anesth Analg 1998;86: 598–612. - Liu SS, Wu CL: Effect of postoperative analgesia on major postoperative
complications: A systemic update of the evidence. Anesth Analg 2007;104:689–702. - Manion SC, Brennan TJ: Thoracic epidural analgesia and acute pain management. Anesthesiology 2011;115:181–188.
- Nygård E, Kofoed KF, Freiburg J, et al: Effects of high thoracic epidural analgesia on myocardial blood flow in patients with ischemic heart disease. Circulation 2005;111:2165–2170.
- Scott NB, Turfrey DJ, Ray DA, et al: A prospective randomized study of the potential benefits of thoracic epidural anesthesia and analgesia in patients
undergoing coronary artery bypass grafting. Anesth Analg 2001;93:
528–535. - Loick HM, Schmidt C, Van Aken, et al: High thoracic epidural anesthesia, but not clonidine, attenuates the perioperative stress response via sympatholysis and reduces the release of troponin T in patients undergoing coronary artery bypass grafting. Anesth Analg 1999;88:701–709.
- Liu SS,Nerve Block BM, Wu CL: Effects of perioperative central neuraxial analgesia on outcome after coronary artery bypass surgery: A metaanalysis. Anesthesiology 2004;101:153–161.
- Svircevic V, Nierich AP, Moons KG, et al: Thoracic epidural anesthesia for cardiac surgery: A randomized trial. Anesthesiology 2011;114:
262–270. - Caputo M, Alwair H, Rogers CA, et al: Thoracic epidural anesthesia improves early outcomes in patients undergoing off-pump coronary artery bypass surgery: A prospective, randomized, controlled trial. Anesthesiology 2011;114:380–390.
- Møiniche S, Kehlet H, Dahl JB: A qualitative and quantitative systematic review of preemptive analgesia for postoperative pain relief: The role of timing of analgesia. Anesthesiology 2002;96:725–741.
- Groeben H, Schäfer B, Pavlakovic G, et al: Lung function under high thoracic segmental epidural anesthesia with ropivacaine or bupivacaine in patients with severe obstructive pulmonary disease undergoing breast surgery. Anesthesiology 2002;96:536–541.
- Diaz N, Wong CA: Neuraxial anesthesia for general surgery. In Wong CA (ed): Spinal and Epidural Anesthesia. McGraw-Hill, 2007, pp 221–227.
- Matot I, Scheinin O, Eid A, et al: Epidural anesthesia and analgesia in liver resection. Anesth Analg 2002;95:1179–1181.
- Zhang HW, Chen YJ, Cao MH, et al: Laparoscopic cholecystectomy under epidural anesthesia: A retrospective comparison of 100 patients. Am Surg 2012;78:107–110.
- Asakura K, Nakazawa K, Tanaka N, et al. Case of cardiac arrest due to
coronary spasm during laparoscopic distal gastrectomy. Masui 2011; 60:75–79. - Shir Y, Raja SN, Frank SM, et al: Intraoperative blood loss during radical retropubic prostatectomy. Epidural versus general anesthesia. Urology 1995;45:993–999.
- Gottschalk A, Smith DS, Jobes DR, et al: Preemptive epidural analgesia and recovery from radical prostatectomy: A randomized controlled trial. JAMA 1998;279:1076–1082.
- Stevens RA, Mikat-Stevens M, Flanigan R, et al: Does the choice of anesthetic technique affect the recovery of bowel function after radical prostatectomy? Urology 1998;52:213–218.
- Ozyuvaci E, Altan A, Karadeniz T, et al: General anesthesia versus epidural and general anesthesia in radical cystectomy. Urol Int 2005;74: 62–67.
- Micali S, Jarrett TW, Pappa P, et al: Efficacy of epidural anesthesia for retroperitoneoscopic renal biopsy. Urology 2000;55:590.
- Richards JT, Read JR, Chambers WA. Epidural anaesthesia as a method of pre-emptive analgesia for abdominal hysterectomy. Anaesthesia 1998;53:296–298.
- Mihic DN, Abram SE: Optimal regional anaesthesia for abdominal hysterectomy: Combined subarachnoid and epidural nerve block compared with other regional techniques. Eur J Anaesthesiol 1993;10:
297–301. - Holte K, Kehlet H: Epidural analgesia and risk of anastomotic leakage. Reg Anesth Pain Med 2001;26:111.
- Kehlet H, Mogensen T: Hospital stay of 2 days after open sigmoidectomy with a multimodal rehabilitation programme. Br J Surg 1999;86: 227–230.
- Bardram L, Funch-Jensen P, Kehlet H: Rapid rehabilitation in elderly patients after laparoscopic colonic resection. Br J Surg 2000;87: 1540–1545.
- Blichfeldt-Lauridsen L, Hansen BD: Anesthesia and myasthenia gravis. Acta Anaesthesiol Scand 2012;56:17–22.
- Kirsh JR, Diringer MN, Borel CO, et al: Preoperative lumbar epidural morphine improves postoperative analgesia and ventilatory function after transsternal thymectomy in patients with myasthenia gravis. Crit Care Med 1991;19:1474–1479.
- Bagshaw O: A combination of total intravenous anesthesia and thoracic epidural for thymectomy in juvenile myasthenia gravis. Paediatr Anaesth 2007;17:370–374.
- Hambly PR, Martin B: Anaesthesia for chronic spinal cord lesions. Anaesthesia 1998;53:273–289.
- Lambert DH, Deane RS, Mazuzan JE: Anesthesia and the control of blood pressure in patients with spinal cord injury. Anesth Analg 1982;61: 344–348.
- Ording H: Incidence of malignant hyperthermia in Denmark. Anesth Analg 1985;64:700–704.
- van Lier F, van der Geest PJ, Hoeks SE, et al: Epidural analgesia is associated with improved health outcomes of surgical patients with chronic obstructive pulmonary disease. Anesthesiology 2011;115:315–321.
- Mulroy MF, Salinas FV: Neuraxial techniques for ambulatory anesthesia. Int Anesthesiol Clin 2005;43:129–141.
