Celiac plexus block has been used in various upper abdominal malignant and nonmalignant pain syndromes with variable success. Pain signals stemming from visceral structures that are innervated by the celiac plexus can be interrupted by blocking the celiac plexus or the splanchnic nerves. These structures include the pancreas, liver, gallbladder, mesentery, omentum, and gastrointestinal tract from the lower esophagus to the transverse colon. The most common application of neurolytic celiac plexus block is upper abdominal malignancy, especially pancreatic cancer; this was first described by Kappis in 1914 [1].
1. ANATOMY OF THE CELIAC PLEXUS
The celiac plexus is a dense network of autonomic nerves that lies anterior to the aorta and the crus of the diaphragm at L1 level. The plexus extends for few centimeters in front of the aorta surrounding the celiac trunk and the superior mesenteric artery (SMA).
Fibers within the plexus arise from efferent sympathetic preganglionic nerves (greater splanchnic nerve T5–T9, lesser splanchnic nerve T10–T11, least splanchnic nerve T12), parasympathetic preganglionic nerves (vagus, posterior trunk), sensory nerves from the phrenic and vagus nerves, and sympathetic postganglionic fibers. Afferent nociception fibers from the abdominal viscera pass diffusely through the celiac plexus and accompany the sympathetic fibers.
Three pairs of ganglia exist within the plexus; these include the celiac ganglia, the superior mesenteric ganglia, and the aortic renal ganglia (Fig. 1). Postganglionic sympathetic nerves from these ganglia accompany blood vessels to the upper abdominal visceral structures. These fibers may play an important role in sympathetically mediated pain syndromes [2].
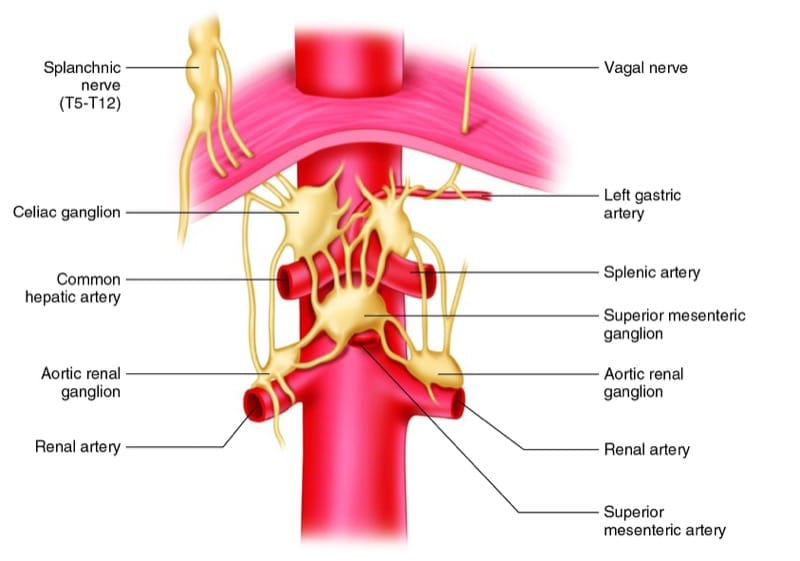
Fig.1 Celiac plexus anatomy. AP view. (Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography© 2010. All rights reserved)
2. CURRENT TECHNIQUES OF THE CELIAC PLEXUS BLOCK
There are two basic methods of performing CPB, which are different depending on the final needle placement relative to the diaphragm: retrocrural or anterocrural. The retrocrural technique also referred to as the deep splanchnic nerve block is considered the classic approach. This technique results in the spread of injectate cephalad and posterior to the diaphragmatic crura (Fig. 2). On the other hand, the anterocrural technique typically involves the insertion of the needle from a posterior approach to a final position anterior to the aorta at the level of the celiac plexus.
