Colin J. L. McCartney and Stephen Choi
INTRODUCTION
Peripheral nerve blocks provide many benefits for patients, including superior pain control and reduction in general anesthesia-related side effects. To optimize pain relief while reducing the total dose of local anesthetic, it would be of use to add a drug that both speeds onset and prolongs sensory block or analgesic effect. Improvements in our knowledge of peripheral nervous system (PNS) pain mechanisms allow us to develop methods of prolonging analgesia while reducing central and peripherally mediated adverse effects. In the last 20 years, a number of drugs have been tested, and several have proven clinically useful when added to local anesthetic for peripheral nerve block or when used for local infiltration or intra-articular analgesia. These drugs are known as analgesic adjuvants. This chapter examines the rationale and current evidence base for use of analgesic adjuvants and summarizes the best strategies for optimizing pain control and reducing adverse effects after surgery under peripheral nerve block, local infiltration, or injection of drugs in the intra-articular space.
RATIONALE FOR USE
Pain transmission in the CNS and PNS involves a complex array of neurotransmitters and pathways that are not easily blocked by one drug type or technique alone. Involvement of several classes of neurotransmitter at the injury site, peripheral nerve, dorsal horn of the spinal cord, and supraspinal sites is responsible for the transmission of nociception. Use of agonists at inhibitory receptors and antagonists at excitatory receptors allows a “multimodal” approach with optimization of pain control and reduction of adverse effects. In 1645, Descartes proposed a mechanism for pain transmission, suggesting that a peripheral pain impulse was transmitted directly from the periphery to the brain by a “hardwired” system without any intermediate modulation (Figure 1).
This theory of pain transmission was widely held as true until as recently as 40 years ago. In 1965, Melzack and Wall proposed their groundbreaking gate control theory of pain that suggested that pain could be modulated or “gated” at a number of points in the pain pathway. Subsequent research identified the dorsal horn (lamina II) of the spinal cord as an important site of potential modulation, and subsequent treatments for acute and chronic pain have utilized this knowledge to good effect. Treatments such as the use of spinal opioids and transcutaneous electrical nerve stimulation (TENS) have been developed in the light of this knowledge. The gate theory also changed many (often unsuccessful) pain management strategies from techniques where we tried to ablate pain pathways either chemically or surgically to more recent modulation techniques by which we attempt to inhibit excitatory influences and enhance inhibitory influences within the pain pathway. In the last few decades, important advances have also occurred in our knowledge of how pain is generated and transmitted from the PNS to the central nervous system (CNS). Modulation of pain in the PNS also involves numerous trans-mitters and mechanisms that both excite and inhibit nociceptive pathways. In the PNS, under normal physiologic conditions nociceptive signals are produced when A-α and C fibers are stimulated by heat, pressure, or several chemicals produced by tissue damage and inflammation (potassium, histamine, bradykinin, prostaglandins, adenosine triphosphate [ATP]). Nociceptive signals are transmitted to the superficial layers of lamina II of the dorsal horn in the spinal cord, where they are modulated at both the presynaptic and postsynaptic level and also by excitatory and inhibitory descending control pathways form the brainstem (Figure 2). Signals that are successful in crossing this gate travel on to the brainstem and thalamus before reaching the cerebral cortex to produce a pain stimulus. Chemical mediators in a wide array are produced in the PNS and have both excitatory and inhibitory influences on peripheral sensory nerve transmission in both the acute and the chronic phase of injury (Figure 3). These can directly activate the nerve (ATP, glutamate, 5-hydroxytryptamine [5-HT], histamine, bradykinin); enhance depolarization by sensitizing the nerve to other stimuli (prostaglandins, prostacyclin, and cytokines such as interleukins); or provide a regulatory role on the sensory neuron, inflammatory cells, and sympathetic fibers (bradykinin, tachykinin, and nerve growth factor).
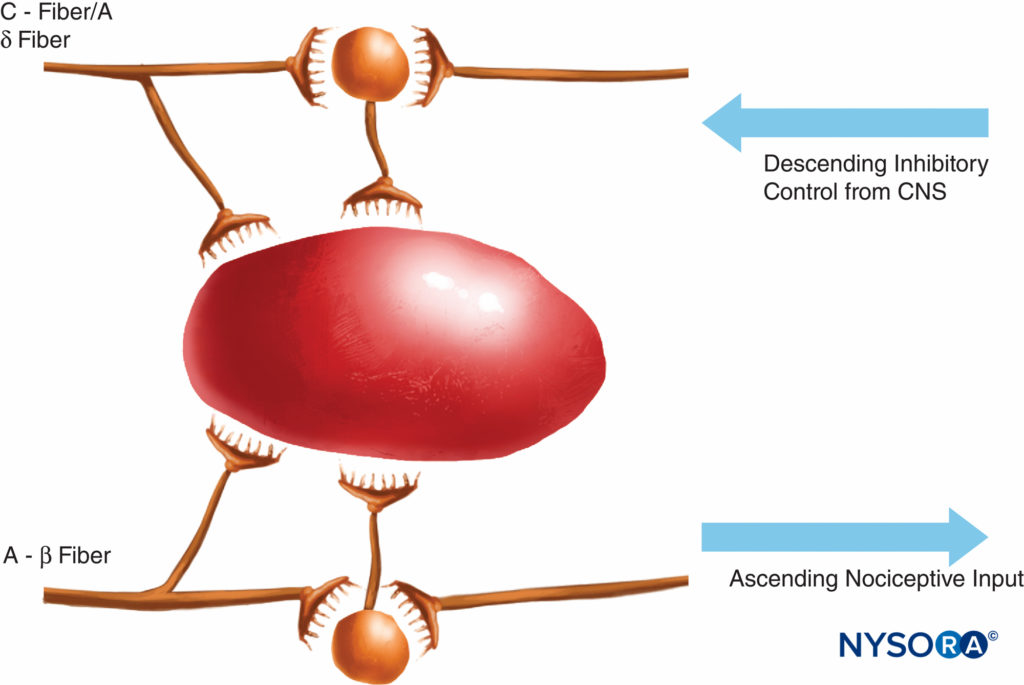
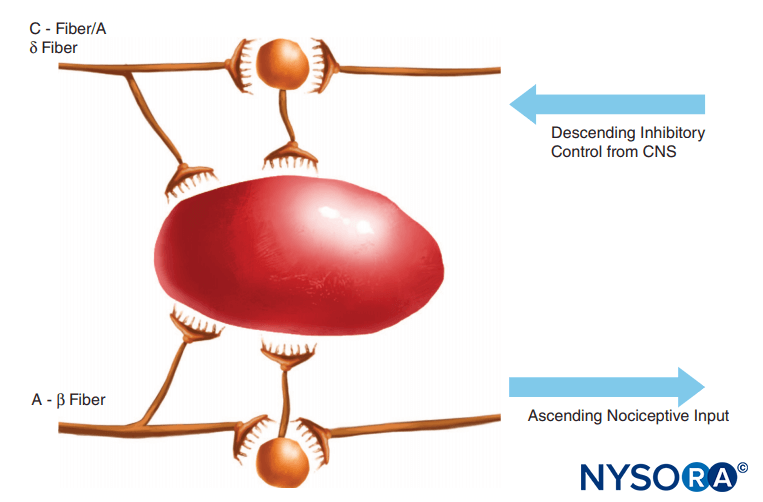
FIGURE 2. The gate theory proposed that small (C) fibers activated excitatory systems (black neuron) that subsequently excited output cells; these latter cells had their activity controlled by the balance of large fiber (A-β)-mediated inhibitions (mediated by endogenous opioids) and also by descending control systems from the central nervous system (mediated by norepinephrine and serotonin).
