Steven L. Orebaugh
INTRODUCTION
Injury to the peripheral nerve is a relatively uncommon but potentially serious complication of regional anesthesia. The fear of neurologic injury with nerve blocks may influence some practitioners as well as patients to avoid peripheral nerve blocks. The mechanisms by which nerve blocks may cause neural injury, along with evaluation and management, are discussed in separate chapters. Instead, this chapter discusses other potential causes of nerve injury as a number of possible factors may result in neurologic symptoms in the perioperative period.
To understand how the perioperative period may adversely influence nerves in extremities, even in subtle ways, ulnar nerve injuries reported in the anesthesiology literature over a decade ago are discussed. Injury to the ulnar nerve may be the most common nerve injury associated with general anesthesia and a significant source of litigation. These injuries appear to occur in the absence of obvious trauma to the involved extremity and are often delayed in their clinical presentation. The compression, pressure, and stretch at the level of the elbow all likely play a role in the pathophysiology, and preexisting neural compromise may also be a consideration. The deleterious effects of stretching or pressure on the ulnar nerve in an anesthetized patient can be prevented by simple maneuvers; for example, placing the extended forearm in supination, rather than pronation, was found to protect an unconscious patient against ulnar nerve injury.
However, when an extremity is itself the site of surgical intervention, many more additional factors may conspire to result in a nerve injury. Initially, the skin is subjected to harsh antimicrobial solutions after clipping or shaving. A pneumatic tourniquet is often placed for these surgeries, with resultant distal ischemia and high pressures on the nerves of the proximal extremity. The surgery itself offers potential for sharp, blunt, or thermal trauma, which could adversely affect nerves, both at the level of small, local cutaneous branches near the incisions and at the level of peripheral nerve trunks. Non-physiologic body position may occur and held for long periods, typically involving the surgical extremity, but sometimes the nonsurgical ones as well. In the postoperative phase, long periods of immobilization in nonphysiologic positions have the potential to cause nerve stretch or compression, as do the immobilizing devices, especially in the presence of unavoidable dependent, posttraumatic edema. Combined with the lack of perception due to general anesthesia or postoperative opioid analgesics, as well as any loss of sensation caused by local anesthetics, there is risk for neural dysfunction or injury or alterations in sensory function (Tables 1 and 2).
SURGICAL TOURNIQUETS
Use of the pneumatic tourniquet for extremity surgery has several benefits, including control of blood loss and improved operating conditions for surgeons (Figure 1). However, the pressure created by these devices may result in muscle or nerve injury, and recommendations for safe use (and safe technology) continue to evolve. The reported incidence of complications related to the use of a tourniquet in one report was as high as 0.15%. However, other large databases have reported a lower risk of injury. If electrophysiologic, subclinical abnormalities are used as a criterion for incidence of neurologic disturbances, incidence could be much higher, especially with high tourniquet pressures. For instance, Saunders et al noted electromyography (EMG) changes, lasting on average 51 days, in 62.5% of knee arthrotomy patients subjected to tourniquet pressures set at 350 to 450 mm Hg. In a randomized, controlled study of 48 patients undergoing knee arthroscopy, Dobner et al noted denervation on EMG in 71% of cases with tourniquets, which had a mean cuff pressure of 393 mm Hg, versus no such changes in the control group, which had no tourniquet for the surgery. These electrophysiologic abnormalities correlated with delayed return of function and lasted several months.
TABLE 1. Potential intraoperative causes of nerve injury.
| Surgical tourniquet (pressure, duration, cuff size/fit) |
| Positioning of operative extremity |
| Positioning of extremities |
| Incision/sharp dissection |
| Retraction/stretch/pressure on nerves |
| Electrocautery thermal injury |
| Insertion of fixators or other sharp instrumentation |
| Limb/joint over-extension or malposition |
While ischemia may contribute to nerve injury with tourniquets, actual physical compression of tissue beneath the cuff may be the dominant insult. In primate studies, the injury to the nerve was primarily found deep to, and at the edges of, the cuff. Such nerve injuries are characterized by microvascular injury, edema formation, disruption of myelin, and axonal degeneration.
The inflation pressure, the duration of cuff inflation, and the shape and size of the cuff are all significant variables related to tissue trauma with pneumatic tourniquets. Existing evidence is insufficient to establish exact recommendations for duration of inflation to ensure that no nerve damage will occur. In general, longer durations of inflation seem to predispose to a higher frequency of nerve injury; most animal studies suggested that 2 hours is a threshold beyond which cellular injury may become irreversible. Beyond this time, periodic deflation and reinflation is recommended, although there is no clinical evidence linking this to improved outcomes.
Tourniquet cuff pressures are frequently set at 150 mm Hg above systolic pressure for the lower extremity and 100 above systolic pressure for the upper extremity. However, absolute safe levels are difficult to determine. Simple prescriptions to inflate to 250 mm Hg for the lower extremity, with a somewhat lower level for the upper extremity, for up to 2 hours fail to take into account all of the potential hazards of these devices. If incorrectly incorrectly applied, inappropriate size, or utilization for prolonged duration are used, tourniquets can lead to neuropraxia.
TABLE 2. Potential postoperative causes of nerve injury.