- Retrocrural approach or “classic” celiac plexus block (deep splanchnic block): This is commonly performed with fluoroscopy or CT guidance at L1 level in the prone position [3]. However, the classic technique of Kappis was performed with surface landmarks in the lateral decubitus position [1].
- Transcrural approach or “true” celiac plexus block: The needle is advanced through the diaphragmatic crura, and the tip is positioned on each side of the anterior aspect of the aorta with CT guidance [4]. Or the needle is advanced through the aorta with fluoroscopic guidance “transaortic approach” [5].
- Anterocrural approach or true “anterior” approach: This was described initially using anatomical landmarks [6], then later with CT [7] and ultrasound guidance [8].
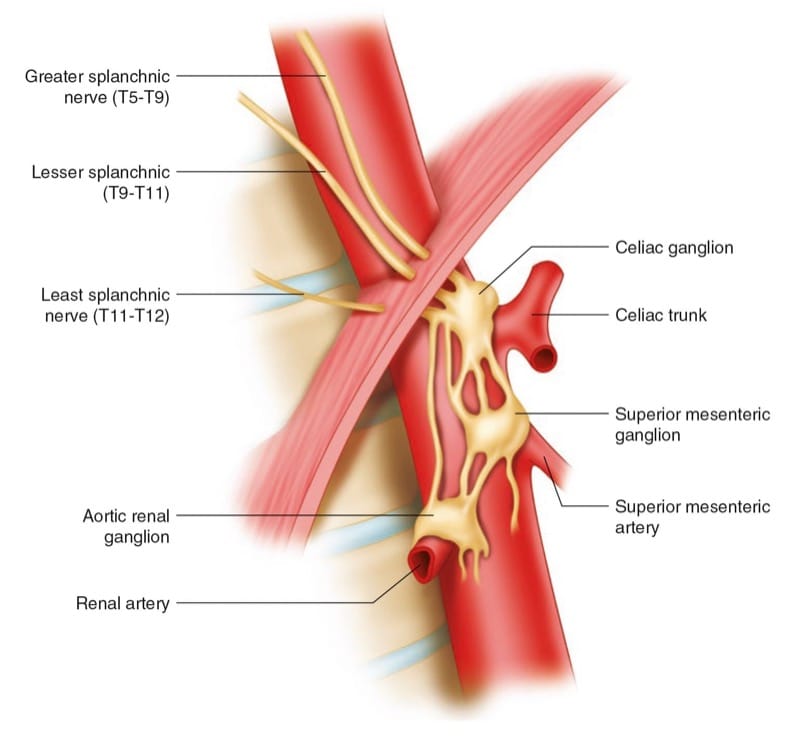
Fig.2 Celiac plexus anatomy. Lateral view showing the celiac plexus, the diaphragm, and the splanchnic nerves. (Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography© 2010. All rights reserved)
3. THE ANTERIOR PERCUTANEOUS APPROACH
Wendling reported the first anterior percutaneous approach to the splanchnic nerves [6]. a thin needle is inserted through the abdominal wall just below, and slightly to the left, of the xiphoid process. The needle is inserted perpendicular to the skin and advanced through the left lobe of the liver and the lesser omentum (occasionally bowel) toward the T12 vertebral body. Interest in this technique has been revived with the introductions of the CT-guided [7] and US-guided approaches [8].
4. ADVANTAGES OF US-GUIDED ANTERIOR APPROACH
- It is the true anterocrural approach. The needle tip location is anterior to the aorta at the exact position of the celiac plexus.
- More suitable for patients who cannot lie prone because of the abdominal pain or other reasons.
- No radiation exposure compared to fluoroscopy or CT.
- More suitable for terminal cancer patients that cannot be transferred to the radiology suite. The US machine is portable, and the block can be performed, with adequate monitoring, in a regular procedure room.
- Avoid injury to nerve roots and neuroaxial structures during needle placement with the posterior approach.