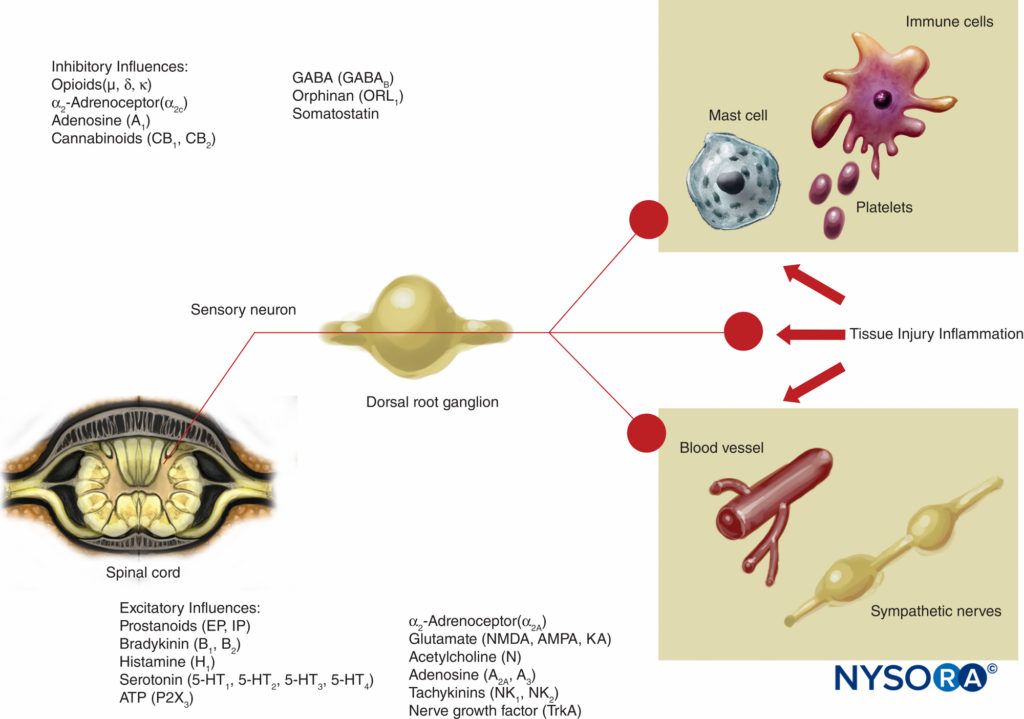
FIGURE 3. Excitatory and inhibitory influences on peripheral nerve activity by mediators released by tissue injury and inflammation and by a variety of agents acting on neuroreceptors. AMPA = α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid; KA = kainic acid; NMDA = N-methyl-d-aspartate; NK = neurokinin; TrkA = Tropomyosin receptor kinase A.
RATIONALE FOR USE OF ANALGESIC ADJUVANTS
As previously noted, pain transmission in the CNS and PNS involves a complex array of neurotransmitters and pathways that are not easily blocked by one drug type or technique alone. A number of drugs in the anesthesiologist’s armamentarium, including opioids, nonsteroidal anti-inflammatory drugs (NSAIDs), α2-agonists, dexamethasone, and N-methyl-aspartate (NMDA) antagonists, have activity at these sites of action and may have benefit if applied in the PNS. Importantly, none has demonstrated neurotoxicity at clinically relevant concentrations.
This knowledge can aid the regional anesthesiologist in a number of ways:
- In the selection of adjuvants to local anesthetics to speed onset, prolong effect, and reduce total required dose.
- Suggest agents that can enhance postoperative analgesia without prolonging adverse effects of local anesthetics.
- Suggest agents that predominantly act at peripheral sites without central effects, thereby optimizing analgesia while minimizing CNS side effects.
OPIOID ANALGESICS
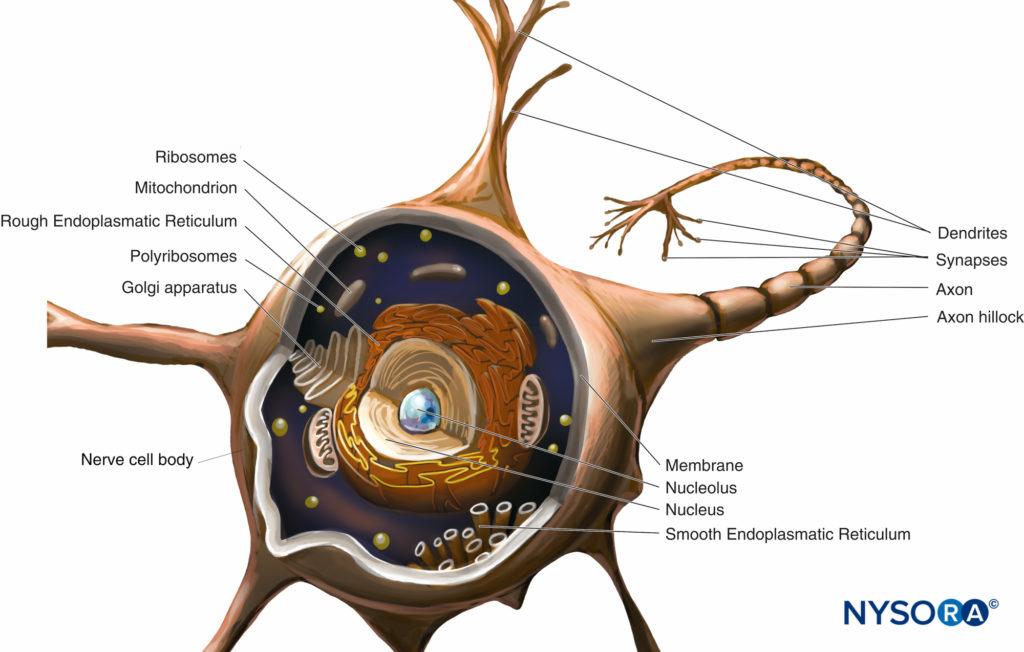
During inflammation, opioid receptors are expressed in peripheral sensory fibers and immune cells; moreover, endogenous opioids are released from these cells and balance the increased nociceptive state produced by inflammation. An increasing body of work suggests an intimate relationship between endogenous opioids and the immune system. Christoph Stein and colleagues in Berlin have performed a number of pioneering studies that described the ability of the immune system to deliver endogenous opioids and the ability of inflammation to stimulate movement of opioid receptors to the site of injury, thereby allowing antinociception to occur. However, these changes do not occur immediately after injury and can take up to 96 hours to occur. Opioid receptors and neuropeptides (eg, substance P) are synthesized in the dorsal root ganglion and transported along intra-axonal microtubules into central and peripheral processes of the primary afferent neuron (Figure 4). At the terminals, opioid receptors are incorporated into the neuronal membrane and become functional receptors. On activation by exogenous or endogenous opioids (released by immune cells), opioid receptors couple to inhibitory G proteins. This leads to direct or indirect (through decrease of cyclic adenosine monophosphate) suppression of Ca2+ or Na+ currents and subsequent attenuation of substance P release.