| Inflammatory changes/postsurgical inflammatory neuropathy |
| Immobilization devices, such as casts/braces, with direct compression |
| Positioning of extremities |
| Prolonged immobilization in nonphysiologic extremity position |
| Edema of extremity, within an immobilizing device |
| Lack of pain or pressure perception due to opioids or numb extremity |
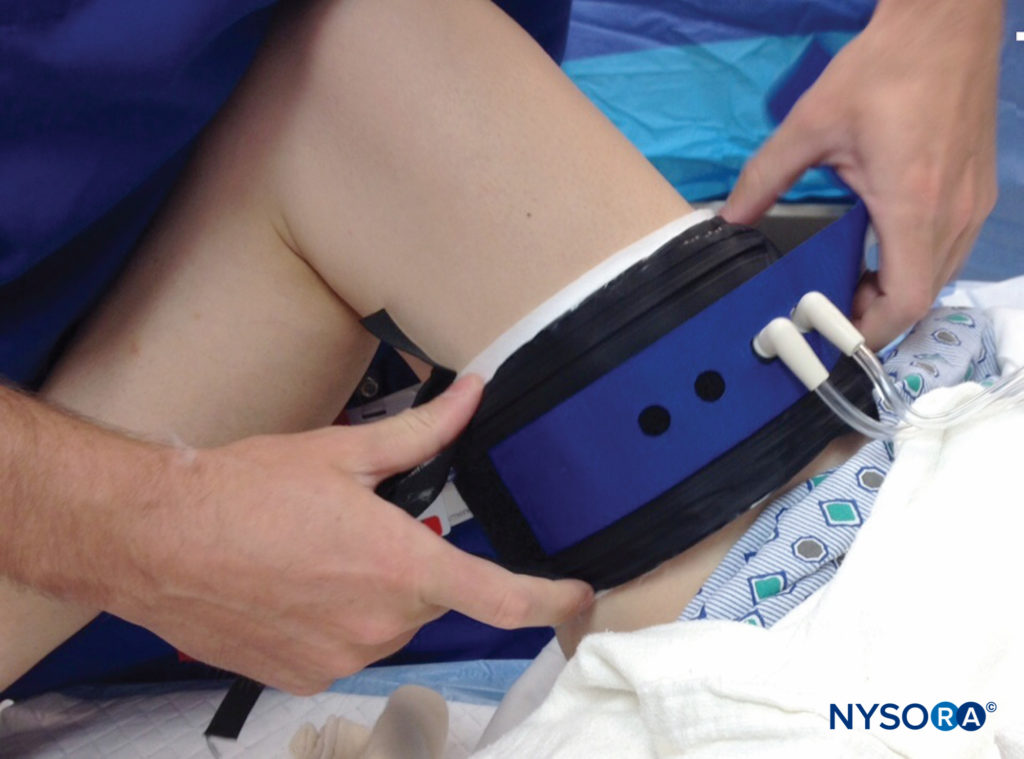
FIGURE 1. Application and use of a surgical tourniquet should take into account limb size, cuff size and shape, and arterial pressure. If possible, a limb occlusion pressure should be obtained, which allows for lower intraoperative cuff pressures while maintaining a bloodless field.
The recognition that higher pressures cause more tissue damage and increase the risk of nerve injury has led to a recommendation for use of lower tourniquet pressures in the past two decades, as well as an interest in finding ways to diminish blood flow to the surgical site while keeping cuff pressures low. Cessation of blood flow to an extremity is actually a function of the limb occlusion pressure (LOP), rather than simply of the systolic arterial pressure; the LOP is determined by the shape and size of the extremity and the site and conformation of the tourniquet, together with the arterial inflow pressure. Interestingly, LOP does not vary directly with the arterial pressure. As such, it is unique for each patient and extremity, suggesting that it is difficult to prescribe universal recommendations for setting cuff pressure based on systolic blood pressure.
Existing pneumatic tourniquets may be modified to determine LOP. Some newer tourniquet systems also feature an integrated means of determining LOP, as well as recommendations, based on this parameter, for setting the optimal tourniquet cuff pressure. Wider, contoured cuffs allow lower pressures as well, which may contribute to patient safety.
While there are no specific guidelines suggested by orthopedic specialty societies for tourniquet management, other specialty societies have issued recommendations for safe use of these devices. Table 3 summarizes existing guidelines and recommendations from the literature. The Association of Surgical Technicians recommends that tourniquets on the lower extremity not be inflated higher than 100 mm Hg above the systolic arterial pressure for the lower extremity and 50 mm Hg above systolic pressure for the upper extremity—significantly lower than the prevailing wisdom might suggest.
TABLE 3. Recommendations for tourniquet inflation pressures.
| AST25 UE: 50 mm Hg above the systolic pressure |
| LE: 100 mm Hg above the systolic pressure |
| AORN27 Determine LOP; 40 mm Hg above LOP for an LOP less than 130 mm Hg, 60 mm Hg above LOP for LOP between 130 and 190 mm Hg, 80 mm Hg above LOP if LOP more than 190 mm Hg |
| Crenshaw57 50-75 mm Hg above systolic pressure for UE 100-150 mm Hg above systolic pressure for LE |
| Noordin22 Determine LOP; base the cuff pressure on level of LOP |
| Estersohn58 90-100 mm Hg above systolic pressure for LE |
Some guidelines for tourniquet management rely specifically on the determination of the LOP. Setting the tourniquet at this level of pressure, with the addition of a safety factor (in case of blood pressure elevation during the case), allows for an overall lower cuff pressure to control blood flow, with a potentially beneficial effect on patient safety. In one series, when LOP was utilized in patients undergoing anterior cruciate ligament reconstruction, tourniquet cuff pressures were dropped by over half compared to the use of standard inflation pressures based solely on systolic blood pressures. The American Society of Operating Room Nurses (AORN) recommends determination of LOP, with addition of a variable degree of pressure, depending on the patient’s systolic blood pressure (greater pressures are added for higher patient blood pressures). Some authors in the orthopedic literature have suggested the use of LOPs as well to favorably affect patient outcomes (Table 3).
Since transmission of pressure to deep tissues is related to the quantity of tissue located directly beneath the cuff, the pressure/shearing effect of the tourniquet is mitigated by a greater thickness of tissue between the cuff and the nerve. This explains the need for higher cuff pressures in larger extremities to control blood flow into the surgical field and the recommendation for use of lower cuff pressures in the arm of adults (as compared to the leg) and in pediatric patients. In general, the lowest pressures that are effective for control of blood flow, coupled with the shortest duration possible, are likely to be safest for the patient. The use of LOPs, which take into account limb size and shape, as well as prevailing arterial inflow pressures, allows for this.
Tourniquet-related nerve injury due to the pressure imparted directly by the cuff on the underlying nerve (as opposed to the distal ischemic insult) frequently results in a greater degree of motor loss than sensory loss, hence the historic term tourniquet paralysis. In the lower extremity, tourniquet injury most commonly affects the sciatic nerve, while in the arm, the radial nerve appears to be most vulnerable.
Fortunately, many of these injuries resolve over time, and permanent injury is uncommon. It should also be noted that, while pneumatic tourniquet use has been the subject of much research, the combination of shear stress and ischemia from the tourniquet, coupled with temporary disruption of normal nerve physiology by local anesthetic administration, has not been sufficiently studied.
POSTSURGICAL INFLAMMATORY NEUROPATHY
Another potential cause of nerve injury that may occur in the wake of surgery, with no apparent relationship to peripheral nerve block, is postsurgical inflammatory neuropathy (PSIN). In this pathologic entity, surgical trauma with tissue damage results in immune stimulation, which is primarily expressed as inflammation of neural tissue. This inflammatory nerve dysfunction may occur in the region of the surgery, at a distant site in the same extremity, or at a completely remote site in the body.
PSIN may even develop diffusely at multiple sites. Affected nerves show evidence of edema, microvascular derangements, myelin injury and loss, and axonal injury, with influx of acute inflammatory cells. Biopsy is required for definitive diagnosis of PSIN; however, magnetic resonance imaging is supportive of the diagnosis and, together with clinical evidence, may allow for presumptive diagnosis and therapy (Figures 2 and 3). Treatment with corticosteroids is helpful in many cases, and while most episodes of PSIN improve gradually over time, permanent sequelae have been reported. In 2011, Staff et al. summarized the most extensive database of cases of PSIN to date. A variety of different surgical types were involved, including orthopedic procedures, general surgery, and even dental cases. None of the 33 patients had received peripheral nerve block. The typical presentation was pain and weakness in the territory of the affected nerves; sensory changes were common as well. Twenty-one of the cases were confirmed by biopsy.