- The authors believe that the most important advantage of the anterior approach is decreasing or even eliminating the potential risk of paraplegia with neurolytic celiac plexus block. Paraplegia and serious neurologic morbidity have been reported after celiac neurolysis [9]. Paraplegia now has been reported with essentially every posterior approach to celiac and splanchnic nerve technique except block by the anterior percutaneous approach [2].
The most accepted postulated mechanism of neurologic injury is spinal cord ischemia or infarction as a consequence of disruption of small nutrient vessels by spasm, direct injury, or accidental intravascular injection [2, 10]. Adamkiewicz’s arteries, the largest spinal cord ventral radicular arteries, supply the lower ventral two-thirds of the spinal cord. After leaving the aorta, they run laterally, about 80% of the time on the left, and typically reach the cord between T8 through L4, making it vulnerable to injury during celiac block by the posterior approach. Also, the posterior retrocrural approach may allow the neurolytic agent to spread or leak posteriorly toward the neuroaxial structures.
5. LIMITATIONS OF US-GUIDED ANTERIOR APPROACH
- Technically challenging in obese patients.
- Patients with large pancreatic tumors or intra-abdominal masses anterior to the plexus will distort the anatomy and will make it very challenging to identify the aorta and the celiac trunk.
- If the mass anterior to the plexus is vascular (by a prior CT or MRI) or as identified by ultrasound examination, the anterior approach should be abounded.
6. SONOANATOMY OF THE EPIGASTRIUM AND RELEVANT STRUCTURES
The celiac plexus is a conglomerate of tiny nerve fibers and autonomic ganglia which form a rather heterogeneous tissue that is – to date – not visualized clearly by high-resolution ultrasound. One should be familiar with the relevant sonoanatomy in order to safely and accurately perform the procedure. One landmark is the abdominal aorta, which is usually well visualized by ultrasound (best use 2–5 MHz broadband probes) in the median epigastrium as tubular pulsating more or less anechoic structure (Fig. 3a, b). The US probe has to be tilted upward to meet the aorta as it enters the abdomen, where it is bordered by the muscular arcade of the diaphragm (Fig. 4a, b). The first artery that arises from the aorta is the celiac trunk (CT), which provides arterial supply to the liver, stomach, spleen, and the pancreatic head. It is the “ultimate landmark” for the celiac plexus. As the celiac trunk exits the aorta, it presents as a typical ram-horn-like symmetric bifurcation (Fig. 5a, b). The second artery arising from the aorta is the SMA, which provides arterial supply to the proximal parts of the bowel – in combination with the CT – the pancreatic head, and the duodenum. The origins of the CT and the SMA are very close to each other that may provoke a wrong allocation in the axial scan. That is why we also perform a sagittal (longitudinal) scan to accurately identify both arteries (Fig. 6a, b). In some rather rare cases, a common trunk for the CT and SMA is found, and it serves as the unique landmark for the celiac plexus. The next artery after the SMA is the left renal artery (Fig. 7).
So the area of the celiac plexus is broadly bordered by several organs (Fig. 8a, b): by the left lobe of the liver, which is rather covering the scene to the ventral right; by the stomach, which frames the area to the ventral left up to its transition to the distal esophagus; and by the pancreas, which is more or less riding on the lienal vein. The cranial boundary is the diaphragm with its exiting aorta (muscular arcade of the aortic hiatus) and the esophagus (esophageal foramen). Inferiorly, the celiac plexus continues with the renal plexus around the origin of the renal arteries.