- Anghelescu DL, Faughnan LG, Baker JN, et al: Use of epidural and peripheral nerve blocks at the end of life in children and young adults with cancer: The collaboration between a pain service and a palliative care service. Paediatr Anaesth 2010;20:1070–1077.
- Sielenkämper AW, Van Aken H: Epidural analgesia in sepsis: Too early to judge a new concept. Intensive Care Med 2004;30:1987–1989.
- Strümper D, Louwen F, Durieux ME, et al: Epidural local anesthetics: A novel treatment for fetal growth retardation? Fetal Diagn Ther 2005; 20:208–213.
- Hong JY, Lim KT: Effect of preemptive epidural analgesia on cytokine response and postoperative pain in laparoscopic radical hysterectomy for cervical cancer. Reg Anesth Pain Med 2008;33:44–51.
- Exadaktylos AK, Buggy DJ, Moriarty DC, et al: Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology 2006;105:660–664.
- Biki B, Mascha E, Moriarty DC, et al. Anesthetic technique for radical prostatectomy surgery affects cancer recurrence: A retrospective analysis. Anesthesiology 2008;109:180–187.
- Freise H, Van Aken H: Risks and benefits of thoracic epidural anaesthesia. Br J Anaesth 2011;107:859–868.
- Mutz C, Vagts DA: Thoracic epidural anesthesia in sepsis—is it harmful or protective? Crit Care 2009;13:182.
- Su TM, Lan CM, Yang LC, et al: Brain tumor presenting with fatal herniation following delivery under epidural anesthesia. Anesthesiology 2002;96:508–509.
- Grocott HP, Mutch WA: Epidural anesthesia and acutely increased intracranial pressure: Lumbar epidural space hydrodynamics in a porcine model. Anesthesiology 1996;85:1086–1091.
- Leffert LR, Schwamm LH: Neuraxial anesthesia in parturients with intracranial pathology: A comprehensive review and reassessment of risk. Anesthesiology 2013;119:703–718.
- Vandermeulen EP, Van Aken H, Vermylen J: Anticoagulants and spinal-epidural anesthesia. Anesth Analg 1994;79:1165–1177.
- Horlocker TT, Wedel DJ, Rowlingson JC, et al: Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine evidence-based guidelines (third edition). Reg Anesth Pain Med 2010;35:64–101.
- O’Rourke, N, Khan K, Hepner DL: Contraindications to neuraxial anesthesia. In Wong CA (ed): Spinal and Epidural Anesthesia. McGraw-Hill, 2007, pp 127–149.
- Choi S, Brull R: Neuraxial techniques in obstetric and non-obstetric patients with common bleeding diatheses. Anesth Analg 2009;109: 648–660.
- Bader AM, Hunt CO, Datta S, et al: Anesthesia for the obstetric patient with multiple sclerosis. J Clin Anesth 1988;1:21–24.
- Confavreux C, Hutchinson M, Hours MM, et al: Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N Engl J Med 1998;339:285–291.
- Hebl JR, Horlocker TT, Schroeder DR: Neuraxial anesthesia and analgesia in patients with preexisting central nervous system disorders. Anesth Analg 2006;103:223–228.
- Lirk P, Birmingham B, Hogan Q: Regional anesthesia in patients with preexisting neuropathy. Int Anesthesiol Clin 2011;49:144–165.
- Wiertlewski S, Magot A, Drapier S, et al: Worsening of neurologic symptoms after epidural anesthesia for labor in a Guillain-Barré patient. Anesth Analg 2004;98:825–827.
- Toledano RD, Pian-Smith MC: Human immunodeficiency virus: Maternal and fetal considerations and management. In Suresh MS, Segal BS, Preston RL, et al (eds): Shnider and Levinson’s Anesthesia for Obstetrics, 5th ed. Lippincott Williams & Wilkins, 2013, pp 595–605.
- Crosby ET, Halpern SH, Rolbin SH: Epidural anaesthesia for caesarean section in patients with active recurrent genital herpes simplex infections: A retrospective review. Can J Anaesth 1989;36:701–704.
- Bader AM, Camann WR, Datta S: Anesthesia for cesarean delivery in patients with herpes simplex virus type-2 infections. Reg Anesth 1990; 15:261–263.
- Brown NW, Parsons APR, Kam PCA: Anaesthetic considerations in a parturient with varicella presenting for caesarean section. Anaesthesia 2003;58:1092–1095.
- Hebl JR, Horlocker TT, Kopp SL, et al: Neuraxial block in patients with preexisting spinal stenosis, lumbar disk disease, or prior spine surgery: Efficacy and neurologic complications. Anesth Analg 2010;111: 1511–1519.
- McDonald SB: Is neuraxial block contraindicated in the patient with aortic stenosis? Reg Anesth Pain Med 2004;29:496–502.
- Ferguson EA, Paech MJ, Veltman MG: Hypertrophic cardiomyopathy and caesarean section: Intraoperative use of transthoracic echocardiography. Int J Obstet Anesth 2006;15:311–316.
- Rosenquist RW, Birnbach DJ: Epidural insertion in anesthetized adults: Will your patients thank you? Anesth Analg 2003;96:1545–1546.
- Horlocker TT, Abel MD, Messick JM, et al: Small risk of serious neurologic complications related to lumbar epidural catheter placement in anesthetized patients. Anesth Analg 2003;96:1547–1552.
- Han KR, Kim C, Park SK, et al: Distance to the adult cervical epidural space. Reg Anesth Pain Med 2003;28:95–97.
- Stonelake PS, Burwell RG, Webb JK: Variation in vertebral levels of the vertebra prominens and sacral dimples in subjects with scoliosis. J Anat 1988;159:165–172.
- Hughes RJ, Saifuddin A: Imaging of lumbosacral transitional vertebrae. Clin Radiol 2004;59:984–991.
- Kim JT, Bahk JH, Sung J: Influence of age and sex on the position of the conus medullaris and Tuffier’s line in adults. Anesthesiology 2003;99: 1359–1363.
- Saifuddin A, Burnett SJ, White J: The variation of position of the conus medullaris in an adult population. A magnetic resonance imaging study. Spine 1998;23:1452–1456.