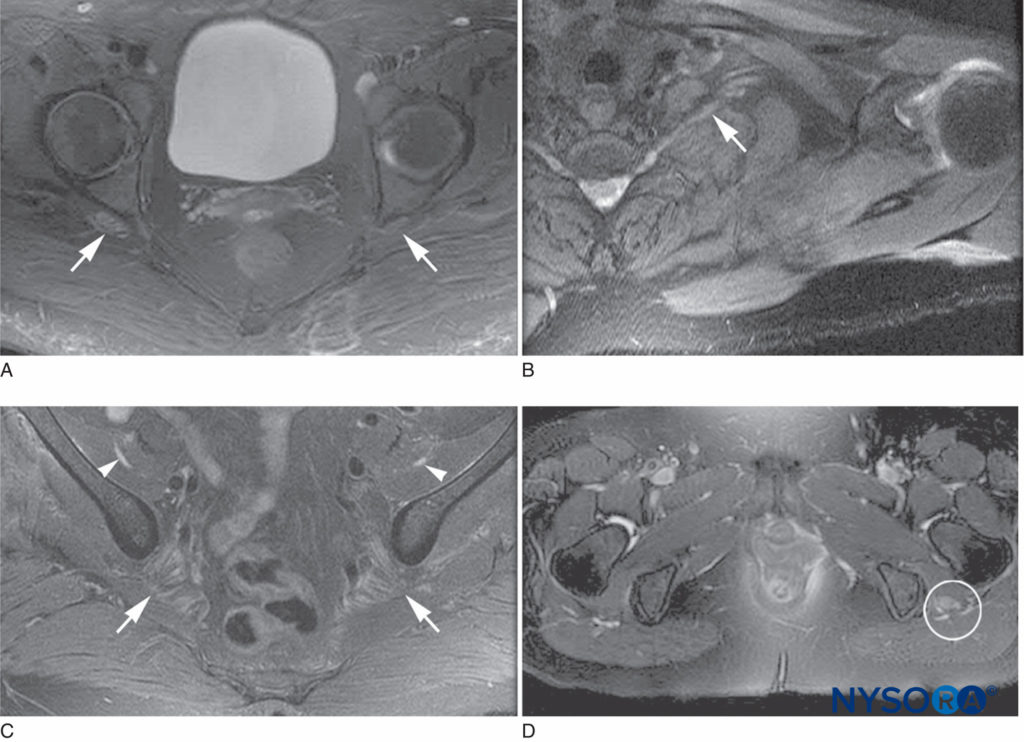
FIGURE 2. Magnetic resonance imaging characteristics of postsurgical inflammatory neuropathy. A: T2 hyperintensity and mild enlargement of bilateral sciatic nerves, right more than left (arrows). B: T2 hyperintensity and mild enlargement of left C8 root and lower trunk (arrow). C: T2 hyperintensity and moderate enlargement of the bilateral femoral nerves (arrowheads) and mild enlargement of the sciatic nerves (arrows). D: T2 hyperintensity and severe enlargement of left sciatic nerve (circled).
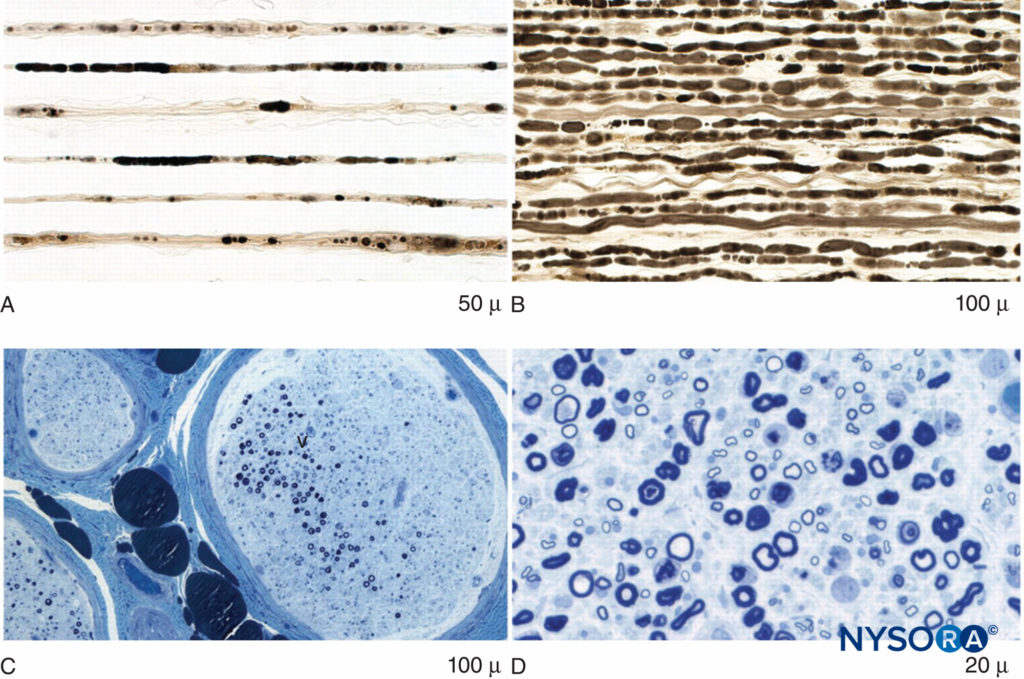
FIGURE 3. Axonal degeneration and focal fiber loss in postsurgical inflammatory neuropathy. A: Teased fiber preparation showing multiple strands with fulminant late axonal degeneration. B: Teased fiber preparation showing multiple closely aligned strands of fulminant early axonal degeneration. C: Low-power methylene blue epoxy section of nerve illustrating multifocal fiber loss. D: High-power methylene blue epoxy sections showing prominent axonal degeneration of large myelinated fibers.
The authors noted that nerve injuries may sometimes be inappropriately ascribed to mechanical causes during surgery, when immune mechanisms are actually the unsuspected cause, and that PSINs may underlie such symptoms of neural compromise much more commonly than is recognized. Given this potential, serious nerve injuries should likely be evaluated, not only with EMG and nerve conduction studies, which are relatively nonspecific unless a level of injury can be clearly established, but also with magnetic resonance neurography, which may provide additional information regarding the severity, extent, and location of the neural insult(s). If a diagnosis cannot be established, nerve biopsy should be considered.