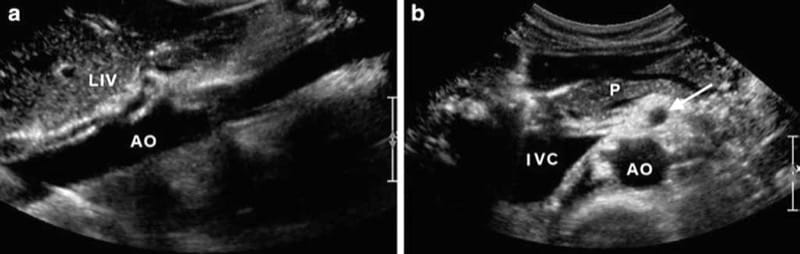
Fig.3 (a) Sagittal scan showing the epigastric segment of the aorta (AO) which is covered by liver tissue (LIV) anteriorly. (b) Axial scan showing the epigastric aorta (AO) as an anechoic disk neighbored by the anechoic cross-section of the inferior vena cava (IVC) and covered by the pancreas (P). The arrow is pointing at the superior mesenteric artery (SMA)
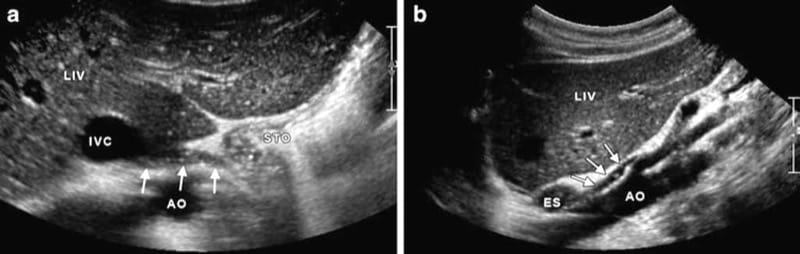
Fig.4 (a) Axial scan showing the muscular arcade of the aortic hiatus of the diaphragm (three arrows) which borders the aorta in the inferior vena cava (IVC), liver (LIV), and stomach (STO). (b) Corresponding sagittal scan showing the aorta (AO) with the first exit-ing arterial branches which are partially covered by the muscular arcade of the aortic hiatus (arrows). ES esophagus
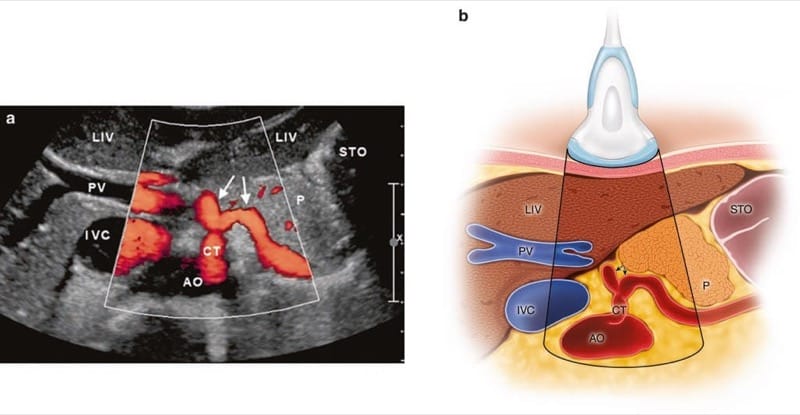