- Vandermeersch E, Kick O, Mollmann M, et al: CSE—The combination of spinal and epidural anesthesia. Reg Anaesth 1991;14:108–112.
- Lirk P, Hogan Q: Spinal and epidural anatomy. In Wong CA (ed): Spinal and Epidural Anesthesia. McGraw-Hill, 2007, 1–25.
- Lee AJ, Ranasinghe JS, Chehade JM, et al: Ultrasound assessment of the vertebral level of the intercristal line in pregnancy. Anesth Analg 2011;113:559–564.
- Van Gessel EF, Forster A, Gamulin Z: Continuous spinal anesthesia: Where do spinal catheters go? Anesth Analg 1993;76:1004–1007.
- Broadbent CR, Maxwell WB, Ferrie R, et al: Ability of anaesthetists to identify a marked lumbar interspace. Anaesthesia 2000;55: 1122–1126.
- Lirk P, Messner H, Deibl M, et al: Accuracy in estimating the correct intervertebral space level during lumbar, thoracic and cervical anaesthesia. Acta Anaesthesiol Scand 2004;48:347–349.
- Hogan QH: Lumbar epidural anatomy. A new look by cryomicrotome section. Anesthesiology 1991;75:767–775.
- Heylings DJ: Supraspinous and interspinous ligaments of the human lumbar spine. J Anat 1978;125:127–131.
- Han KR, Kim C, Park SK, et al: Distance to the adult cervical epidural space. Reg Anesth Pain Med 2003;28:95–97.
- Zarzur E: Anatomic studies of the human ligamentum flavum. Anesth Analg 1984;63:499–502.
- Williams DM, Gabrielsen TO, Latack JT, et al: Ossification in the cephalic attachment of the ligamentum flavum. An anatomical and CT study. Radiology 1984;150:423–426.
- Brown DL: Spinal, epidural, and caudal anesthesia. In Miller RD, Fleisher LE, Johns RA, et al (eds): Miller’s Anesthesia, 6th ed. Elsevier, 2005, pp 1653–1683.
- Westbrook JL, Renowden SA, Carrie LE: Study of the anatomy of the extradural region using magnetic resonance imaging. Br J Anaesth 1993; 71:495–498.
- Lirk P, Kolbitsch C, Putz G, et al: Cervical and high thoracic ligamentum
flavum frequently fails to fuse in the midline. Anesthesiology 2003;99: 1387–1390. - Lirk P, Colvin J, Steger B, et al: Incidence of lower thoracic ligamentum
flavum midline gaps. Br J Anaesth 2005;94:852–855. - Lirk P, Moriggl B, Colvin J, et al: The incidence of lumbar ligamentum flavum midline gaps. Anesth Analg 2004;98:1178–1180.
- Reina MA, Villanueva MC, Machés F, et al: The ultrastructure of the human spinal nerve root cuff in the lumbar spine. Anesth Analg 2008; 106:339–344.
- Bernards CM: Sophistry in medicine: Lessons from the epidural space.
Reg Anesth Pain Med 2005;30:56–66. - Reina MA, De León Casasola O, Villanueva MC, et al: Ultrastructural findings in human spinal pia mater in relation to subarachnoid anesthesia. Anesth Analg 2004;98:1479–1485.
- Blomberg RG: The lumbar subdural extraarchnoid space of humans: An anatomical study using spinaloscopy in autopsy cases. Anesth Analg 1987;66:177–180.
- Haines DE: On the question of a subdural space. Anat Rec 1991;230: 3–21.
- Boon JM, Abrahams PH, Meiring JH, et al: Lumbar puncture: Anatomical review of a clinical skill. Clin Anat 2004;17:544–553.
- Hogan QH: Epidural anatomy: New observations. Can J Anaesth 1998;45:R40–48.
- Bernards CM: Epidural anesthesia. In Mulroy MF, Bernards CM, McDonald SB, et al (eds): A Practical Approach to Regional Anesthesia, 4th ed. Lippincott Williams & Wilkins, 2009, p 104.
- Igarashi T, Hirabayashi Y, Shimizu R, et al: The lumbar extradural structure changes with increasing age. Br J Anaesth 1997;78:149–152.
- Blomberg R: The dorsomedian connective tissue band in the lumbar epidural space of humans: An anatomical study using epiduroscopy in autopsy cases. Anesth Analg 1986;65;747–752.
- Hogan QH, Toth J: Anatomy of soft tissues of the spinal canal. Reg Anesth Pain Med 1999;24;303–310.
- Hogan QH: Distribution of solution in the epidural space: Examination by cryomicrotome section. Reg Anesth Pain Med 2002;27:150–156.
- Fujinaka MK, Lawson EF, Schukteis G, Wallace MS: Cervical epidural depth: correlation between needle angle, cervical anatomy, and body surface area. Pain Med 2012;13:665–669.
- Aldrete JA, Mushin AU, Zapata JC, et al: Skin to cervical epidural space distances as read from magnetic resonance imaging films: Consideration of the “hump pad.” J Clin Anesth 1998;10:309–313.
- Segal S, Beach M, Eappen S: A multivariate model to predict the distance from the skin to the epidural space in an obstetric population. Reg Anesth 1996;21:451–455.
- Sutton DN, Linter SP: Depth of extradural space and dural puncture.
Anaesthesia 1991;46:97–98. - Reynolds AF, Roberts PA, Pollay M, et al: Quantitative anatomy of the
thoracolumbar epidural space. Neurosurgery 1986;17:905–907. - Hogan QH: Epidural anatomy examined by cryomicrotome section. Influence of age, vertebral level, and disease. Reg Anesth 1996;21: 395–406.
- Toprak HI, Ozpolat Z, Ozturk E, et al: Hyperbaric bupivacaine affects the doses of midazolam required for sedation after spinal anaesthesia. Eur J Anaesthesiol 2005;22:904–906.
- Shono A, Sakura S, Saito Y, et al: Comparison of 1% and 2% lidocaine epidural anaesthesia combined with sevoflurane general anaesthesia utilizing a constant bispectral index. Br J Anaesth 2003;91:825–829.