SURGICAL CAUSES OF NERVE INJURY
Given the invasive nature of surgical procedures, unintended injuries to anatomic structures are not surprising. Injury to nerves from surgical trauma, whether by sharp dissection or insertion of surgical or fixation devices, is a potential risk of many types of procedures. For instance, in shoulder surgery, injuries may occur to the suprascapular, axillary, musculocutaneous, subscapular, or spinal accessory nerves from either open or arthroscopic procedures. Femoral nerve injuries in the perioperative period are commonly related to ischemia from stretch or retraction occurring during abdominal or pelvic procedures.During hip arthroscopy, sciatic nerve injury may occur and is most closely related to the force of distraction on the operative leg40 (Figure 4). During hamstring tendon harvest for autograft anterior cruciate ligament reconstruction, injury to the infrapatellar or sartorial branch of the saphenous nerve, with consequent sensory deficits, occurs in as many as 74% of patients. Fixation devices, such as K wires, may inadvertently cause trauma to nerves as well. Aberrant anatomy may result in unpredictable positions of nerves, putting them at risk during otherwise-routine procedures.
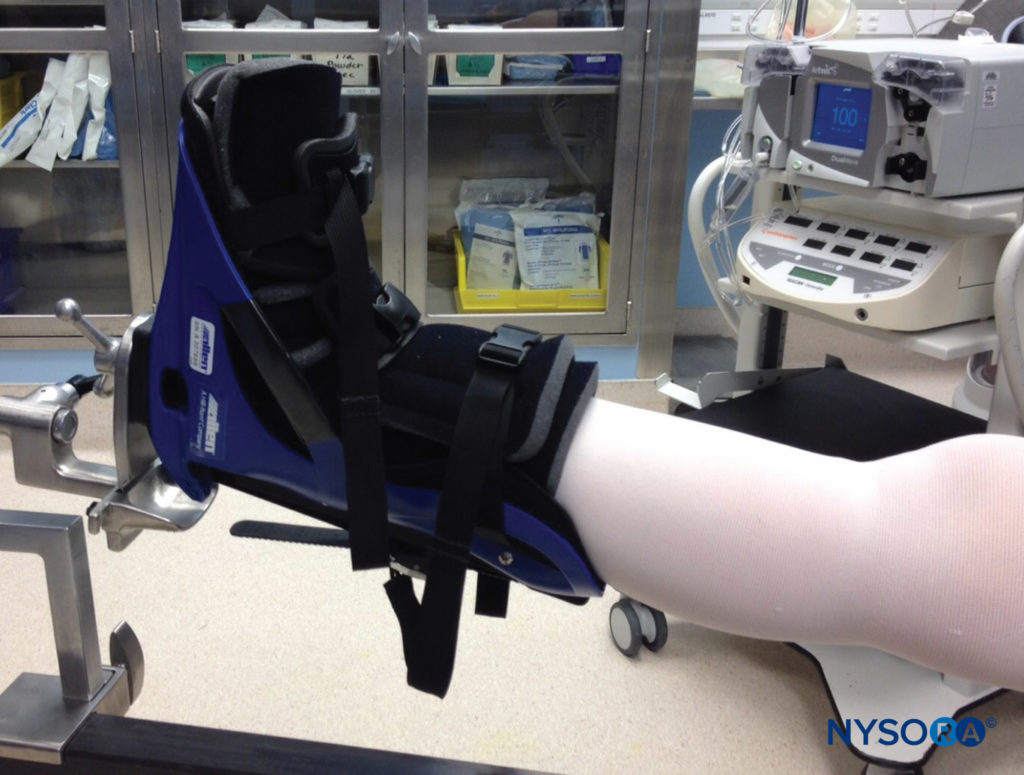
FIGURE 4. Hip arthroscopy surgery requires forceful distraction of the operative leg, which poses a risk for sciatic nerve injury.
Positioning for Surgery
Surgical positioning in the operating room may play a crucial role in nerve injury and should be considered when new nerve symptoms are reported, especially when positions other than supine are utilized. Prone position, lithotomy, and severe degrees of Trendelenburg are all known to predispose to nerve injury. In addition, the lateral position is more likely to result in brachial plexus nerve injury than beach-chair position for shoulder procedures (Figure 5). In the sitting position, prolonged cases have resulted in neuropraxia to either or both sciatic nerves, including sensory loss and disabling motor weakness (Figure 6). Lateral tilting of the head in the sitting position may result in stretch of the brachial plexus, with the potential for nerve compromise as well.
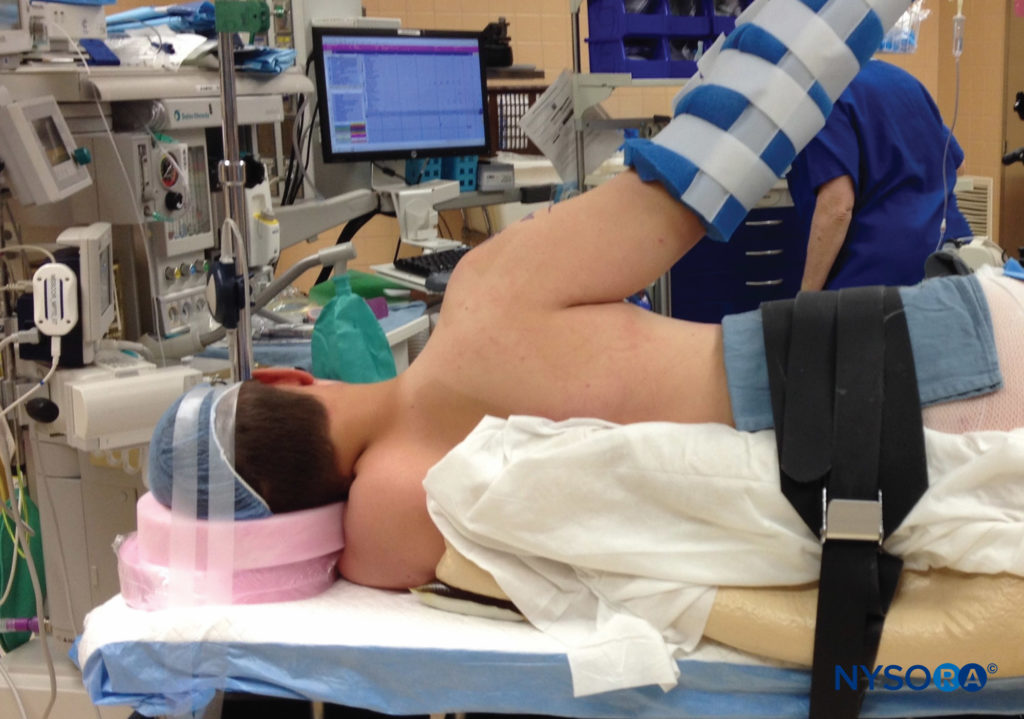
FIGURE 5. Lateral position for shoulder surgery is associated with a higher incidence of nerve injury.