The permeability of the perineurium is increased within inflamed tissue, enhancing the ability of opioids to reach target receptors. Numerous studies have applied opioids in the PNS to either peripheral nerves or the intra-articular space. Although many studies claimed an analgesic benefit of peripherally applied opioids, few studies incorporated a control group with a systemically applied opioid for comparison. Without inclusion of a control, it is impossible to interpret whether the peripheral opioid is having a true peripheral effect or is instead being carried to the CNS to induce analgesia. True peripherally mediated opioid analgesia may be beneficial if this is associated with improved analgesia or reduced adverse effects compared with systemic administration. If the effect is mediated centrally, then there is no clear benefit over systemic administration.
Perineural Opioids
Opioid receptors identified on primary afferent fibers are transported from the dorsal root ganglion to the site of inflammation; however, while they are undergoing axonal transport, they may not be easily reached by opioid agonists. This may explain the reason that two recent systematic reviews published in 1997 and 2000 found little evidence for the benefit of adding opioids to local anesthetics in peripheral nerve block. An updated table of studies examining perineuronal administration of opioids (excluding buprenorphine and tramadol) shows that analgesic benefit remains equivocal (Table 1). In addition, Peng and Choyce reviewed the use of opioids in intrave-nous regional anesthesia (IVRA) with similar disappointing conclusions.
TABLE 1. Outcomes of studies examining the effect of perineuronal/perineural opioids (excluding tramadol and buprenorphine).
| Total Studies | Overall Outcomes | Systemic Control Outcomes |
|---|---|---|
| 19 studies | 10 supportive | 7 systemic control: 5 supportive; 2 negative |
| 9 negative | 12 no systemic control: 5 supportive; 7 negative. |
Despite these disappointing results, the two opioid agonists that have demonstrated analgesic efficacy when administered perineuronally are buprenorphine and tramadol. Buprenorphine is a partial µ-receptor agonist with a very high receptor affinity compared with fentanyl (24-fold) or morphine (50-fold). In addition, it has intermediate lipid solubility, which allows it to cross the neural membrane. Candido and colleagues added 0.3 mg buprenorphine (a partial opioid agonist) to a combination of mepivacaine and tetracaine in axillary block and found an almost 100% increase in the duration of analgesia compared with the administration of axillary block plus the same dose of intramuscular buprenorphine with no significant increase in adverse effects. This supports the peripheral analgesic effect of buprenorphine and the earlier findings of two studies that examined buprenorphine without a systemic control group. Studies examining buprenorphine are presented in greater detail in Table 2.
TABLE 2. Studies examining buprenorphine as an analgesic adjuvant with local anesthetics.
| Author/Date | Patients/ Groups | Block Type | Dose | Local Anesthetic | Systemic Control | Results |
|---|---|---|---|---|---|---|
| Viel 1989 | 20/2 | Supraclavicular | 3 mcg/kg | Bupivacaine 0.5% 40 mL | No | Prolonged analgesia compared to morphine group (35 vs. 18.25 h); no difference in sensory block. |
| Bazin 1997 | 89/4 | Supraclavicular | 3 μg/kg | Bupivacaine 0.5% Lidocaine 1% | No | Prolonged analgesia compared to control group (20 vs. 11.5 h) |
| Candido 2001 | 40/2 | Supraclavicular | 0.3 mg | Mepivacaine 1% Tetracaine 0.2% | No | Prolonged analgesia compared to control group (17.4 vs. 5.3 h) |
| Candido 2002 | 60/3 | Axillary | 0.3 mg | Mepivacaine 1% | Yes | The mean duration of postoperative analgesia was 22.3 h in axillary group vs. 12.5 h in IM group and 6.6 h in placebo group. |
| Tetracaine 0.2% | IM |
Tramadol is a weak opioid agonist with some selectivity for the µ-receptor that also inhibits norepinephrine reuptake and stimulates serotonin release in the intrathecal space. Norepinephrine and serotonin are transmitters for the descending control pathway in the spinal cord and enhance analgesia. Kapral and coworkers used a 100-mg dose of tramadol as an adjuvant to mepivacaine in axillary brachial plexus block. They divided 60 patients into three groups: One group received mepivacaine 1% with 2 mL saline, the second group received mepivacaine 1% with 100 mg tramadol, and the third group received mepivacaine 1% with 2 mL saline and 100 mg tramadol intravenously. This study demonstrated an increased duration of motor and sensory block in the axillary tramadol group that significantly (p < .01) outlasted both an intravenous and a placebo group. Robaux and colleagues subsequently performed a dose-response study with placebo and 40-, 100-, and 200-mg doses of tramadol added to a fixed dose of mepivacaine 1.5% in axillary block and found that the 200-mg dose provided the best analgesia with no increased adverse effects. Alemanno and colleagues used a 1.5-mg/kg dose of tramadol as an adjuvant to 0.5% levobupivacaine (0.5 mL/kg) for interscalene block. Here, 120 patients were divided into three groups: One group received local anesthetic alone, the second group received local anesthetic with systemic tramadol, and the third group received local anesthetic with perineural tramadol. While both groups receiving tramadol experienced prolonged analgesia compared to placebo, the group receiving perineural tramadol experienced prolonged analgesia compared to systemic tramadol (14.5 vs. 10.1 hours; p < .001).
Intra-articular Opioids and Other Peripheral Routes of Administration
Opioid agonists administered into inflamed tissue will bind to opioid receptors on sensory terminals and induce analgesia. Animal studies indicated that these peripheral opioid receptors are expressed 96 hours after the initial inflammatory injury. Intra-articular administration of opioids will therefore only produce analgesia in patients with preexisting inflammation. Kalso and coworkers systematically examined the role of intra-articular opioids in 1997 and established that there existed evidence for a prolonged benefit from intra-articular morphine without significant adverse effects at doses of 1 to 5 mg. No dose response was detected. Recent articles supported this finding and showed the benefit of intra-articular morphine, tramadol, buprenorphine, and sufentanil. However, a systematic review of the effects of intra-articular morphine demonstrated only a mild analgesic effect [visual analogue scale for pain (VAS) 12–17 mm reduction] but could not exclude that the effect was mediated by systemic absorption.