- Koo M, Sabaté A, Dalmau A, et al: Sevoflurane requirements during coloproctologic surgery: Difference between two different epidural regimens. J Clin Anesth 2003;15:97–102.
- Eappen S, Kissin I: Effect of subarachnoid bupivacaine nerve block on anesthetic requirements for thiopental in rats. Anesthesiology 1998;88: 1036–1042.
- Tverskoy M, Shagal M, Finger J, et al: Subarachnoid bupivacaine block decreases midazolam and thiopental hypnotic requirements. J Clin Anesth 1994;6:487–490.
- Tverskoy M, Shifrin V, Finger J, et al: Effect of epidural bupivacaine nerve block on midazolam hypnotic requirements. Reg Anesth 1996;21: 209–213.
- Hodgson PS, Liu SS, Gras TW: Does epidural anesthesia have general anesthetic effects? A prospective, randomized, double-blind, placebocontrolled trial. Anesthesiology 1999;91:1687–1692.
- Reinoso-Barbero F, Martínez-García E, Hernández-Gancedo MC, et al: The effect of epidural bupivacaine on maintenance requirements of sevoflurane evaluated by bispectral index in children. Eur J Anaesthesiol 2006;23:460–464.
- Holte K, Foss NB, Svensén C, et al: Epidural anesthesia, hypotension, and
changes in intravascular volume. Anesthesiology 2004;100:281–286. - Meisner A, Rolf A, Van Aken H: Thoracic epidural anesthesia and the patient with heart disease: Benefits, risks, and controversies. Anesth Analg 1997;85, 517–528.
- Missant C, Claus P, Rex S, Wouters PF: Differential effects of lumbar and thoracic epidural anaesthesia on the haemodynamic response to acute right
ventricular pressure overload. Br J Anaesth 2010;104:143–149. - Buggy DJ, Doherty WL, Hart EM, Pallet EJ: Postoperative wound oxygen tension with epidural or intravenous analgesia: A prospective, randomized, single-blind clinical trial. Anesthesiology 2002;97:952–958.
- Kabon B, Fleischmann E, Treschan T, et al: Thoracic epidural anesthesia
increases tissue oxygenation during major abdominal surgery. Anesth Analg 2003;97:1812–1817. - Olausson K, Magnusdottir H, Lurje L, et al: Anti-ischemic and antianginal
effects of thoracic epidural anesthesia versus those of conventional medical therapy in the treatment of severe refractory unstable angina pectoris. Circulation 1997;96:2178–2182. - Richter A, Cederholm I, Fredrikson M, et al: Effect of long-term thoracic epidural analgesia on refractory angina pectoris: A 10 year experience. J Cardiothorac Vasc Anesth 2012;26:822–828.
- Rolf N, Van de Velde M, Wouters PF, et al: Thoracic epidural anesthesia improves functional recovery from myocardial stunning in conscious dogs. Anesth Analg 1996;83:935–940.
- Rolf N, Meissner A, Van Aken H, et al: The effects of thoracic epidural
anesthesia on functional recovery from myocardial stunning in propofol anesthetized dogs. Anesth Analg 1997;84:723–729. - Freise H, Van Aken HK: Risks and benefits of thoracic epidural anaesthesia. Br J Anaesth 2011;107:859–868.
- Svircevic V, van Dijk D, Nierich AP, et al: Meta-analysis of thoracic epidural anesthesia versus general anesthesia for cardiac surgery. Anesthesiology 2011;114:271–282.
- Gu WJ, Wei CY, Huang DQ, et al: Meta-analysis of randomized controlled trials on the efficacy of thoracic epidural anesthesia in preventing atrial fibrillation after coronary artery bypass grafting. BMC Cardiovasc Disord 2012;12:1–7.
- Freund FG, Bonica JJ, Ward RJ, et al: Ventilatory reserve and level of motor nerve block during high spinal and epidural anesthesia. Anesthesiology 1967;28:834–837.
- Groeben H: Epidural anesthesia and pulmonary function. J Anesth 2006;20:290–299.
- Lirk P, Hollmann MW: Outcome after regional anesthesia: Weighing risks and benefits. Minerva Anestesiol 2014;80:610–618.
- Sielenkämper AW, Van Aken H: Thoracic epidural anesthesia: More than just anesthesia/analgesia. Anesthesiology 2003;99:523–525.
- Brodner G, Van Aken H, Hertle L, et al: Multimodal perioperative management—combining thoracic epidural analgesia, forced mobilization, and oral nutrition—reduces hormonal and metabolic stress and improves convalescence after major urologic surgery. Anesth Analg 2001;92:1594–1600.
- Frank SM, Beattie C, Christopherson R, et al: Epidural versus general anesthesia, ambient operating room temperature, and patient age as predictors of inadvertent hypothermia. Anesthesiology 1992;77: 252–257.
- Matsukawa T, Sessler DI, Christensen R, et al: Heat flow and distribution
during epidural anesthesia. Anesthesiology 1995;83: 961–967. - Szmuk P, Ezri T, Sessler D, et al: Spinal anesthesia speeds active postoperative rewarming. Anesthesiology 1997;87:1050–1054.
- Backman SB: Regional anesthesia: sympathectomy-mediated vasodilation. Can J Anaesth 2009;56:702–703.
- Greene NM: Area of differential nerve block in spinal anesthesia with hyperbaric tetracaine. Anesthesiology 1958;19:45–50.
- Brull SJ, Greene NM: Zones of differential sensory nerve block during extradural anaesthesia. Br J Anaesth 1991;66:651–655.
- Valley MA, Bourke DL, Hamil MP, et al: Time course of sympathetic block during epidural anesthesia: Laser Doppler flowmetry studies or regional skin perfusion. Anesth Analg 1993;76:289–294.
- Ginosar Y, Weiniger CF, Kurz V, et al: Sympathectomy mediated vasodilation: A randomized concentration ranging study of epidural bupivacaine. Can J Anaesth 2009;56:213–221.
- Bengsston M: Changes in skin blood flow and temperature during spinal analgesia evaluated by laser Doppler flowmetry and infrared thermography. Acta Anaesthesiol Scand 1984;28:625–630.