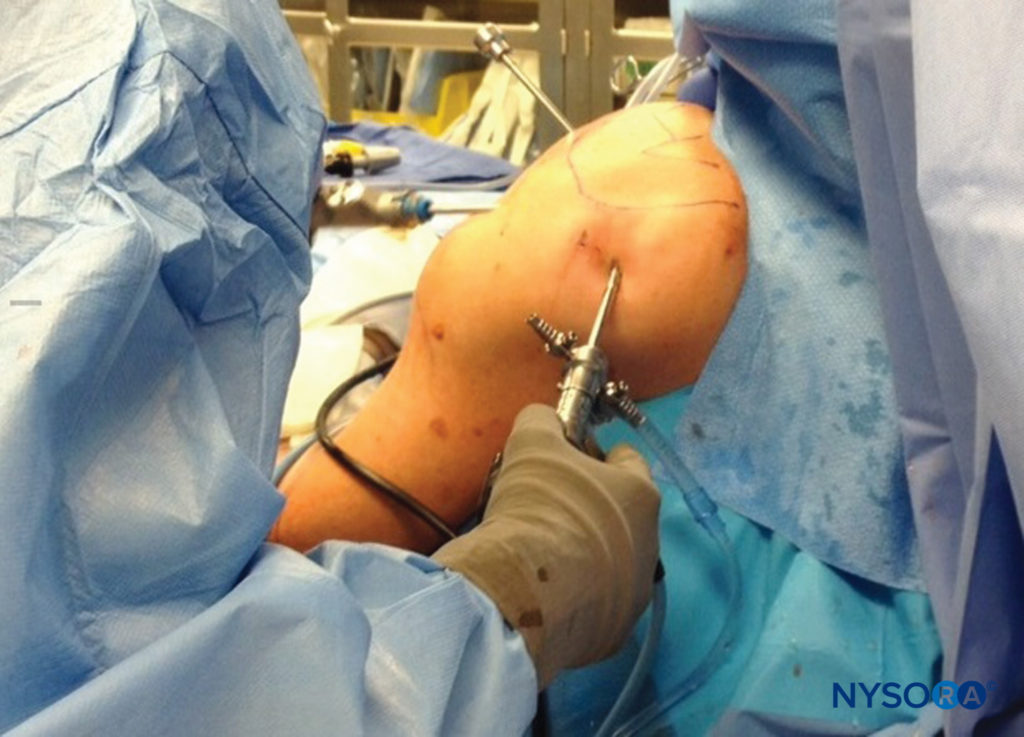
FIGURE 6. The beach-chair position, when adopted for prolonged periods, may result in sciatic nerve compression injury.
Postoperative Immobilization
The positioning of the extremity after an operation may also contribute to nerve compromise. While immobilization in a relatively neutral position at the hip, knee, and ankle is usual for lower extremity procedures, this is not the case for the upper extremity. In orthopedic procedures for hand, wrist, shoulder, and some elbow conditions, holding the extremity in flexion at the elbow for long periods, in a sling or shoulder immobilizer, helps to protect the injured extremity and reduce the severity of postoperative edema. However, prolonged immobilization in flexion, sometimes for weeks, may be deleterious to the ulnar nerve, which is placed in a degree of stretch (Figure 7).
The combination of this position with relative immobility and the inevitable postoperative edema that occurs may predispose to ulnar entrapment, compression, and sulcus ulnaris syndrome Another concern in the postoperative period is the immobilizing device itself. Splints, casts, and braces, if applied without regard to underlying nerves, may present a hazard. Even when placed with care for potential pressure or constriction, the unavoidable edema that occurs in the aftermath of surgical trauma, especially with dependency, may serve to make a comfortable device quite tight (Figure 8). Compartment syndrome may result when such appliances completely extinguish blood flow to the underlying tissues, and this is discussed more fully in Acute Compartment Syndome of the Limb: Implications for Regional Anesthesia.
However, even in the absence of such severe circulatory embarrassment, pressure over a nerve, with resultant palsy, may occur. One example is the potential for a knee brace, placed after anterior cruciate ligament reconstruction, to impinge on the peroneal nerve over the neck of the fibula, with resultant numbness over the top of the foot and weakness of dorsiflexion (Figure 9). Shoulder immobilization devices, with snug straps over the distal extremity or circular cutouts that lie at the base of the thumb, may lead to sensory changes in the tip of a digit, which typically resolve rapidly when this constriction is addressed, as experienced in my own practice
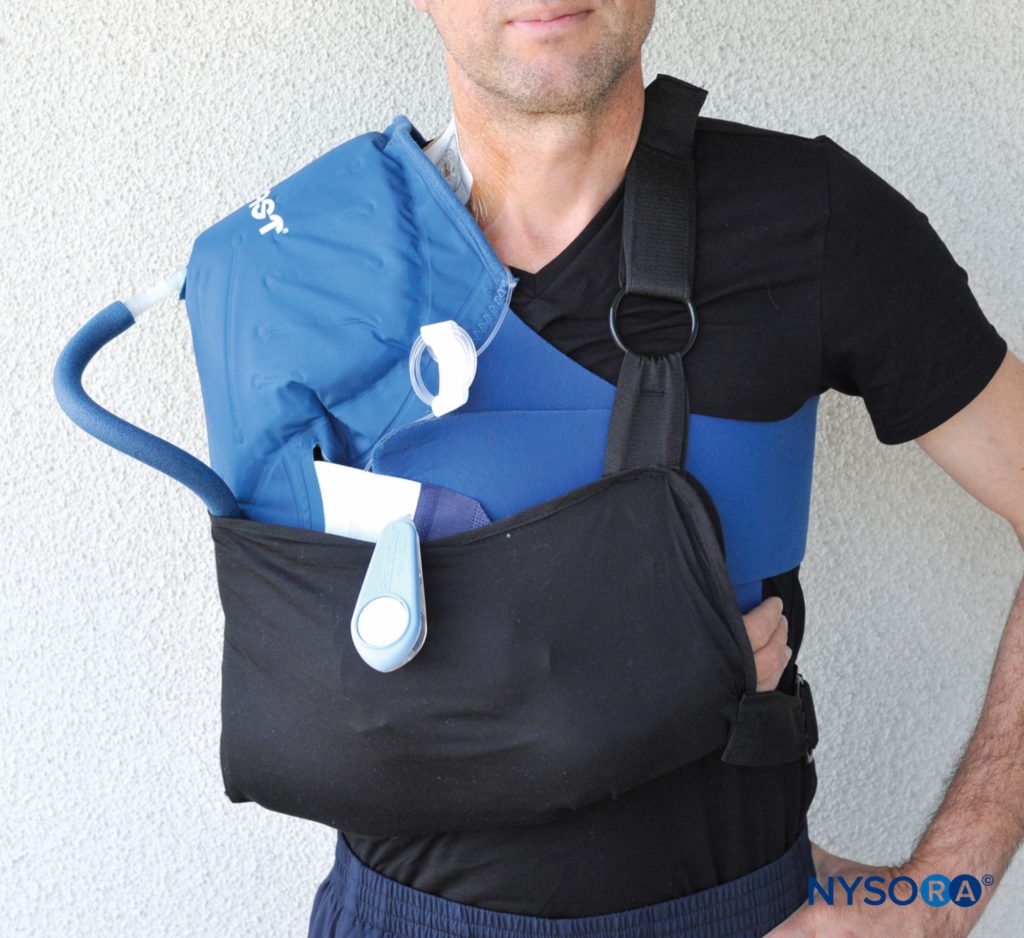
FIGURE 7. Shoulder surgeries and other upper extremity procedures usually require a prolonged period of immobilization in flexion at the elbow, in slings or other devices. This may result in ulnar nerve dysfunction or injury.