ALPHA2-AGONISTS AND CLONIDINE
Clonidine is an α2-agonist with some α1-stimulatory effects. It has traditionally been used as an antihypertensive agent and has been noted to have sedative and analgesic effects for many years. More recently, it was determined that α2-receptors exist in the dorsal horn of the spinal cord, and stimulation of these receptors produces analgesic effects by inhibiting the presynaptic release of excitatory transmitters, including substance P and glutamate. Intrathecal clonidine mediates analgesia by increasing acetylcholine levels, which in turn stimulates muscarinic receptors. Muscarinic excitation increases γ-amino butyric acid levels onto the primary afferent fiber, inhibiting the release of the excitatory neurotransmitter glutamate. Clonidine injected close to peripheral nerves with or without local anesthetic drugs appears to mediate analgesia in a number of ways. Clonidine has local anesthetic properties and tonically inhibited compound action potentials of C fibers greater then A-α fibers in rat sciatic nerve and was comparable to lidocaine in its ability to inhibit C fibers in rabbit vagus nerve. Clonidine also has a pharmacokinetic effect on local anesthetic redistribution mediated by a vasoconstrictor effect at the α1-receptor. Recent animal models have demonstrated and supported earlier work that clonidine predominantly facilitates peripheral nerve block through hyperpolarization-activated cationic current and that this effect is independent of any vasoconstrictor effect. A more recent addition to the selection of α2-agonists is dexmedetomidine, which is selective for the α2-receptor and which at present is mainly studied as a sedative agent in intensive care units. Dexmedetomidine may be expected to produce not only more profound analgesia but also greater adverse effects because of the selectivity of action. Stimulation of the α2-receptor produces hypotension, bradycardia, and sedation at higher doses, and these effects may outweigh any analgesic benefits produced by the use of these agents.
Perineuronal Application
Over 30 studies in humans have now examined the effect of clonidine on local anesthetics in peripheral nerve block. There is good evidence from these studies that clonidine in doses up to 1.5 µg/kg prolongs sensory block and analgesia when administered with local anesthetics for peripheral nerve block. This supports the early opinion of Murphy and colleagues that clonidine is a beneficial adjuvant when added to peripheral nerve block and that the effect is most likely mediated in the PNS. Although a number of studies have examined the effect of clonidine added to peripheral nerve block, only a few have controlled for a systemic effect. Singelyn and coworkers evaluated 30 patients receiving an axillary brachial plexus block with 40 mL of 1% mepivacaine plus epinephrine 5 µg/mL. Patients were randomized to three groups and received (1) local anesthetic alone, (2) local anesthetic plus 150 µg of clonidine administered subcutaneously, or (3) 150 µg of clonidine in the brachial plexus block with local anesthetic. Clonidine added to the axillary brachial plexus block delayed the onset of pain twofold, without adverse effects when compared with systemic control. Hutschala and coworkers have recently demonstrated the peripheral analgesic effect of clonidine in volunteers when added to brachial plexus block with 0.25% bupivacaine.
However, other recent studies demonstrated no overall benefit of adding clonidine to long-acting local anesthetics such as bupivacaine and ropivacaine. More recently, a meta-analysis by Popping and colleagues estimated that clonidine prolonged postoperative analgesia, sensory block, and motor block by 122, 74, and 141 minutes, respectively. Clonidine, however, also increased the probability of hypotension (odds ratio [OR] 3.61), fainting (OR 5.07), sedation (OR 2.28), and bradycardia (OR 3.09). There was no observed dose response between a range of 30 and 300 µg, with the majority receiving 150 µg. The addition of clonidine to continuous peripheral nerve blocks is not beneficial. Ilfeld and colleagues have demonstrated in two studies that both 0.1 and 0.2 µg/mL of clonidine added to a continuous infusion of ropivacaine 0.2% failed to reduce pain scores or oral analgesic use after upper extremity surgery. Dexmedetomidine, as previously postulated, does indeed produce a more profound effect on analgesia when applied perineurally in conjunction with local anesthetics. Four studies have recently examined this, and a meta-analysis suggested that dexmedetomidine prolongs the analgesic effects of brachial plexus blocks by 284 minutes. Interestingly, despite initial concerns that dexmedetomidine may have greater hemodynamic effects than clonidine, this does not appear to be the case.
Intravenous Regional Anesthesia
Intravenous regional anesthesia is a useful, simple regional anesthetic technique especially for minor peripheral upper limb procedures that is limited by tourniquet tolerance and poor postoperative analgesia. Clonidine has been demonstrated in a number of studies to improve onset time and intraoperative tourniquet tolerance. Only one study has demonstrated improved postoperative analgesia in the early postoperative period compared with placebo. Reuben and coworkers randomized 45 patients to 40 mL 0.5% lidocaine with clonidine 1 µg/kg, lidocaine alone with intravenous clonidine, and lidocaine alone with intravenous saline. Patients who were given clonidine with lidocaine experienced significantly less pain and requested fewer analgesics then patients in the other two groups. Higher doses of clonidine (150 µg) produced significantly more sedation and incidence of hypotension. To date only one study has used dexmedetomidine in IVRA. Memis and colleagues added 0.5 µg/kg of dexmedetomidine to 0.5% lidocaine and demonstrated reduction in onset time and improvement in postoperative analgesia compared with placebo with no significant adverse effects.
Intra-articular Techniques
The intra-articular effect of clonidine has been examined when administered with and without local anesthetic and been found to have beneficial effects on postoperative analgesia. The addition of morphine and clonidine may be expected to have additive effects. Two studies have examined this question, with one demonstrating improved analgesia and the other no difference. Preclinical trials have demonstrated that, similar to opioids, clonidine-mediated analgesia is enhanced by inflammation, although at the present time the mechanism is not evident.
DEXAMETHASONE
Dexamethasone is a potent synthetic corticosteroid with approximately seven times the anti-inflammatory potency of prednisolone and very little mineralocorticoid activity. The half-life is approximately 36 to 54 hours in the perioperative setting. The effectiveness of dexamethasone as a postoperative antiemetic (4 to 10 mg intravenously) has been confirmed by over 60 randomized controlled trials, with a recent meta-analysis estimating on OR of 0.31 and a 3.7 number needed to treat (NNT). Given its systemic anti-inflammatory properties, the analgesic effects of a single preoperative intravenous dose of dexamethasone have been investigated in over 24 randomized trials with modest effects up to 24 hours. This meta-analysis, published in 2011, included 2751 patients and estimated that verbal rating scale for pain (VRS) scores were reduced to a maximum of 0.64 points up to 24 hours after dexamethasone administration. Prior to these reviews, in vitro and murine studies of the specific pharmacologic action of dexamethasone yielded several novel applications in addition to systemic administration.
Perineural Application
Perineural corticosteroids are thought to exert their effect by several mechanisms, including attenuating the release of inflammatory mediators, reducing ectopic neuronal discharge, and inhibiting potassium channel–mediated discharge of nociceptive C fibers. It is widely believed that dexamethasone improves the quality and duration of peripheral nerve block when administered in conjunction with local anesthetics. The US Food and Drug Administration (FDA) (or any other regulatory body), however, does not approve dexamethasone for perineural administration. Nonetheless, multiple studies have assessed the effects of combining dexamethasone (4 to 10 mg) with local anesthetic for peripheral nerve blocks. Upper and lower extremity peripheral nerve blocks performed with dexamethasone demonstrated prolongation of analgesia or sensory/motor block ranging from approximately 50% to 75% beyond that performed with local anesthetic alone. Only one study to date has compared perineural to systemic dexamethasone in the context of peripheral nerve blocks. This study randomized patients to interscalene brachial plexus block with placebo or 8 mg of perineural or systemic dexamethasone. The authors demonstrated block prolongation in both dexamethasone groups from 12 hours to approximately 20 and 22 hours for systemic and perineural administration, respectively, and concluded that systemic and perineural dexamethasone administration were equivalent. Further study is required comparing the effects of perineural and systemic dexamethasone before final conclusions can be drawn. Concerns over complications related to dexamethasone, such as effects on blood glucose and neurotoxicity from the preservative used in multidose vials, have not been apparent in practice. In particular, a single dose of dexamethasone, whether administered perineurally or systemically, did not increase blood glucose to a clinically significant degree. Murine studies of sodium bisulfite demonstrated no neurotoxicity with intrathecal administration.