- Kimura T, Goda Y, Kemmotsu O, et al: Regional differences in skin blood flow and temperature during total spinal anaesthesia. Can J Anaesth 1992;39:123–127.
- Bromage PR, Joyal AC, Binney JC: Local anesthetic drugs: penetration from the spinal extradural space into the neuraxis. Science 1963; 140: 392–394.
- Moore D, Spierdijk J, VanKleef J, et al: Chloroprocaine neurotoxicity: Four additional cases. Anesth Analg 1982;61:155–159.
- Lynch C 3rd: Depression of myocardial contractility in vitro by bupivacaine, etidocainem, and lidocaine. Anesth Analg 1986;65: 551–559.
- Graf B, Martin E, Bosnjak Z: Stereospecific effect of bupivacaine isomers on atrioventricular conduction in the isolated perfused guinea pig heart. Anesthesiology 1997;86:410–419.
- McClure JH: Ropivacaine. Br J Anaesth 1996;76:300–307.
- Bader AM, Datta A, Flanagan H, et al: Comparison of bupivacaine- and ropivacaine-induced conduction block in isolated rabbit vagus nerve. Anesth Analg 1989;68:724–727.
- Curatolo M, Petersen-Felix S, Arendt-Nielsen L, et al: Adding sodium bicarbonate to lidocaine enhances the depth of epidural block. Anesth Analg 1998;86:341–347.
- Corke BC, Carlson CG, Dettbarn WD: The influence of 2-chloroprocaine on the subsequent analgesic potency of bupivacaine. Anesthesiology 1984;60:25–27.
- Stanton-Hicks M, Berges PU, Bonica JJ: Circulatory effects of peridural nerve block. IV. Comparison of the effects of epinephrine and phenylephrine. Anesthesiology 1973;39:308–314.
- Bouguet D: Caudal clonidine added to local anesthetics enhances post-operative analgesia after anal surgery in adults. Anesthesiology 1994; 81:A942.
- Kroin JS, Buvanendran A, Beck DR, et al: Clonidine prolongation of lidocaine analgesia after sciatic nerve block in rats is mediated via the hyperpolarization-activated cation current, not by alpha-adrenoreceptors. Anesthesiology 2004;101:488–494.
- Landau R, Schiffer E, Morales M, et al: The dose-sparing effect of clonidine added to ropivacaine for labor epidural analgesia. Anesth Analg 2002;95:728–734.
- Kayacan N, Arici G, Karsli B, et al: Patient-controlled epidural analgesia in labour: The addition of fentanyl or clonidine to bupivacaine. Agri 2004;16:59–66.
- Novak-Jankovic V, Paver-Eržen V, Bovill JG, et al: Effect of epidural and intravenous clonidine on the neuro-endocrine and immune stress response in patients undergoing lung surgery. Eur J Anaesthesiol 2000; 17:50–56.
- Matot I, Drenger B, Weissman C, et al: Epidural clonidine, bupivacaine and methadone as the sole analgesic agent after thoracotomy for lung resection. Anaesthesia 2004;59:861–866.
- Wu CT, Jao SW, Borel CO: The effect of epidural clonidine on perioperative cytokine response, postoperative pain, and bowel function in patients undergoing colorectal surgery. Anesth Analg 2004;99: 502–509.
- Roelants F, Lavand’homme PM, Mercier-Fuzier V: Epidural administration of neostigmine and clonidine to induce labor analgesia: Evaluation of efficacy and local anesthetic-sparing effect. Anesthesiology 2005;102:1205–1210.
- Boogmans T, Vertommen J, Valkenborgh T, et al: Epidural neostigmine and clonidine improves the quality of combined spinal epidural analgesia in labour: A randomised, double-blind controlled trial. Eur J Anaesthesiol 2014;31:190–196.
- Roelants F, Lavand’homme PM: Epidural neostigmine combined with sufentanil provides balanced and selective analgesia in early labor. Anesthesiology 2004;101:439–444.
- Omais M, Lauretti G, Paccola CA: Epidural morphine and neostigmine for postoperative analgesia after orthopedic surgery. Anesth Analg 2002;95:1698–1701.
- Habib AS, Gan TJ. Use of neostigmine in the management of acute postoperative pain and labour pain: A review. CNS Drugs 2006;20: 821–839.
- Lauretti GR, Gomes JM, Reis MP, Pereira NL:. Low doses of epidural ketamine or neostigmine, but not midazolam, improve morphine analgesia in epidural terminal cancer pain therapy. J Clin Anesth 1999; 11(8):663–668.
- Lauretti GR, de Oliveira R, Perez MV, et al: Postoperative analgesia by intraarticular and epidural neostigmine following knee surgery. J Clin Anesth 2000;12:444–448.
- Johansen MJ, Gradert TL, Sattefield WC, et al: Safety of continuous intrathecal midazolam infusion in the sheep model. Anesth Analg 2004;98:1528–1535.
- Bharti N, Madan R, Mohanty PR, et al: Intrathecal midazolam added to bupivacaine improves the duration and quality of spinal anaesthesia. Acta Anaesthesiol Scand 2003;47:1101–1105.
- Tucker AP, Mezzatesta J, Nadeson R, et al: Intrathecal midazolam II: Combination with intrathecal fentanyl for labor pain. Anesth Analg 2004;98:1521–1527.
- Ugur B, Basaloglu K, Yurtseven T, et al: Neurotoxicity with single dose intrathecal midazolam administration. Eur J Anaesthesiol 2005;22: 907–912.
- Yaksh TL, Allen JW: Preclinical insights into the implementation of intrathecal midazolam: a cautionary tale. Anesth Analg 2004;98: 1509–1511.
- Gambling D, Hughes T, Martin G, et al: A comparison of Depodur, a novel, single-dose extended-release epidural morphine, with standard epidural morphine for pain relief after lower abdominal surgery. Anesth Analg 2005;100:1065–1074.
- Viscusi ER, Martin G, Hartrick CT, et al: Forty-eight hours of postoperative pain relief after total hip arthroplasty with a novel, extended-release epidural morphine formulation. Anesthesiology 2005;102:1014–1022.