Fig.5 Axial scan with duplex mode (a) and corresponding illustration (b) showing the typical ram-horn-like appearance of the celiac trunk (CT). AO aorta, IVC inferior vena cava, LIV liver, PV portal vein, P pancreas, and STO stomach. (Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography© 2010. All rights reserved)
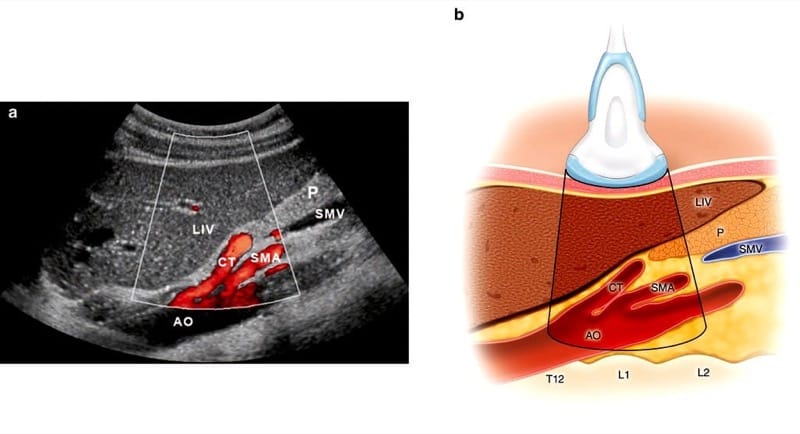
Fig.6 Sagittal scan with duplex mode (a) and corresponding illustration (b) showing the aorta (AO) with the first arterial branches: the celiac trunk (CT) and the superior mesenteric artery (SMA). LIV liver, P pancreas, and SMV superior mesenteric vein. (Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography© 2010. All rights reserved)
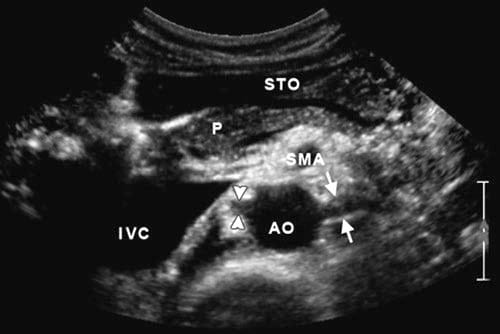
Fig.7 Axial scan more caudally showing the aorta (AO) and with the left renal artery (arrows) and the right renal artery (arrowheads). IVC inferior vena cava, P pancreas, STO stomach, and SMA superior mesenteric artery.
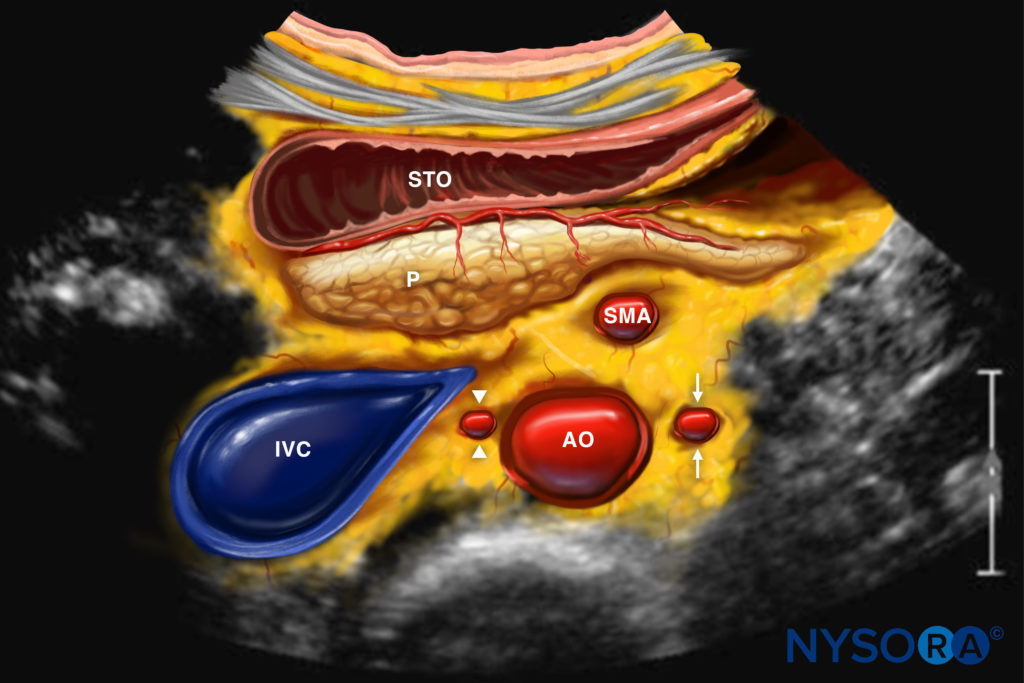
Reverse Ultrasound Anatomy illustration of figure 7. AO, aorta; IVC, inferior vena cava; P, pancreas; STO, stomach; SMA, superior mesenteric artery.