Intravenous Regional Anesthesia
Bigat and colleagues investigated the effects of adding dexamethasone to lidocaine IVRA in a randomized trial. Seventy-five patients were randomized to lidocaine with placebo, 8 mg of dexamethasone with lidocaine, or 8 mg of systemic dexamethasone. In this study, systemic dexamethasone exerted no effect on the efficacy of IVRA, while lidocaine plus dexamethasone demonstrated improved block characteristics.
N-METHYL-ASPARTATE RECEPTOR ANTAGONISTS
Within the dorsal horn of the spinal cord both ionotropic [N-methyl- -aspartate (NMDA)], α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA), kainic acid (KA), and metabotropic glutamate receptors are involved in nociceptive signaling and central sensitization in conditions of chronic pain. Recently, multiple glutamate receptors have been found in peripheral nerve terminals and may contribute to peripheral pain signaling. Injection of the NMDA receptor agonist glutamate into masseter muscle produces pain in both rats and humans. Subsequent injection of NMDA receptor antagonists such as ketamine and dextromethorphan attenuates the pain.
A number of studies have examined the effect of NMDA antagonists in producing peripherally mediated analgesia in patients. Tverskoy and colleagues infiltrated bupivacaine with 0.3% ketamine or placebo for patients having inguinal herniorraphy and found that ketamine significantly enhanced the anesthetic and analgesic actions of a local anesthetic administered for infiltration anesthesia. Ketamine has been used as the sole anesthetic in IVRA, but patients suffered excessive adverse effects on tourniquet deflation. Other workers have added ketamine (0.1 mg/mL) or clonidine (1 µg/kg) to lidocaine for IVRA. Patients in the ketamine group had the best pain control, although both clonidine and ketamine significantly reduced analgesic consumption compared with lidocaine alone, with mild psychomimetic side effects in the ketamine group. Two studies have examined the use of intra-articular ketamine. Dal and coworkers randomized patients to intra-articular ketamine (0.5 mg/kg), neostigmine, bupivacaine, or placebo. Patients receiving all three drugs had similar improvements in analgesia with knee flexion compared with placebo; however, the ketamine group had the longest duration of analgesia. Brill and colleagues performed a dose-response study using up to 1 mg/kg intra-articular ketamine after knee arthroscopy and found that the analgesic benefit only occurred in the first hour after surgery compared with placebo. Magnesium has NMDA-blocking effects and blocks the ion channel on the NMDA receptor during normal physiologic states. Persistent nociceptive input in the dorsal horn of the spinal cord removes magnesium, allowing calcium influx and intracellular changes leading to persistent pain states. Turan et al exploited this analgesic potential in the PNS by adding 1.5 g magnesium to lidocaine 0.5% for IVRA. Magnesium reduced onset time and significantly prolonged analgesic effect up to 6 hours after surgery with no difference in adverse effects. Overall, NMDA antagonists may have significant potential for producing peripherally mediated analgesia in the future, although currently available agents (except magnesium in IVRA) have limited effects and at higher doses produce excessive adverse effects.
CYCLOOXYGENASE INHIBITION
Prostaglandins sensitize peripheral nerve endings to the effects of endogenous chemical mediators released during tissue injury. NSAIDs inhibit the production of prostaglandins through their well-known effect of inhibiting cyclooxygenase (COX). Application of NSAIDs directly in the PNS would therefore appear to make sense as a means of reducing pain by peripheral mechanism.
Intravenous Regional Anesthesia
A number of authors have added ketorolac to IVRA in doses from 5 to 60 mg, producing an improvement in intraoperative tourniquet tolerance and postoperative analgesia. Steinberg and colleagues performed a dose-response study with ketorolac in IVRA using placebo, 5-, 10-, 15-, 20-, 30-, and 60-mg doses of ketorolac. It was found that 20 mg was the ideal dose, with lower doses producing less analgesia and higher doses being no more effective. Lysine acetylsalicylic acid 90 mg (equivalent to 50 mg acetylsalicylic acid) has been added to prilocaine for IVRA with prolongation of postoperative analgesia.
Intra-articular Administration
The use of ketorolac alone, with local anesthetic or local anesthetic and morphine, is no more effective than local anesthetic alone when administered in the intraarticular space. Read more about Intra-articular and Periarticular Infiltration of Local Anesthetics.
Infiltration
Ketorolac has been successfully infiltrated in a dose of 30 to 60 mg following hernia repair, giving an effect similar to infiltration with bupivacaine. However, local infiltration was found to be no more effective than systemic administration.
CHOLINERGIC ANALGESIA
Muscarinic receptors mediate analgesia in the dorsal horn of the spinal cord, and neostigmine has produced analgesia when administered to both the intrathecal and epidural space. Neostigmine has also been applied in the PNS in a number of studies, with generally disappointing results. Van Elstraete and coworkers and Bone and colleagues added neostigmine 500 µg to local anesthetic in axillary brachial plexus block. One study demonstrated no difference, and the other found only significant reduction in pain at 24 hours, with no difference at other time points. Neostigmine added to local anesthetic for IVRA has also been disappointing. Turan and coworkers added 500 µg neostigmine to prilocaine 0.5% and found improvement in sensory and motor block onset and offset with prolonged time to first analgesic request. However, McCartney and colleagues performed a similar study using neostigmine 1 mg added to lidocaine 0.5%, with no differences found between groups. Overall, neostigmine appears disappointing as an analgesic adjuvant for peripheral nerve block or IVRA. Neostigmine, however, has been used successfully as an analgesic adjuvant for intra-articular use after knee arthroscopy. Yang and coworkers performed a dose-response study and found 500 µg to be most effective, which was more effective than 2 mg of intra-articular morphine. The effectiveness of the intra-articular cholinergic analgesic pathway compared with the poor results with perineuronal application may be related to the presence of the inflammatory response in the intra-articular space, increasing the analgesic efficacy of acetylcholine by a mechanism that is yet to be defined.
SUMMARY
Peripheral nerve blocks provide significant anesthetic and analgesic benefits for our patients. Analgesic adjuvants such as opioids, α2-agonists, NMDA receptor antagonists, and other agents can be added to local anesthetics both to facilitate onset and to prolong anesthetic and analgesic effects by mechanisms existing in the PNS. Several agents are effective when administered in the perineuronal or intra-articular space and when given in IVRA or local infiltration (Table 3). The effect size of each particular adjuvant is variable, with dexamethasone producing the largest effect size. Our evolving knowledge of nociceptive mechanisms in the PNS will allow novel techniques to be developed in the future to further improve pain management.
TABLE 3. Best analgesic adjuvants in the peripheral nervous system by route of administration.
| Route | Agent and Dose |
|---|---|
| Perineuronal/perineural | Dexamethasone 4–10 mg; buprenorphine 0.3 mg; clonidine 1–2 μg/kg; tramadol 200 mg |
| IVRA | Dexmedetomidine 0.5 μg/kg; magnesium 1.5 g |
| Intra-articular | Clonidine 150 μg; morphine 5 mg |
| Local infiltration | Ketamine 3 mg/mL |
NB: Several studies authored by Dr. S. Reuben that have since been retracted were referenced in the previous edition of this text. These references have been removed. All references remaining in which Dr. Reuben was involved that have not been retracted are still referenced.