- Carvalho B, Riley E, Cohen SE, et al: Single-dose, sustained release epidural morphine in the management of postoperative pain after elective cesarean delivery: Results of a multicenter randomized controlled study. Anesth Analg 2005;100:1150–1158.
- Magnusdottir H, Kimo K, Ricksten S: High thoracic epidural analgesia does not inhibit sympathetic nerve activity in the lower extremities. Anesthesiology 1999;91:1299–1304.
- Basse L, Werner M, Kehlet H: Is urinary drainage necessary during continuous epidural anesthesia after colonic resection. Reg Anesth Pain Med 2000;25:498–501.
- Galindo A, Hernandez J, Benavides O, et al: Quality of spinal extradural anesthesia: The influence of spinal nerve root diameter. Br J Anaesth 1975;47:41–47.
- Duggan J, Bowler GM, McClure JH, et al: Extradural nerve block with bupivacaine: Influence of dose, volume, concentration and patient characteristics. Br J Anaesth 1998;61:324–331.
- Sakura S, Sumi M, Kushizaki H, et al: Concentration of lidocaine affects intensity of sensory nerve block during lumbar epidural anesthesia. Anesth Analg 1999;88:123–127.
- Hodgkinson R, Husain FJ: Obesity, gravity, and spread of epidural anesthesia. Anesth Analg 1981;60:421–424.
- Seow LT, Lips FJ, Cousins MJ: Effect of lateral position on epidural block for surgery. Anaesth Intensive Care 1983;11:97–102.
- Park WY, Hagins FM, Rivat EL, et al: Age and epidural dose response in adult men. Anesthesiology 1982;56:318–320.
- Park WY, Massengale M, Kim SI, et al: Age and spread of local anesthetic solutions in the epidural space. Anesth Analg 1980;59:768–771.
- Veering BT, Burm AG, van Kleef JW, et al: Epidural anesthesia with bupivacaine: Effects of age on neural block and pharmacokinetics. Anesth Analg 1987;66:589–593.
- Hirabayashi Y, Shimizu R, Matsuda I, et al: Effect of extradural compliance and resistance on spread of extradural analgesia. Br J Anaesth 1990;65:508–513.
- Bromage PR, Bufoot MF, Crowell DE, et al: Quality of epidural block. I. Influence of physical factors. Br J Anaesth 1964;36: 342–352.
- Grundy EM, Zamora AM, Winnie AP: Comparison of spread of epidural anesthesia in pregnant and nonpregnant women. Anesth Analg 1978;57:544–546.
- Arakawa M: Does pregnancy increase the efficacy of lumbar epidural anesthesia? Int J Obstet Anesth 2004;13:86–90.
- Henke VG, Bateman BT, Leffert LR: Spinal anesthesia in severe preeclampsia. Anaesth Analg 2013;117:686–693.
- Spiegel JE, Vasudevan A, Li Y, et al: A randomized prospective study comparing two flexible epidural catheters for labour analgesia. Br J Anaesth 2009;103:400–405.
- Frölich MA, Zhang K, Ness TJ: Effect of sedation on pain perception. Anesthesiology 2013;118:611–621.
- Frölich MA, Burchfield DJ, Euliano TY, et al: A single dose of fentanyl and midazolam prior to Cesarean section have no adverse neonatal effects. Can J Anaesth 2006;53:79–85.
- Varelmann D, Pancaro C, Cappiello EC, et al: Nocebo-induced hyperalgesia during local anesthetic injection. Anesth Analg 2010;110: 868–870.
- Marica LS, O’Day T, Janosky J, et al: Chloroprocaine is less painful than lidocaine for skin infiltration anesthesia. Anesth Analg 2002;94: 351–354.
- Cepeda MS, Tzortzopoulou A, Thackrey M, et al: Adjusting the pH of lidocaine for reducing pain on injection. Cochrane Database Syst Rev 2010;12:CD006581.
- Al Shahwan MA: Prospective comparison between buffered 1% lidocaine-epinephrine and skin cooling in reducing the pain of local anesthetic infiltration. Dermatol Surg 2012;38:1654–1659.
- Hamilton CL, Riley ET, Cohen SE: Changes in the position of epidural catheters associated with patient movement. Anesthesiology 1997;86: 778–784.
- Richardson MG, Thakur R, Abramowicz JS, et al: Maternal posture influences the extent of sensory nerve block produced by intrathecal dextrosefree bupivacaine with fentanyl for labor analgesia. Anesth Analg 1996;83:1229–1233.
- Tsen LC: Neuraxial techniques for labor analgesia should be placed in the lateral position. Int J Obstet Anesth 2008;17:146–149.
- American Society of Anesthesiologists Task Force on Infectious Complications Associated With Neuraxial Techniques: Practice advisory for the prevention, diagnosis, and management of infectious complications associated with neuraxial techniques. Anesthesiology 2010;112:530–545.
- Malhotra S, Dharmadasa A, Yentis SM: One vs two applications of chlorhexidine/ethanol for disinfecting the skin: Implications for regional anaesthesia. Anaesthesia 2011;66:574–578.
- Bogod DS: The sting in the tail: Antisepsis and the neuraxis revisited.
Anaesthesia 2012;67:1305–1309. - Segal S, Arendt KW: A retrospective effectiveness study of loss of resistance to air or saline for identification of the epidural space. Anesth Analg 2010;110:558–563.
- Laviola S, Kirvelä M, Spoto M: Pneumocephalus with intense headache and unilateral pupillary dilatation after accidental dural puncture during epidural anesthesia for cesarean section. Anesth Analg 1999;88: 582–583.
- Van de Velde M: Identification of the epidural space: Stop using the loss of resistance to air technique! Acta Anaesthiol Belg 2006;57: 51–54.
- Dalens B, Bazin JE, Haberer JP: Epidural bubbles as a cause of incomplete analgesia during epidural anesthesia. Anesth Analg 1987;66: 679–683.
- Naulty JS, Ostheimer GW, Datta S, et al: Incidence of venous air embolism during epidural catheter insertion. Anesthesiology 1982;57: 410–412.