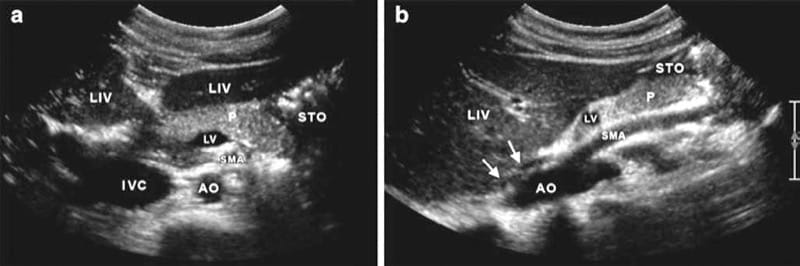
Fig.8 (a) Axial scan showing the left and right liver lobes sepa-rated by the echoic falciform ligament (LIV), the pancreas body (P), and the stomach at the left (STO). The lienal vein entering the portal confluens (LV). SMA superior mesenteric artery, IVC inferior vena cava, and AO Aorta. (b) Corresponding sagittal scan showing the structures of interest. The aorta (AO) with the exiting superior mesenteric artery (SMA), and the liver (LIV). The pancreas (P) covered by the distal parts of the stomach (STO). The proximal part of the lienal vein (LV) is depicted as well as the muscular arcade of the aortic hiatus (arrows).
7. PERCUTANEOUS ULTRASOUND-GUIDED CELIAC PLEXUS BLOCK TECHNIQUE
The patient is in the supine position, and the ultrasound transducer is positioned over the epigastrium, just caudal to the xiphoid process (Fig. 9). A scout scanning is performed so the operator will be familiar with the relevant anatomy especially in malignant cases where the anatomy may be distorted and accordingly plan on the safest and shortest path for the needle (usually out-of-plane). We obtain both short-axis and long-axis views to correctly identify the celiac trunk (CT) and the SMA (see above). A 20- or 22-gauge needle is then introduced under direct vision in the short axis or the long axis. We prefer to advance the needle from the lateral side of the transducer (short-axis view) to lie just cephalad to the origin of the CT and not between the CT and SMA, to avoid injury to those vessels or their branches. The injection is carried out with real-time sonography after negative aspiration and negative test dose as ultrasound is not accurate in recognizing intravascular injections at such depth.
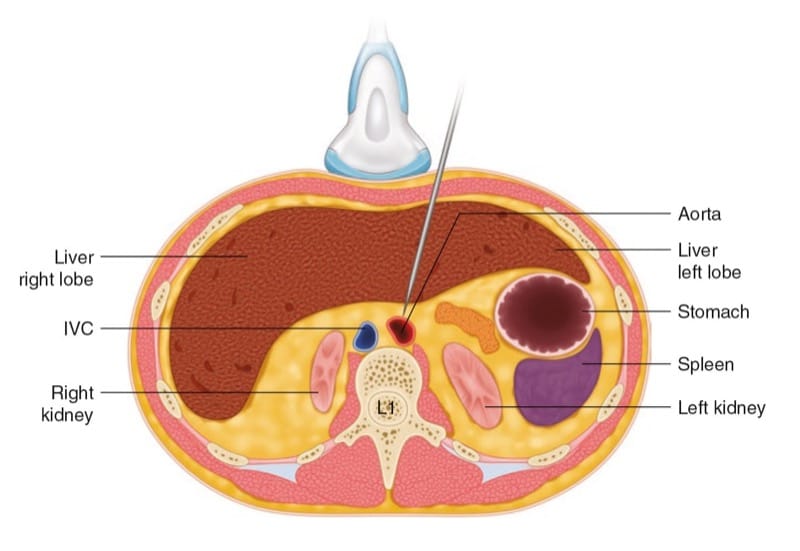
Fig.9 Short-axis illustration showing the position of the US transducer and the needle to perform celiac plexus block.
(Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography© 2010. All rights reserved)