REFERENCES
- Kehlet H, Dahl JB: The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth Analg 1993;77:1048–1056.
- Raja SN, Meyer RA, Ringkamp M, et al: Peripheral neural mechanisms of nociception. In Wall PD, Melzack R (eds): Textbook of Pain, 4th ed. Churchill-Livingstone, 1999, pp 11–57.
- Dickenson AH: Gate control theory of pain stands the test of time. Br J Anaesth 2002;88:755–757.
- Millan MJ: The induction of pain: An integrative review. Prog Neurobiol 1999;57:1–164.
- Sawynok J: Topical and peripherally acting analgesics. Pharmacol Rev 2003;55:1–20.
- Williams BA, Hough KA, Tsui BY, Ibinson JW, Gold MS, Gebhart GF: Neurotoxicity of adjuvants used in perineural anesthesia and analgesia in comparison with ropivacaine. Reg Anesth Pain Med 2011; 36(3): 225–230.
- Likar R, Mousa SA, Philippitsch G, et al: Increased numbers of opioid expressing inflammatory cells do not affect intraarticular morphine analgesia. Br J Anaesth 2004;93:375–380.
- Brack A, Rittner HL, Machelska H, et al: Control of inflammatory pain by chemokine-mediated recruitment of opioid-containing polymorphonuclear cells. Pain 2004;112:229–238.
- Machelska H, Cabot PJ, Mousa SA, et al: Pain control in inflammation governed by selectins. Nat Med 1998;4:1425–1428.
- Stein C, Schafer M, Machelska H: Attacking pain at its source: New perspectives on opioids. Nat Med 2003;9:1003–1008.
- Mousa SA, Zhang Q, Sitte N, et al: beta-Endorphin-containing memory-cells and mu-opioid receptors undergo transport to peripheral inflamed tissue. J Neuroimmunol 200;115:71–78.
- Picard PR, Tramer MR, McQuay HJ, et al: Analgesic efficacy of peripheral opioids (all except intra-articular): A qualitative systematic review of randomised controlled trials. Pain 1997;72:309–318.
- Murphy DB, McCartney CJ, Chan VW: Novel analgesic adjuncts for brachial plexus block: A systematic review. Anesth Analg 2000;90: 1122–1128.
- Fanelli G, Casati A, Magistris L, et al: Fentanyl does not improve the nerve block characteristics of axillary brachial plexus anaesthesia performed with ropivacaine. Acta Anaesthesiol Scand 2001;45:590–594.
- Karakaya D, Buyukgoz F, Baris S, et al: Addition of fentanyl to bupivacaine prolongs anesthesia and analgesia in axillary brachial plexus block. Reg Anesth Pain Med 2001;26:434–438.
- Likar R, Koppert W, Blatnig H, et al: Efficacy of peripheral morphine analgesia in inflamed, non-inflamed and perineural tissue of dental surgery patients. J Pain Symptom Manage 2001;21:330–337.
- Nishikawa K, Kanaya N, Nakayama M, et al: Fentanyl improves analgesia but prolongs the onset of axillary brachial plexus block by peripheral mechanism. Anesth Analg 2000;91:384–387.
- Choyce A, Peng P: A systematic review of adjuncts for intravenous regional anesthesia for surgical procedures. Can J Anaesth 2002;49: 32–45.
- Gutstein H, Akil H: Opioid analgesics. In Hardman J, Limbird L (eds): Goodman & Gilman’s The Pharmacologic Basis of Therapeutics, 10th ed. McGraw-Hill, 2001, p 601.
- Lanz E, Simko G, Theiss D, et al: Epidural buprenorphine—A double-blind study of postoperative analgesia and side effects. Anesth Analg 1984;63:593–598.
- Candido KD, Winnie AP, Ghaleb AH, et al: Buprenorphine added to the local anesthetic for axillary brachial plexus block prolongs postoperative analgesia. Reg Anesth Pain Med 2002;27:162–167.
- Candido KD, Franco CD, Khan MA, et al: Buprenorphine added to the local anesthetic for brachial plexus block to provide postoperative analgesia in outpatients. Reg Anesth Pain Med 2001;26:352–356.
- Viel EJ, Eledjam JJ, De La Coussaye JE, et al: Brachial plexus block with opioids for postoperative pain relief: Comparison between buprenorphine and morphine. Reg Anesth 1989;14:274–278.
- Bazin JE, Massoni C, Bruelle P, et al: The addition of opioids to local anaesthetics in brachial plexus block: The comparative effects of morphine, buprenorphine and sufentanil. Anaesthesia 1997;52: 858–862.
- Alhashemi JA, Kaki AM: Effect of intrathecal tramadol administration on postoperative pain after transurethral resection of prostate. Br J Anaesth 2003;91:536–540.
- Kapral S, Gollmann G, Waltl B, et al: Tramadol added to mepivacaine prolongs the duration of an axillary brachial plexus block. Anesth Analg 1999;88:853–856.
- Robaux S, Blunt C, Viel E, et al: Tramadol added to 1.5% mepivacaine for axillary brachial plexus block improves postoperative analgesia dose-dependently. Anesth Analg 2004;98:1172–1177.
- Alemmano F, Ghisi D, Fanelli A, et al: Tramodol and 0.5% levobupivacaine for single shot interscalene block. Minerva Anestesiol 2013;78(3): 291–296.
- Kalso E, Tramer MR, Carroll D, et al: Pain relief from intra-articular morphine after knee surgery: A qualitative systematic review. Pain 1997;71:127–134.
- Brandsson S, Karlsson J, Morberg P, et al: Intraarticular morphine after arthroscopic ACL reconstruction: A double-blind placebo-controlled study of 40 patients. Acta Orthop Scand 2000;71:280–285.
- Rasmussen S, Larsen AS, Thomsen ST, et al: Intra-articular glucocorticoid, bupivacaine and morphine reduces pain, inflammatory response and convalescence after arthroscopic meniscectomy. Pain 1998;78:131–134.
- Alagol A, Calpur OU, Kaya G, et al: The use of intraarticular tramadol for postoperative analgesia after arthroscopic knee surgery: A comparison of different intraarticular and intravenous doses. Knee Surg Sports Traumatol Arthrosc 2004;12:184–188.
- Varrassi G, Marinangeli F, Ciccozzi A, et al: Intra-articular buprenorphine after knee arthroscopy. A randomised, prospective, double-blind study. Acta Anaesthesiol Scand 1999;43:51–55.
- Vranken JH, Vissers KC, de Jongh R, et al: Intraarticular sufentanil administration facilitates recovery after day-case knee arthroscopy. Anesth Analg 2001;92:625–628.
- Gupta A, Bodin L, Holmstrom B, Berggren: A systematic review of the peripheral analgesic effects of intraarticular morphine. Anesth Analg 2001;93(3):761–770.
- Unnerstall JR, Kopajtic TA, Kuhar MJ: Distribution of alpha 2 agonist binding sites in the rat and human central nervous system: Analysis of some functional, anatomic correlates of the pharmacologic effects of clonidine and related adrenergic agents. Brain Res 1984;319:69–101.