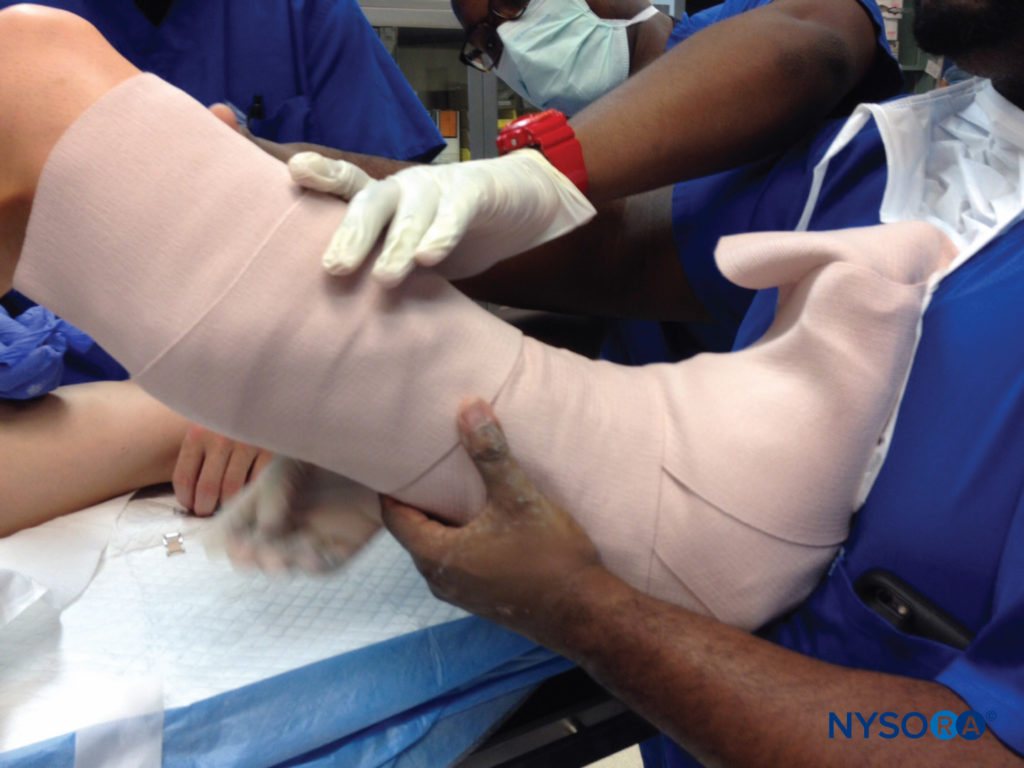
FIGURE 8. Postoperative casts or splints should be placed with care to avoid firm apposition against skin or pressure over bony prominences or superficial nerves, with consideration of likely edema of the affected extremity.
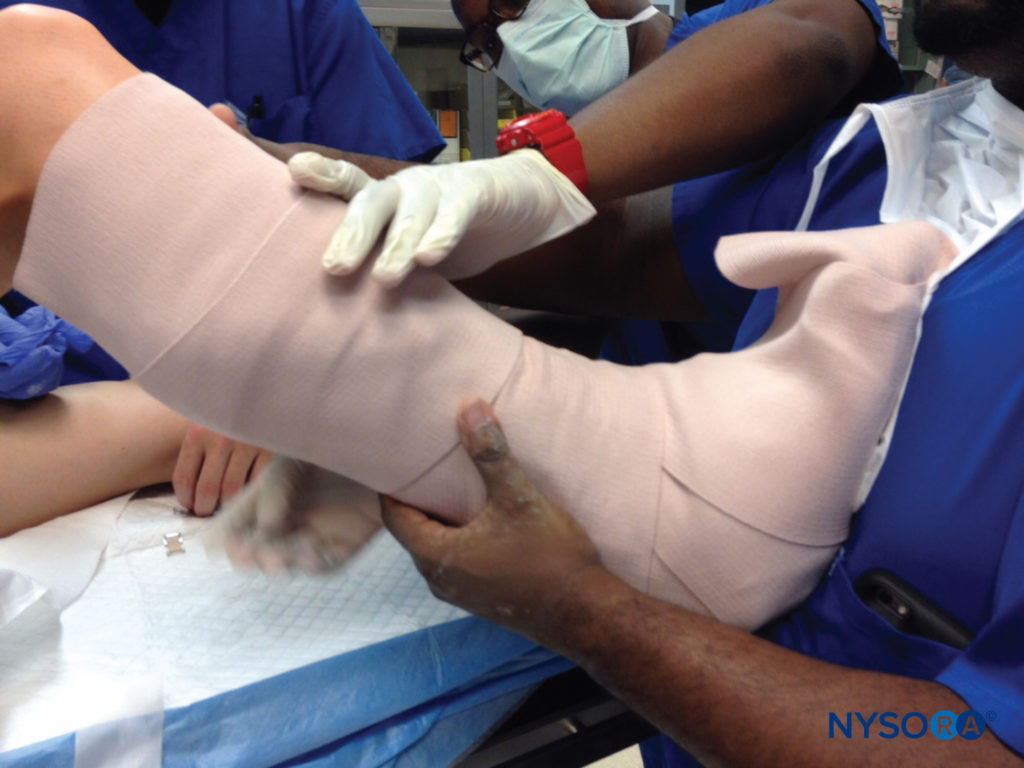
FIGURE 9. During placement of a knee brace after surgery, care should be taken to avoid a tight fit or pressure directly over the common peroneal nerve, which may result in sensory or motor loss in the foot.