- Evron S, Sessler D, Sadan O, et al: Identification of the epidural space: Loss of resistance with air, lidocaine, or the combination of air and lidocaine. Anesth Analg 2004;99:245–250.
- Balki M, Lee Y, Halpern S, et al: Ultrasound imaging of the lumbar spine in the transverse plane: The correlation between estimated and actual depth to the epidural space in obese parturients. Anesth Analg 2009;108: 1876–1881.
- Mhyre JM: Why do pharmacologic test doses fail to identify the unintended intrathecal catheter in obstetrics? Anesth Analg 2013; 116:4–7.
- Tsui B, Gupta S, Finucane B: Confirmation of epidural catheter placement using nerve stimulation. Can J Anaesth 1998;45: 640–644,1998.
- Lyons GR, Kocarev MG, Wilson RC, et al: A comparison of minimum local anesthetic volumes and doses of epidural bupivacaine (0.125% w/v and 0.25% w/v) for analgesia in labor. Anesth Analg 2007;104: 412–415.
- Wong CA, Ratliff JT, Sullivan JT, et al: A randomized comparison of programmed intermittent epidural bolus with continuous epidural infusion for labor analgesia. Anesth Analg 2006;102:904–909.
- Heesen M, Klohr S, Rossaint R, et al: Can the incidence of accidental dural puncture in laboring women be reduced? A systematic review and meta-analysis. Minerva Anestesiol 2013;79:1187–1197.
- Van de Velde M, Schepers R, Berends N, et al: Ten years of experience with accidental dural puncture and post-dural puncture headache in a tertiary obstetric anaesthesia department. Int J Obstet Anesth 2008;17: 329–335.
- Agerson AN, Scavone BM: Prophylactic epidural blood patch after unintentional dural puncture for the prevention of postdural puncture headache in parturients. Anesth Analg 2012;115:133–136.
- Bauer ME, Kountanis JA, Tsen LC, et al: Risk factors for failed conversion of labor epidural analgesia to cesarean delivery anesthesia: A systematic review and meta-analysis of observational trials. Int J Obstet Anesth 2012;21:294–309.
- Toledano RD, Kodali BS, Camann WR: Anesthesia drugs in the obstetric and gynecologic practice. Rev Obstet Gynecol 2009;2: 93–100.
- Mhyre JM, Greenfield ML, Tsen LC, et al: A systematic review of randomized controlled trials that evaluate strategies to avoid epidural vein cannulation during obstetric epidural catheter placement. Anesth Analg 2009;108:1232–1242.
- Neal JM, Mulroy MF, Weinberg GL: American Society of Regional Anesthesia and Pain Medicine checklist for managing local anesthetic systemic toxicity: 2012 version. Reg Anesth Pain Med 2012;37:16–18.
- Bhole MV, Manson AL, Seneviratne SL, et al: IgE-mediated allergy to local anaesthetics: Separating fact from perception: A UK perspective. Br J Anaesth 2012;108:903–911.
- Rice I, Wee MY, Thomson K: Obstetric epidurals and chronic adhesive arachnoiditis. Br J Anaesth 2004;92:109–120.
- Taniguchi M, Bollen AW, Drasner K: Sodium bisulfite: Scapegoat for chloroprocaine neurotoxicity? Anesthesiology 2004;100:85–91.
- Checketts MR: Wash & go—but with what? Skin antiseptic solutions for central neuraxial nerve block. Anaesthesia 2012;67:819–822.
- Na EH, Han SJ, Kim MH: Delayed occurrence of spinal arachnoiditis following a caudal nerve block. J Spinal Cord Med 2011;34:616–619.
- Ross JS, Masaryk T, Modic M, et al: MR imaging of lumbar arachnoiditis. Am J Roentgenol 1987;149:1025–1032.
- Wang YL, Hsieh JR, Chung HS, et al: The local addition of tenoxicam reduces the incidence of low back pain after lumbar epidural anesthesia. Anesthesiology 1998;89:1414–1417.
- Stevens RA, Urmey WF, Urquhart, et al: Back pain after epidural anesthesia with chloroprocaine. Anesthesiology 1993;78:492–497.
- Hakim SH, Narouze S, Shaker NN, et al: Risk factors for new-onset persistent low-back pain following nonobstetric surgery performed with epidural anesthesia. Reg Anesth Pain Med 2012;37:175–182.
- Wilkinson HA: Field nerve block anesthesia for lumbar puncture. JAMA 1983;249:2177.
- Wang YL, Tan PP, Yang CH, et al: Epidural dexamethasone reduces the incidence of backache after lumbar epidural anesthesia. Anesth Analg 1997;84:376–378.
- Breen TW: Epidural analgesia and back pain. In Halpern Sh, Douglas MJ (eds): Evidence-Based Obstetric Anesthesia. BMJ Books, 2005, pp 208–216.
- Arendt K, Demaerschalk BM, Wingerchuck DM, et al: Atraumatic lumbar puncture needles: After all these years, are we still missing the point? Neurologist 2009;15:17–20.
- Brownridge P: The management of headache following accidental dural puncture in obstetric patients. Anaesth Intensive Care 1983;11:4–15.
- Headache Classification Committee of the International Headache Society: The international classification of headache disorders, third edition. Cephalalgia 2013;33:629–808.
- Webb CA, Weyker PD, Zhang L, et al: Unintentional dural puncture with a Tuohy needle increases risk of chronic headache. Anesth Analg 2012;115:124–132.
- Apfel CC, Saxena A, Cakmakkaya OS, et al: Prevention of postdural puncture headache after accidental dural puncture: A quantitative systematic review. Br J Anaesth 2010;105:255–263.
- Oedit R, van Kooten F, Bakker SL, et al: Efficacy of the epidural blood patch for the treatment of post lumbar puncture headache BLOPP: A randomised, observer-blind, controlled clinical trial. BMC Neurol 2005;
5:12. - van Kooten F, Oedit R, Bakker SL, et al: Epidural blood patch in post dural puncture heachache: A randomized, observed-blind, controlled clinical trial. J Neurol Neurosurg Psychiatry 2008;79:553–559.