- Kuraishi Y, Hirota N, Sato Y, et al: Noradrenergic inhibition of the release of substance P from the primary afferents in the rabbit spinal dorsal horn. Brain Res 1985;359:177–182.
- Fleetwood-Walker SM, Mitchell R, Hope PJ, et al: An alpha 2 receptor mediates the selective inhibition by noradrenaline of nociceptive responses of identified dorsal horn neurones. Brain Res 1985;334:243–254.
- Baba H, Kohno T, Okamoto M, et al: Muscarinic facilitation of GABA release in substantia gelatinosa of the rat spinal dorsal horn. J Physiol 1998;508:83–93.
- Butterworth JF 5th, Strichartz GR: The alpha 2-adrenergic agonists clonidine and guanfacine produce tonic and phasic block of conduction in rat sciatic nerve fibers. Anesth Analg 1993;76:295–301.
- Gaumann DM, Brunet PC, Jirounek P: Clonidine enhances the effects of lidocaine on C-fiber action potential. Anesth Analg 1992;74:719–725.
- Eisenach JC, Gebhart GF: Intrathecal amitriptyline. Antinociceptive interactions with intravenous morphine and intrathecal clonidine, neostigmine, and carbamylcholine in rats. Anesthesiology 1995;83: 1036–1045.
- Kroin JS, Buvanendran A, Beck DR, et al: Clonidine prolongation of lidocaine analgesia after sciatic nerve block in rats is mediated via the hyperpolarization-activated cation current, not by alpha-adrenoreceptors. Anesthesiology 2004;101:488–494.
- Singelyn FJ, Dangoisse M, Bartholomee S, et al: Adding clonidine to mepivacaine prolongs the duration of anesthesia and analgesia after axillary brachial plexus block. Reg Anesth 1992;17:148–150.
- Hutschala D, Mascher H, Schmetterer L, et al: Clonidine added to bupivacaine enhances and prolongs analgesia after brachial plexus block via a local mechanism in healthy volunteers. Eur J Anaesthesiol 2004; 21:198–204.
- Culebras X, Van Gessel E, Hoffmeyer P, et al: Clonidine combined with a long acting local anesthetic does not prolong postoperative analgesia after brachial plexus block but does induce hemodynamic changes. Anesth Analg 2001;92:199–204.
- Popping DM, Elia N, Marret E, Wenk M, Tramer MR. Clonidine as an adjuvant to local anesthetics for peripheral nerve and plexus blocks: A meta-analysis of randomized trials. Anesthesiology 2009;111(2): 406–415.
- Ilfeld BM, Morey TE, Enneking FK: Continuous infraclavicular perineural infusion with clonidine and ropivacaine compared with ropivacaine alone: A randomized, double-blinded, controlled study. Anesth Analg 2003;97:706–712.
- Ilfeld BM, Morey TE, Thannikary LJ, et al: Clonidine added to a continuous interscalene ropivacaine perineural infusion to improve postoperative analgesia: A randomized, double-blind, controlled study. Anesth Analg 2005;100:1172–1178.
- Abdallah FW, Brull R: Facilitatory effects of perineural dexmedetomidine on neuraxial and peripheral nerve block: a systematic review and meta-analysis. Br J Anaesth 2013;110(6):915–925.
- Alayurt S, Memis D, Pamukcu Z: The addition of sufentanil, tramadol or clonidine to lignocaine for intravenous regional anaesthesia. Anaesth Intensive Care 2004;32:22–27.
- Gentili M, Bernard JM, Bonnet F: Adding clonidine to lidocaine for intravenous regional anesthesia prevents tourniquet pain. Anesth Analg 1999;88:1327–1330.
- Reuben SS, Steinberg RB, Klatt JL, et al: Intravenous regional anesthesia using lidocaine and clonidine. Anesthesiology 1999;91:654–658.
- Lurie SD, Reuben SS, Gibson CS, et al: Effect of clonidine on upper extremity tourniquet pain in healthy volunteers. Reg Anesth Pain Med 2000;25:502–505.
- Memis D, Turan A, Karamanlioglu B, et al: Adding dexmedetomidine to lidocaine for intravenous regional anesthesia. Anesth Analg 2004;98:835–840.
- Joshi W, Reuben SS, Kilaru PR, et al: Postoperative analgesia for outpatient arthroscopic knee surgery with intraarticular clonidine and/or morphine. Anesth Analg 2000;90:1102–1106.
- Tan PH, Buerkle H, Cheng JT, et al: Double-blind parallel comparison of multiple doses of apraclonidine, clonidine, and placebo administered intra-articularly to patients undergoing arthroscopic knee surgery. Clin J Pain 2004;20:256–260.
- Gentili M, Juhel A, Bonnet F: Peripheral analgesic effect of intraarticular clonidine. Pain 1996;64:593–596.
- Gentili M, Houssel P, Osman M, et al: Intra-articular morphine and clonidine produce comparable analgesia but the combination is not more effective. Br J Anaesth 1997;79:660–661.
- Buerkle H, Schapsmeier M, Bantel C, et al: Thermal and mechanical antinociceptive action of spinal vs peripherally administered clonidine in the rat inflamed knee joint model. Br J Anaesth 1999;83:436–441.
- Adrenal cortical steroids. In Drug Facts and Comparisons, 5th ed. Facts and Comparisons, 1997, pp 122–128.
- De Oliveira GS Jr, Castro-Alves LJ, Ahmad S, Kendall MC, McCarthy RJ: Dexamethasone to prevent postoperative nausea and vomiting: An updated meta-analysis of randomized controlled trials. Anesth Analg 2013;116(1):58–74.
- De Oliveira GS Jr, Almeida MD, Benzon HT, McCarthy RJ: Perioperative single dose systemic dexamethasone for postoperative pain: A meta-analysis of randomized controlled trials. Anesthesiology 2011;115(3): 575–588.
- Attardi B, Takimoto K, Gealy R, Severns C, Levitan ES: Glucocorticoid induced up-regulation of a pituitary K+ channel mRNA in vitro and in vivo. Receptors Channels 1993;1:287–293.
- Eker HE, Cok OY, Aribogan A, Arslan G: Management of neuropathic pain with methylprednisolone at the site of nerve injury. Pain Med 2012;13:443–451.
- Johansson A, Hao J, Sjolund B: Local corticosteroid application blocks transmission in normal nociceptive C-fibres. Acta Anaesthesiol Scand 1990;34:335–338.
- Cummings KC 3rd, Napierkowski DE, Parra-Sanchez I, et al: Effect of dexamethasone on the duration of interscalene nerve blocks with ropivacaine or bupivacaine. Br J Anaesth 2011;107:446–453.
- Fredrickson MJ, Danesh-Clough TK, White R: Adjuvant dexamethasone for bupivacaine sciatic and ankle blocks: Results from 2 randomized placebo-controlled trials. Reg Anesth Pain Med 2013;38(4):300–307.
- Movafegh A, Razazian M, Hajimaohamadi F, Meysamie A: Dexamethasone added to lidocaine prolongs axillary brachial plexus block. Anesth Analg 2006;102:263–267.