Prolonged Skin Pressure
Pressure over a digital or more substantial nerve may lead to sensory or motor deficits in the territory of that nerve. However, prolonged contact with a patch of underlying skin caused by an immobilizing splint, brace, or cast may lead to sensory deficits in that region, simply as a result of longterm compression of sensory receptors in the skin. Such abnormalities of sensation would not be expected to cause changes in either EMG or nerve conduction studies. While these effects on nerve function are not, of themselves, related to anesthesia interventions, the mere presence of a protracted period of sensory alteration due to peripheral nerve block (whether applied by the anesthesiologist or the surgeon) may make it difficult for the patient to perceive the pressure caused over skin or a subcutaneous nerve, contributing to the potential for injury or temporary dysfunction of these tiny nerves. Of particular concern is heel ischemia and ulceration after prolonged heel rest in patients who have received sciatic nerve block. Thus, careful home-going instructions and follow-up of such patients is essential.
Complex Regional Pain Syndrome
Complex regional pain syndrome (CRPS) after surgery is usually the result of the traumatic event itself, although it may also occur as a result of nerve injury, so called type 2 CRPS. While this entity usually manifests with pain and limb dysfunction, sensory disturbances may be primary symptoms early in its course. Severe cases may result in atrophy and weakness as well. Distinguishing early CRPS from nerve injury can be accomplished with careful neurologic exam, quantitative sensory testing, the quantitative sudomotor axon reflex test (QSART), and appreciation of other changes that come with this disease. Sensory disturbances in CRPS will not likely be limited to the territory of a single peripheral nerve, as is expected with peripheral nerve injury.
POSTOPERATIVE EVALUATION
Determining the etiology of a nerve injury requires integration of the physical examination, electrophysiology or imaging. When all diagnostic modalities are carefully scrutinized, the majority of postoperative nerve injuries in fact are caused by factors other than regional technique. The utility of EMG as a test depends on both patient tolerance of the procedure and the skill and experience of the examiner. Physical findings may add further specific information about the level of the actual nerve lesion.
For instance, in femoral nerve injury, the level of the lesion may be reliably determined to be above or below the inguinal ligament by assessing whether the hip flexor muscles (iliacus and psoas muscles), which are innervated high in the pelvis, are affected, along with the knee extensors, which are innervated in the thigh itself, below the level of arborization of the nerve. A lesion that occurs proximally, in the pelvis—such as an inflammatory lumbar plexopathy—with weakness of hip flexion as well as knee extension cannot be related to structural damage to the femoral nerve caused by a peripheral block at the level of the femoral crease. Similarly, a sciatic nerve injury with loss of hamstring innervation could not be attributable to trauma from a popliteal/sciatic block, which occurs at a significant distance below the release of branches to these muscles.
SUMMARY
There are numerous potential causes of neurologic injury or dysfunction in the perioperative period. Anesthesiologists should assume the leading role in establishing the cause of postoperative neurologic injury to guide therapy as well as for medicolegal reasons. This requires a multidisciplinary approach with detailed motor and sensory examination, neurology or physical medicine referral, appropriate electrophysiologic testing, as well as imaging, as detailed in Assessment of Neurologic Complications of Regional Anesthesia.
REFERENCES
- Warner MA, Warner ME, Martin JT: Ulnar neuropathy: incidence, outcome, risk factors in sedated or anesthetized patients. Anesthesiology 1994;81:1332–1340.
- Warner MA, Warner DO, Matsumoto JY, et al: Ulnar neuropathy in
surgical patients. Anesthesiology 1999;90:54. - Cheney FW, Domino KB, Caplan RA, et al: Nerve injury associated with anesthesia. Anesthesiology 1999;90:1062.
- O’Driscoll SW, Horii E, Carmichael SW, et al: The cubital tunnel and ulnar neuropathy. J Bone Joint Surg Am 1991;73:613.
- Macnicol MF. Extraneural pressures affecting the ulnar nerve at the elbow. J Hand Surg Eur 1982;14:5.
- Alvine FG, Schurrer ME. Posoperative ulnar nerve palsy: are there predisposing factors? J Bone Joint Surg Am 1987;69:255.
- Prielipp RC, Morell RC, Walker FO, et al: Ulnar nerve pressure: influence of arm position and relationship to somatosensory evoked potentials. Anesthesiology 1999;91:345.
- McEwen JA: Complications of and improvements in pneumatic tourniquet used in surgery. Med Instrum 1981;15:253.
- Odinsson A, Finson V: Tourniquet use and its complications in Norway. J Bone Joint Surg Br 2006;88:1090.
- Middleton RWD, Varian JPW: Tourniquet paralysis. Aust N Z J Surg 1974;44:124.
- Saunders KC, Louis DL, Weingarden SI, et al: Effect of tourniquet time on postoperative quadriceps function. Clin Orthoped Relat Res 1979;143:194.
- Dobner JJ, Nitz AJ: Postmeniscectomy tourniquent palsy and functional sequelae. Am J Sports Med 1982;10:211.
- Ochoa J, Danta G, Fowler TJ, et al: Nature of the nerve lesion caused by a pneumatic tourniquet. Nature 1971;233:265.
- Ochoa J, Fowler TJ, Rudge P, et al: Anatomic changes in peripheral nerves compressed by a pneumatic tourniquet. J Anat 1972;113 (pt 3):433.
- Rydevik B, Lundborg G, Bagge U: Effects of graded compression on intraneural blood flow. J Hand Surg Am 1981;6:3.
- Wakai A, Winter DC, Street JT, et al: Pneumatic tourniquets in extremity surgery. J Am Acad Orthop Surg 2001;9:345.
- Heppenstall RB, Balderston R, Goodwin C: Pathophysiologic effects distal to a tourniquet in the dog. J Trauma 1979;19:234.
- Klenerman L, Biswas M, Hulands GH, et al: Systemic and local effects of application of a tourniquet. J Bone Joint Surg Br 1980;62:385.
- Nitz AJ, Dobner JJ, Matulionis DH. Pneumatic tourniquet application and nerve integrity: motor function and electrophysiology. Exp Neurol 1986;94:264.
- Rorabeck CH: Tourniquet-induced nerve ischemia. An experimental investigation. J Trauma 1980;20:280.
- Pedowitz RA. Tourniquet-induced neuromuscular injury: a review of rabbit and clinical experiments. Acta Orthoped Scand 1991;Suppl 245:1.
- Noordin S, McEwen JA, Kragh JF Jr, et al: Surgical tourniquets in orthopaedics. J Bone Joint Surg Am 2009;91:2958.