- Angle P, Tang SL, Thompson D, et al: Expectant management of postdural puncture headache increases hospital length of stay and emergency room visits. Can J Anaesth 2005;52:397–402.
- Paech MJ, Doherty DA, Christmas T, et al: The volume of blood for epidural blood patch in obstetrics: A randomized, blinded clinical trial. Anesth Analg 2011;113:126–133.
- Haines DE: On the question of a subdural space. Anat Rec 1991; 230:3–21.
- Lubenow T, Keh-Wong E, Kristof K, et al: Inadvertent subdural injection: A complication of epidural nerve block. Anesth Analg 1988;67: 175–179.
- Paech MJ, Godkin R, Webster S: Complications of obstetric epidural analgesia and anaesthesia: A prospective analysis of 10,995 cases. Int J Obstet Anesth 1998;7:5–11.
- Cappiello E, Tsen LC: Complications and side effects of central neuraxial techniques. In Wong CA (ed): Spinal and Epidural Anesthesia. McGraw-Hill, 2007, pp 152–182.
- Grewal S, Hocking G, Wildsmith JA: Epidural abscesses. Br J Anaesth 2006;96:292–302.
- Darouiche R, Spinal epidural abscess: N Engl J Med 2006;355: 2012–2020.
- Connor DE, Chittiboina P, Caldito G, et al: Comparison of operative and nonoperative management of spinal epidural abscess: A retrospective review of clinical and laboratory predictors of neurologic outcome. J Neurosurg Spine 2013;19:119–127.
- Curry WT, Hoh BL, Amin-Hanjani S, et al: Spinal epidural abscess: Clinical presentation, management, and outcome. Surg Neurol 2005;63:364–371.
- Okano K, Kondo H, Tsuchiya R, et al: Spinal epidural abscess associated with epidural catheterization: Report of a case and a review of the literature. Jpn J Clin Oncol 1999;29:49–52.
- Danner RL, Hartman BJ: Update on spinal epidural abscess: 35 cases and review of the literature. Rev Infect Dis 1987;9:265–274.
- Huang PY, Chen SF, Chang WN, et al: Spinal epidural abscess in adults caused by Staphylococcus aureus: Clinical characteristics and prognostic factors. Clin Neurol Neurosurg 2012;114:572–576.
- Baer ET: Post-dural puncture bacterial meningitis. Anesthesiology 2006; 105:381–393.
- Reynolds F: Neurological infections after neuraxial anesthesia. Anesthesiol Clin 2008;26:23–52.
- Usubiaga JE: Neurological complications following epidural anesthesia. Int Anaesthesiol 1975;13:1–153.
- Kane RE: Neurologic deficits following epidural or spinal anesthesia. Anesth Analg 1981;60:150–161.
- Kao M, Tsai S, Tsou M, et al: Paraplegia after delayed detection of inadvertent spinal cord injury during thoracic epidural catheterization in an anesthetized elderly patient. Anesth Analg 2004;99:580–583.
- Loo CC, Irestedt L: Cauda equina syndrome after spinal anaesthesia with hyperbaric 5% lignocaine: A review of six cases of cauda equina syndrome reported to the Swedish Pharmaceutical Insurance 1993–1997. Acta Anaesthesiol Scand 1999;43:371–379.
- Lambert DH, Hurley RJ: Cauda equina syndrome and continuous spinal anesthesia. Anesth Analg 1991;72:817–819.
- Gerancher JC: Cauda equina syndrome following a single spinal administration of 5% hyperbaric lidocaine through a 25-gauge Whitacre needle. Anesthesiology 1997;87:687–689.
- Benson JS: US Food and Drug Administration safety alert: Cauda equine syndrome associated with the use of small-bore catheters in continuous spinal anesthesia. AANA J 1992;60:223.
- Rigler ML, Drasner K, Krejcie, TC, et al: Cauda equina syndrome after continuous spinal anesthesia. Anesth Analg 1991;72:275–281.
- Gaiser RR: Should intrathecal lidocaine be used in the 21st century? J Clin Anesth 2000;12:476–481.
- Walters MA, Van de Velde M, Wilms G: Acute intrathecal haematoma following neuraxial anaesthesia: Diagnostic delay after apparently normal radiological imaging. Int J Obstet Anesth 2012;21:181–185.
- Auroy Y, Narchi P, Messiah A, et al: Serious complications related to regional anesthesia: Results of a prospective survey in France. Anesthesiology 1997;87:479–486.
- Pollard JB: Common mechanisms and strategies for prevention and treatment of cardiac arrest during epidural anesthesia. J Clin Anesth 2002;14:52–56.
- Pollard JB: Cardiac arrest during spinal anesthesia: Common mechanisms and strategies for prevention. Anesth Analg 2001;92:252–256.
- Krismer AC, Hogan QH, Wenzel V, et al: The efficacy of epinephrine or vasopressin for resuscitation during epidural anesthesia. Anesth Analg 2001;93:734–742.
- Curatolo M, Scaramozzino P, Venuti FS, et al: Factors associated with hypotension and bradycardia after epidural block. Anesth Analg 1996;83:1033–1040.
- Liguori GA, Sharrock NE: Asystole and severe bradycardia during epidural anesthesia in orthopedic patients. Anesthesiology 1997;86: 250–257.
- Camann WR, Hortvet LA, Hughes N, et al: Maternal temperature regulation during extradural analgesia for labour. Br J Anaesth 1991;67: 565–568.
- Borgeat A, Ekatodramis G, Schenker CA: Postoperative nausea and vomiting in regional anesthesia: A review. Anesthesiology 2003;98: 530–547.
- Allen TK, Jones CA, Habib AS: Dexamethasone for the prophylaxis of postoperative nausea and vomiting associated with neuraxial morphine administration: A systematic review and meta-analysis. Anesth Analg 2012;114:813–822.
- Turnbull J, Moonesinghe R: Perioperative epidurals: The controversy goes on. Br J Hosp Med 2011;72:538.
- Dong H, Zhang Y, Xi H: The effects of epidural anaesthesia and analgesia on natural killer cell cytotoxicity and cytokine response in patients with epithelial ovarian cancer undergoing radical resection, J Int Med Research 2012;40:1822–1829.