- Parrington SJ, O’Donnell D, Chan VW, et al: Dexamethasone added to mepivacaine prolongs the duration of analgesia after supraclavicular brachial plexus block. Reg Anesth Pain Med 2010;35:422–426.
- Tandoc MN, Fan L, Kolesnikov S, Kruglov A, Nader ND: Adjuvant dexamethasone with bupivacaine prolongs the duration of interscalene block: A prospective randomized trial. J Anesth 2011;25:704–709.
- Vieira PA, Pulai I, Tsao GC, Manikantan P, Keller B, Connelly NR. Dexamethasone with bupivacaine increases duration of analgesia in ultrasound-guided interscalene brachial plexus block. Eur J Anaesthesiol 2010;27:285–288.
- Desmet M, Braems H, Reynvoet M, et al: I.V. and perineural dexamethasone are equivalent in increasing the analgesic duration of a single-shot interscalene block with ropivacaine for shoulder surgery: A prospective, randomized, placebo-controlled study. Br J Anaesth 2013; 111(3):445–452. Epub ahead of print.
- Thangaswamy CR, Rewari V, Trikha A, Dehran M, Chandralekha: Dexamethasone before total laparoscopic hysterectomy: A randomized controlled dose-response study. J Anesth 2010;24:24–30.
- Worni M, Schudel HH, Seifert E, et al: Randomized controlled trial on single dose steroid before thyroidectomy for benign disease to improve postoperative nausea, pain, and vocal function. Ann Surg 2008;248: 1060–1066.
- Taniguchi M, Bollen AW, Drasner K. Sodium bisulfite: Scapegoat for chloroprocaine neurotoxicity? Anesthesiology 2004;100(1):85–91.
- Bigat Z, Boztug N, Hadimioglu N, Cete N, Coskunfirat N, Ertok E: Does dexamethasone improve the quality of intravenous regional anesthesia and analgesia? A randomized, controlled clinical study. Anesth Analg 2006;102(2):605–609.
- Coderre TJ, Katz J, Vaccarino AL, et al: Contribution of central neuroplasticity to pathological pain: Review of clinical and experimental evidence. Pain 1993;52:259–285.
- Price DD, Mao J, Mayer DJ: Central neural mechanisms of normal and abnormal pain states. In Fields HL, Liebskind JC (eds): Progress in Pain Research and Management. IASP Press, 1994;61–84.
- Dickenson AH, Chapman V, Green GM: The pharmacology of excitatory and inhibitory amino acid-mediated events in the transmission and modulation of pain in the spinal cord. Gen Pharmacol 1997;28: 633–638.
- Alfredson H, Forsgren S, Thorsen K, et al: Glutamate NMDAR1 receptors localised to nerves in human Achilles tendons. Implications for treatment? Knee Surg Sports Traumatol Arthrosc 2001;9:123–126.
- Cairns BE, Hu JW, Arendt-Nielsen L, et al: Sex-related differences in human pain and rat afferent discharge evoked by injection of glutamate into the masseter muscle. J Neurophysiol 2001;86:782–791.
- Svensson P, Cairns BE, Wang K, et al: Injection of nerve growth factor into human masseter muscle evokes long-lasting mechanical allodynia and hyperalgesia. Pain 2003;104:241–247.
- Cairns BE, Svensson P, Wang K, et al: Activation of peripheral NMDA receptors contributes to human pain and rat afferent discharges evoked by injection of glutamate into the masseter muscle. J Neurophysiol 2003;90:2098–2105.
- Tverskoy M, Oren M, Vaskovich M, et al: Ketamine enhances local anesthetic and analgesic effects of bupivacaine by peripheral mechanism: A study in postoperative patients. Neurosci Lett 1996;215:5–8.
- Amiot JF, Bouju P, Palacci JH, et al: Intravenous regional anaesthesia with ketamine. Anaesthesia 1985;40:899–901.
- Gorgias NK, Maidatsi PG, Kyriakidis AM, et al: Clonidine versus ketamine to prevent tourniquet pain during intravenous regional anesthesia with lidocaine. Reg Anesth Pain Med 2001;26:512–517.
- Dal D, Tetik O, Altunkaya H, et al: The efficacy of intra-articular ketamine for postoperative analgesia in outpatient arthroscopic surgery. Arthroscopy 2004;20:300–305.
- Brill S, McCartney CJ, Sawyer R, et al: Intra-articular ketamine analgesia following knee arthroscopy: A dose finding study. Pain Clin 2005;17: 25–29.
- Turan A, Memis D, Karamanlioglu B, et al: Intravenous regional anesthesia using lidocaine and magnesium. Anesth Analg 2005;100: 1189–1192.
- Reuben SS, Steinberg RB, Kreitzer JM, et al: Intravenous regional anesthesia using lidocaine and ketorolac. Anesth Analg 1995;81: 110–113.
- Steinberg RB, Reuben SS, Gardner G: The dose-response relationship of ketorolac as a component of intravenous regional anesthesia with lidocaine. Anesth Analg 1998;86:791–793.
- Corpataux JB, Van Gessel EF, Donald FA, et al: Effect on postoperative analgesia of small-dose lysine acetylsalicylate added to prilocaine during intravenous regional anesthesia. Anesth Analg 1997;84:1081–1085.
- Reuben SS, Duprat KM: Comparison of wound infiltration with ketorolac versus intravenous regional anesthesia with ketorolac for postoperative analgesia following ambulatory hand surgery. Reg Anesth 1996;21:565–568.
- Ben-David B, Katz E, Gaitini L, et al: Comparison of IM and local infiltration of ketorolac with and without local anaesthetic. Br J Anaesth 1995;75:409–412.
- Connelly NR, Reuben SS, Albert M, et al: Use of preincisional ketorolac in hernia patients: Intravenous versus surgical site. Reg Anesth 1997; 22:229–232.
- Bosek V, Cox CE: Comparison of analgesic effect of locally and systemically administered ketorolac in mastectomy patients. Ann Surg Oncol 1996;3:62–66.
- Van Elstraete AC, Pastureau F, Lebrun T, et al: Neostigmine added to lidocaine axillary plexus block for postoperative analgesia. Eur J Anaesthesiol 2001;18:257–260.
- Bone HG, Van Aken H, Booke M, et al: Enhancement of axillary brachial plexus block anesthesia by coadministration of neostigmine. Reg Anesth Pain Med 1999;24:405–410.
- Turan A, Karamanlyoglu B, Memis D, et al: Intravenous regional anesthesia using prilocaine and neostigmine. Anesth Analg 2002;95(5): 1419–1422.
- McCartney CJ, Brill S, Rawson R, et al: No anesthetic or analgesic benefit of neostigmine 1 mg added to intravenous regional anesthesia with lidocaine 0.5% for hand surgery. Reg Anesth Pain Med 2003;28: 414–417.
- Yang LC, Chen LM, Wang CJ, et al: Postoperative analgesia by intra-articular neostigmine in patients undergoing knee arthroscopy. Anesthesiology 1998;88:334–339.
- Gentili M, Enel D, Szymskiewicz O, et al: Postoperative analgesia by intraarticular clonidine and neostigmine in patients undergoing knee arthroscopy. Reg Anesth Pain Med 2001;26:342–347.
Colin J. L. McCartney and Stephen Choi