- Kam PCA, Kavanaugh R, Yoong FFY: The tourniquet: pathophysiological consequences and anaesthetic implications. Anaesthesia 2001;56:534.
- Ishii Y, Noguchi H, Matsuda Y, et al: A new tourniquet system that determines pressure in synchrony with systolic blood pressure. Arch Orthop Traum Surg 2008;128:297.
- Association of Surgical Technologists: Recommended standards of practice for safe use of pneumatic tourniquets. 2007. http://www.ast.org//pdf/Standards_of_Practice/RSOP_Pneumatic_Tourniquets.pdf. Accessed June 28, 2015.
- Reilly CW, McEwen JA, Leveille L, et al: Minimizing tourniquet pressure in pediatric anterior cruciate ligament reconstructive surgery. J Pediatr Orthop 2009;29:275.
- Conner R, Blanchard J, Burlingame B, Chard R, Denholm B, Downing D: AORN. Recommended practices for the use of the pneumatic tourniquet. In Perioperative Standards and Recommended Practices. AORN, 2009, p. 373.
- Shaw JA, Murray DG: The relationship between tourniquet pressure and underlying soft-tissue pressure in the thigh. J Bone Joint Surg Am 1982;64:1148.
- Lieberman JR, Staneli CT, Dales MC: Tourniquet pressures on pediatric patients: a clinical study. Orthopedics 1997;20:1143.
- Horlocker TT, Hebl JR, Gali B, et al: Anesthetic, patient and surgical risk factors for neurologic complications after prolonged total tourniquet time during total knee arthroplasty. Anesth Analg 2006;102:950.
- Staff NP, Engelstad J, Klein CJ, et al: Post-surgical inflammatory neuropathy. Brain 2010;133:2866.
- Malamut RI, Marques W, England JD, et al: Postsurgical idiopathic brachial neuritis. Muscle Nerve 1994;17:320.
- Ahn KS, Kopp SL, Watson JC, et al: Postsurgical inflammatory neuropathy. Reg Anesth Pain Med 2011;36:403.
- Barrington MJ, Morrison W, Sutherland T, et al: Case scenario: postoperative brachial plexopathy associated with infraclavicular brachial plexus block. Anesthesiology 2014;121:383.
- Sully WF, Wilson DJ, Parada SA, et al: Iatrogenic nerve injuries in shoulder surgery. J Am Acad Orthop Surg 2013;21:717.
- Rhee PC, Spinner RJ, Bishop AT, et al. Iatrogenic brachial plexus injuries assocaiated with open subpectoral biceps tenodesis. Am J Sports Med 2013;41:2048.
- Carofino BC, Brogan DM, Kircher MF, et al: Iatrogenic nerve injuries during shoulder surgery. J Bone Joint Surg 2013;95:1667.
- Yoo JC, Lee YS, Ahn JH, et al: Isolated suprascapular nerve injury below the spinoglenoid notch after SLAP repair. J Shoulder Elbow Surg 2009;18:e27.
- Moore AE, Stringer MD: Iatrogenic femoral nerve injury. Surg Radiol Anat 2011;33:649.
- Telleria JJ, Safran MR, Harris AH, et al: Risk of sciatic nerve traction injury during hip arthroscopy—is it the amount or duration? J Bone Joint Surg Am 2012;94:2025.
- Sanders B, Rolf R, McClelland W, et al: Prevalence of saphenous nerve injury after autogenous hamstring harvest. Arthroscopy 2007;23:956.
- Glanvill R, Boon JM, Birkholtz F, et al: Superficial radial nerve injury during standard K-wire fixation of uncomplicated distal radius fracture. Orthopedics 2006;29:639.
- Jou IM, Lai KA. Acute median nerve injury due to migration of a Kirschner wire. J Hand Surg Br 1998;23:112.
- Jeon IH, Kim PT, Park IH, et al: High bifurcation of median nerve at the wrist causing common digital nerve injury in endoscopic carpal tunnel release. J Hand Surg Br 2002;27:580.
- Warner ME: Patient positioning. In Barash PG, Cullen BF, Stoelting RK (eds): Clinical Anesthesia, 7th ed. Lipincott, Williams and Wilkins, 2013, p 803.
- Rains DD, Rooke GA, Wahl CJ: Pathomechanisms and complications related to patient positioning and anesthesia during shoulder arthroscopy. Arthroscopy 2011;27:532.
- Wang J-C, Wong T-T, Chen H-H, et al: Bilateral sciatic neuropathy as a complication of craniotomy in the sitting position. Childs Nerv Syst 2012;28:159.
- Lowdon IMR: Neurocirculatory disturbances of the extremities. In Duthie RB, Bentley G (eds): Mercer’s Orthopedic Surgery. Oxford University Press, 1996, p 881.
- Coppieters MW, Van De Velde M, Stappaerts KH: Positioning in anesthesiology. Anesthesiology 2002;97:75.
- Borgeat A, Ekatodramis G, Kalberer F, et al: Acute and nonacute complications associated with interscalene block and shoulder surgery. Anesthesiology 2001;97:1274.
- Arnold WD, Elsheikh BH: Entrapment neuropathies. Neurol Clin 2013;31:405.
- Mauser N, Gissel H, Henderson C, et al: Acute lower-leg compartment syndrome. Orthopedics 2013;36:619.
- Flanigan RM, DiGiovanni BF: Peripheral nerve entrapments of the lower leg, ankle, and foot. Foot Ankle Clin N Am 2011;16:255.
- Guzelkucuk U, Skempes D, Kumnerddee W: Common peroneal nerve palsy caused by compression stockings after surgery. Am J Phys Med Rehab 2014;93:609.
- Todkar M: Sciatic nerve block causing heel ulcer after total knee replacement in 36 patients. Acta Orthop Belg 2005;71(6):724–725.
- Rockett M: Diagnosis, mechanisms and treatment of complex regional pain syndrome. Curr Opin Anesthesiol 2014;27:494–500.
- Gierthmuhlen J, Maier C, Baron R, et al: Sensory signs in complex regional pain syndrome and peripheral nerve injury. Pain 2012; 153:765.
- Barrington MJ, Watts SA, Gledhill SR, et al: Preliminary report of the Australasian Regional Anesthesia Collaboration. Reg Anesth Pain Med 2009;34:534.
- Preston DC, Shapiro BE: Electromyography and Neuromuscular Disorders, 2nd ed. Elsevier, 2005, p 3.
- Preston DC, Shapiro BE: Electromyography and Neuromuscular Disorders, 2nd ed. Elsevier, 2005, p 355.
- Laughlin RS, Dyck P: Electrodiagnostic testing in lumbosacral plexopathies. Phys Med Rehab Clin N Am 2013;24:93, 2013.